Abstract
Fluorine 18 Sodium Fluoride (18F-NaF) (sodium fluoride) PET/CT is a highly sensitive but is a non-specific method for identifying bone metastases. Qualitative scan interpretation using low dose CT for lesion localization is often complicated by the presence of co-existing degenerative joint disease (DJD). A semi-quantitative analysis might help in accurately differentiating benign from metastatic osseous lesions. The aim of the study was to evaluate the clinical utility of 18F-NaF PET/CT in differentiating DJD from metastatic disease in the skeleton using a qualitative analysis as well as a semi-quantitative approach using the SUVmax and to determine if there is an upper limit of SUVmax value that can reliably differentiate metastases from DJD. Baseline 18F-NaF PET/CT scans were performed for 17 castrate resistant prostate cancer patients (CRPC). A qualitative as well as semi-quantitative analysis using maximum standardized uptake value (SUVmax) based on body weight was performed for 65 metastatic and 56 DJD sites identified on the low dose CT scan acquired as a part of whole body PET/CT scan. The SUVmax range in DJD was 2.6-49.9 (mean: 6.2). The SUVmax range for metastatic lesions was 11.2-188 (mean: 160). The SUVmax value for metastatic as well as areas of DJD showed significant variation during treatment. Bone metastases showed statistically significantly higher SUVmax than DJD using a mixed effect regression model. ROC/AUC analysis was performed based on averaging the SUVs over all lesions in each subject. The AUC was found to be fairly high at 0.964 (95% CI: 0.75-0.996). The SUVmax over 50 always represented a bone metastasis and below 12 always represented a site of DJD. The results of our preliminary data show that semi-quantitative analysis is complementary to the qualitative analysis in accurately identifying DJD from metastatic disease. The cut-off SUVmax of 50 can help in differentiating DJD from bone metastases.
Keywords: F-18 NaF, castrate resistant prostate cancer, PET/CT, degenerative joint disease, bone metastases
Introduction
Bone metastases are the most common malignant tumors and occur in 30-70% of all cancer patients. Prostate cancer is the leading cause for bone metastases in men [1]. The most frequent sites of metastases in prostate cancer patients are lymph nodes and bone. It has been found that 90% of patients who die of prostate cancer have bone metastases [2]. The prime objective of imaging in oncology patients is early identification of metastases, to accurately identify the full extent of metastatic disease, to evaluate the complications associated with metastatic disease such as pathologic fractures and cord compression, to monitor response to therapy and in some instances to guide biopsy if histologic confirmation is required [3-5]. The bone scan (BS) is the most commonly used modality for detection of bone metastases because it is widely available and provides an entire skeletal visualization within a reasonable amount of time and cost [5-7]. 99mTc-Methylene diphosphonate (99mTc-MDP) is the most commonly used radiopharmaceutical for skeletal imaging in general nuclear medicine [1,6]. The spine is a common site of skeletal metastases however degenerative changes are also found to be very common in most of the patients which should not be confused with malignant/metatstaic disease [8].
Several reports have raised doubts whether the BS is as effective for identifying metastatic disease as was previously perceived [9-11]. Although the addition of single photon emission tomography (SPECT) and SPECT-CT has improved diagnostic accuracy and is helpful in differentiating benign from malignant disease, the main drawback of SPECT is that data is acquired for a limited region in the skeleton. Hence it is not logistically feasible in a routine clinical practice to perform several SPECT acquisitions tomographically to assess the entire skeleton [12]. 18F-NaF was first introduced as a bone imaging agent by Blau et al [13]. 18F-NaF PET imaging combines the superior pharmacokinetic properties of 18F-NaF compared to 99mTc-polyphosphonates and has improved spatial resolution and lesion contrast of PET technology. 18F-NaF PET/CT provides high target to background ratios but accumulates in both malignant and benign disease similar to 99mTc-MDP bone scan [14-16]. Relatively little data is available using semi quantitative assessment based on SUVmax in differentiating DJD from bone metastases with 18F-NaF PET/CT.
The aim of the study was to evaluate the clinical utility of SUVmax in differentiating DJD from bone metastases in castrate resistant prostate cancer patients (CRPC) undergoing 18F-NaF PET/CT scans. An additional aim of this study was to determine an upper limit of SUVmax that can reliably differentiate DJD from bone metastases in CRPC patients.
Materials & methods
This imaging study was performed as a pilot sub study of a multicenter clinical trial for CRPC patients with bone metastases. The study was approved by the local institutional review board. Seventeen baseline 18F-NaF PET/CT scans were performed between February 2012 and December 2013 for 17 CRPC patients enrolled in the clinical trial. All the patients enrolled in the study had documented bone metastases on 99mTc-MDP bone scans. The age range for patients was 56-76 years (mean 63.3 yrs). A total of 65 metastatic and 56 DJD sites showing increased tracer uptake were analyzed qualitatively as well as semi-quantitatively.
PET/CT technique
No special preparations were needed before 18F-NaF administration. Patients were administered approximately 185 MBq (5 mCi) of 18F-NaF intravenously; whole body PET/CT scans were obtained from toes to vertex 60 minutes post injection using a Discovery VCT PET/CT system (GE Medical Systems). Low dose CT was performed first with 140 kV, auto/smart mA with average 80 mA, 0.5 s per CT rotation, a pitch of 0.516 and a table speed of 20.6 mm/rotation, slice thickness of 5.0 mm, interval of 3.27 mm without any specific breath-holding instructions. A PET emission scan was obtained after the acquisition of the CT scan without changing the patient’s position. PET data was acquired from 12-13 bed positions with an acquisition time of 3 minutes per bed position. PET images were reconstructed using an ordered-subsets expectation maximization algorithm. CT data were used for attenuation correction and lesion localization. F-18 NaF PET/CT scans were interpreted using MIRADA software system on a McKesson workstation.
18F-NaF PET/CT interpretation
18F-NaF PET/CT scans were independently interpreted by 3 experienced nuclear physicians (SP, LH and SM). The readers were unaware of clinical data and findings of other imaging modalities. The inter observer agreement among the three readers was 90%. Discrepant interpretations of the readers were resolved by consensus. 18F-NaF PET/CT scans were interpreted both qualitatively as well as semi quantitatively. In qualitative analysis areas of increased 18F-NaF uptake were categorized as DJD, metastases or equivocal by correlating increased skeletal uptake on corresponding low dose CT images. Lesions were categorized as bone metastases if increased uptake correlated in location with a lytic, sclerotic, mixed lytic-sclerotic change on low dose CT images. Lesions were categorized as DJD if increased uptake correlated to facet joints, endplates, within osteophytes and around the joints on low dose CT images. If the low dose CT did not show any abnormality over areas of increased uptake, such lesions were categorized as inconclusive. The rationale of this categorization is that in clinical practice, areas of increased uptake in the skeleton without definite CT changes may require further validation of the nature of the lesions with either other imaging modality or rarely involves tissue diagnosis especially in baseline scans. Semi quantitative assessment was performed using maximum standardized uptake value calculated from drawing region of interest (ROI) over sites of increased uptake in the skeleton. SUVmax was recorded for five representative bone metastatses lesions (lesions were selected to represent both axial and appendicular skeleton when present) and 2-5 DJD lesions using low dose CT as standard of reference.
Statistical analysis
The differences in baseline SUVmax between DJD and bone metastases within each subject were tested. We used linear mixed effects model with diagnosis as a fixed effect and a subject specific random intercept term. These models take into account that each subject contributes multiple, possibly correlated SUVmax values. We then averaged the SUVmax values within each subject and fitted a logistic regression model to assess whether SUVmax could be used to classify lesions into the two disease groups. To assess the diagnostic ability the receiver operating characteristic curve (ROC) and the area under the curve (AUC) with its associated 95% confidence interval were obtained. P < 0.05 (two-sided) was the criterion for statistical analysis. All statistical graphics and computations were done in R 3.0.1 (R development Core Team, 2013).
Results
The SUVmax in degenerative joint disease (DJD) was found to range from 2.6 to 49.9 SUV with a mean of 22.6 SUV, and the SUVmax for bone metastases ranged from 11.2 to 188 SUV with a mean of 63.4 SUV. There was some overlap of SUVmax range between DJD and metastatic bone lesions, but it was found that a SUVmax over 50 SUV always represented a bone metastasis and that a SUVmax below 11 SUV always represented a site of DJD in our patient population. F18 NaF PET/CT measures bone turnover which is osteoblastic activity [17]. So far in literature there is no generalized consensus on the SUVmax cutoff value with 18F-NaF PET/CT. In our study bone metastases showed statistically significantly higher SUVmax than DJD (p < 0.001) when looking at all lesions (Figure 1) as well as when comparing the average SUVmax for bone metastases to the average SUVmax for DJD within the patient (Figures 2 and 3). Variation of thresholds applied to the average SUVmax values of lesions within each patient gave us a ROC curve, the AUC of which was found to be fairly high at 0.964 (95% CI: 0.75-0.996) (Figure 4).
Figure 1.

This figure shows the box plots for all the representative degenerative joint disease (DJD) and metastases lesions. The distributions were compared using a 2-sided t-test (p < 0.001).
Figure 2.
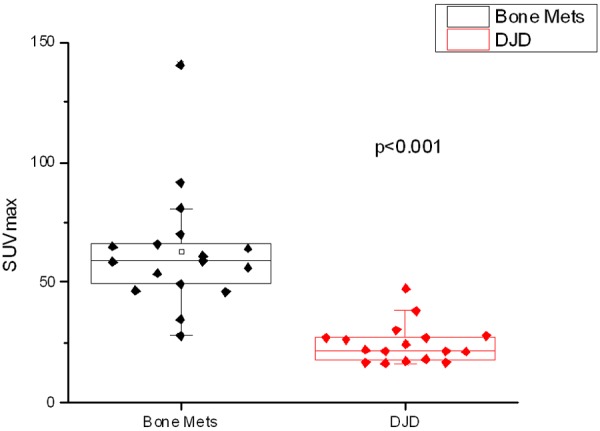
Box plots of the average SUVmax values of the representative degenerative joint disease (DJD) and metastases (Bone Mets) lesions of each patient. The distributions were compared using a 2-sided t-test (p < 0.001)..
Figure 3.
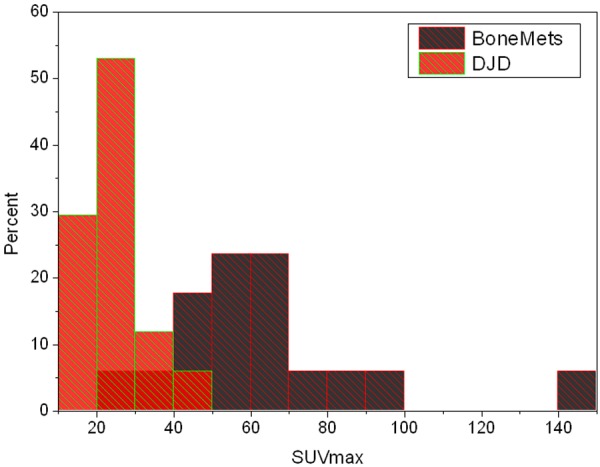
Histograms of the average SUVmax values of the representative degenerative joint disease (DJD) and bone metastases (Bone Mets) lesions of each patient, showing the overlap of the two distributions.
Figure 4.
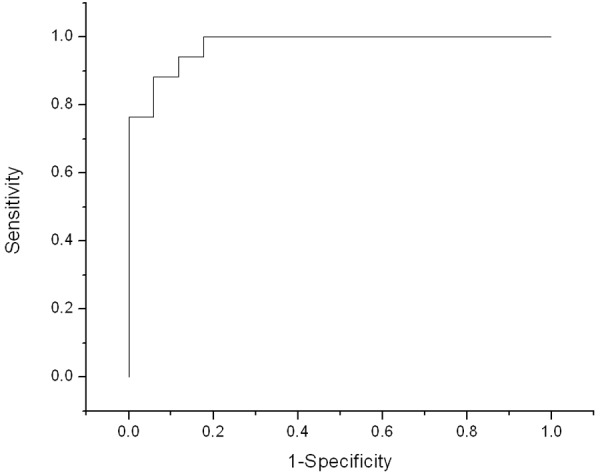
ROC curve coming from using different thresholds of patient averaged SUVmax to discriminate between degenerative and metastatic bone lesions; Area under the curve (AUC) = 0.976 (0.804-0.997).
Discussion
Early detection or exclusion of bone metastases is of high clinical significance in the management of patients with high risk prostate cancer patients [2]. The detection of bone metastases in advanced hormone refractory disease can guide in modifying therapy. It has been known that identification of osseous metastatic disease is often confounded by degenerative bone disease particularly in the spine and periarticular regions. The degenerative bone changes are commonly seen in prostate cancer patients as most of the patients are above 50 years of age and the majority of these patients have co-existing degenerative disease in the skeleton. 18F-NaF is known to be a better imaging agent in identifying bone metastases based on its superior pharmacokinetic characteristics combined with superior resolution of PET imaging compared with conventional bone-seeking agents as 99mTc-MDP. After diffusing through capillaries into bone extracellular fluid, fluoride ions exchange with hydroxyl groups in hydroxyapatite crystal bone to form fluoroapatite which is mainly deposited at the surface where there is greatest bone remodeling and turnover [6]. Although the uptake pattern is similar for both agents, 18F-NaF shows high target to background ratio due to its minimal protein binding, lower molecular weight resulting in faster blood clearance and a 2 fold higher uptake compared to Tc-99m polyphosphonates [18]. 18F-NaF is found to be superior compared to conventional planar or SPECT skeletal scintigraphy as increased uptake is found in both sclerotic and lytic metastases. But high sensitivity of 18F-NaF is also seen in non-malignant bone pathologies such as DJD. This limits the specificity of 18F-NaF PET in differentiating between a benign and a metastatic lesion [19]. The pattern of uptake is somewhat helpful as metastatic lesions typically tend to involve the posterior part of the vertebral body and pedicle or involve the vertebra extensively in advanced cases. Increased uptake in the osteophytes, endplates, spinous processes and around joints is considered benign [20]. In rare cases, the metastasis may be isolated to the lamina or the spinous process [21]. In our study prominent increased uptake was identified in the spinous process in 4 patients, which was found to be metastatic based on radiologic characteristics as well as the intensity of uptake (Figure 5).
Figure 5.
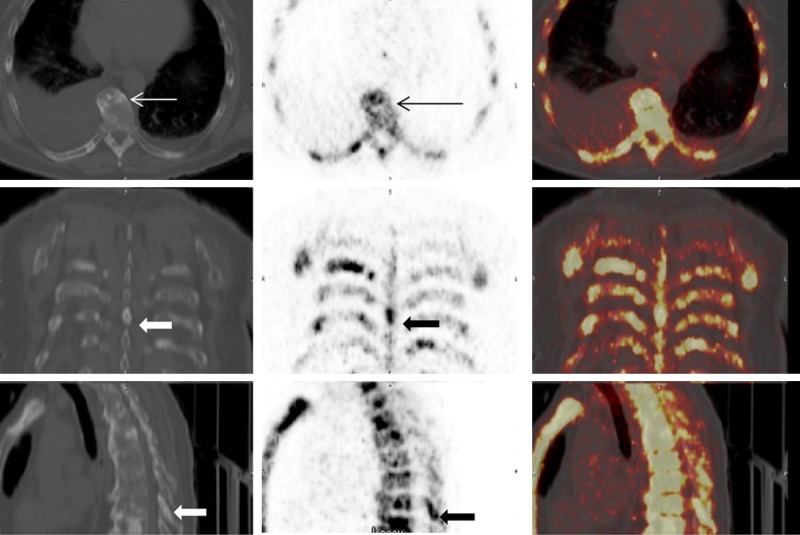
72 year old male with CRPC(Castrate resistant prostate cancer). 18F-NaF PET/CT images of upper thoracic spine. CT (left), PET (middle) and fused PET/CT images (right) in axial, coronal and sagittal views. Prominent increased uptake is seen in the spinous process metastatic lesion on PET images (thick black arrow); CT images show a sclerotic lesion in the spinous process. (Thick white arrow). Increased uptake (thin black arrow) is also seen in a thoracic vertebra which corresponds to an osteophyte on CT images (thin white arrow). The intensity of uptake in the osteophyte is much less compared to the uptake seen in the spinous process.
Studies measuring regional skeletal kinetic parameters using compartmental modeling and nonlinear regression analysis using PET tracers have been reported to have a role in differentiating malignant from benign lesions as well as for monitoring response to therapy [15,22]. The clinical utility of SUVmax in differentiating benign from metastatic disease has not been reported so far for 18F-NaF PET/CT. The use of a semi-quantitative method of measuring SUVmax within a region of interest in oncology imaging using Fluorine-18 Fluorodeoxyglucose (18F-FDG) is well-established and is considered as the most widely used parameter for quantitation of lesions in 18F-FDG PET/CT studies [23]. 18F FDG uptake reflects the metabolic rate of glycolysis in tumors and thus provides additional information to morphologic imaging [24]. However 18F FDG PET/CT is known to be less senitive than bone scintigraphy for osseous metastases in prostate cancer [25].
In our preliminary study we found a statistically significant difference in SUVmax between DJD and bone metastases. This holds promise for a future potential role of measuring the degree oF-NaF uptake in bone metastases and DJD lesions in prostate cancer patients using SUVmax in accurately differentiating between benign and metastatic bone disease. The uptake of 18F-NaF depends on a number of factors including blood flow, regional osteoblastic activity as well as on renal clearance [26]. In our study we found statistically significant difference in the SUVmax value of DJD from bone metastases which could be likely related to more high osteoblastic activity in the cancellous bone in response to bone metastases. Cook et al in 2011 published a study using 18F-NaF to assess response in castrate resistant prostate cancer patients treated with Radium-223 Chloride (Ra-223) by looking at changes in SUVmax values [27]. However they did not look at SUVmax value to differentiate between DJD and bone metastases. To the best of our knowledge no study is currently available using SUVmax in 18F-NaF PET/CT to differentiate DJD from bone metastases.
There were few limitations of this study including the small sample size. There was also some overlap of SUVmax values between DJD and bone metastases. None of the metastatic or DJD lesions were biopsied to confirm histopathology. However in the clinical world this is done not very frequently. The semi-quantitative method of SUVmax has already been criticized especially in FDG PET primarily due to its vulnerability to a large number of factors of variability. The favorable kinetics of fluoride hold promise as it is not affected by patient’s glycemic status suggesting that the SUV of 18F-NaF fluctuates less than the SUV in a FDG PET study [28,29]. Further prospective studies are needed in the future to establish a more robust role of SUVmax as a semi quantitative parameter in accurately identifying metastatic disease to the skeleton from benign lesions with 18F-NaF PET/CT.
Conclusion
This pilot study demonstrated that bone metastases have a significantly higher SUVmax compared to DJD. The SUVmax value along with CT characteristics of the lesions should further enhance the diagnostic accuracy of 18F-NaF PET/CT. Accurate identification and quantification of osseous lesions can be a step towards using 18F-NaF PET/CT as a reliable imaging biomarker in the future.
Acknowledgements
This research was funded by PCF Mazzone Challenge, PCF Creativity, DOD Prostate Cancer Clinical Trials Consortium (W81XWH-09-1-0142) and NCI CCSG grant (P30CA014520).
Disclosure of conflict of interest
None.
References
- 1.Padhani A, Husband J. Bone metastases. In: Husband JES, Reznek RH, editors. Imaging in Oncology. Oxford, U.K.: Isis Medical Media Ltd; 1998. pp. 765–787. [Google Scholar]
- 2.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18FfluoridePET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 3.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64. [PubMed] [Google Scholar]
- 4.Schirrmeister H, Guhlnamm A, Kotzerke J, Santjohanser C, Kühn T, Kreienberg R, Messer P, Nüssle K, Elsner K, Glatting G, Träger H, Neumaier B, Diederichs C, Reske SN. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J. Clin. Oncol. 1999;17:2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 5.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 6.Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med. 2001;31:28–49. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 7.Gottschalk A, Ludema K. Oncologic imaging: interactions of nuclear medicine with CT and MRI using the bone scan as a model. Semin Nucl Med. 1997;27:142–151. doi: 10.1016/s0001-2998(97)80044-x. [DOI] [PubMed] [Google Scholar]
- 8.Rosen RS, Fayad L, Wahl RL. Increased 18F-FDG uptake in degenerative disease of the spine: Characterization with 18F-FDG PET/CT. J Nucl Med. 2006;47:1274–1280. [PubMed] [Google Scholar]
- 9.Schirrmeister H, Guhlmann A, Elsner K, Kotzerke J, Glatting G, Rentschler M, Neumaier B, Träger H, Nüssle K, Reske SN. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18FPET. J Nucl Med. 1999;40:1623–1629. [PubMed] [Google Scholar]
- 10.Schirrmeister H, Glatting G, Hetzel J, Nüssle K, Arslandemir C, Buck AK, Dziuk K, Gabelmann A, Reske SN, Hetzel M. Prospective evaluation of clinical value of planar bone scan, SPECT and 18F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800–1804. [PubMed] [Google Scholar]
- 11.Jacobson AF, Fogelman I. Bone scanning in clinical oncology: does it have a future? Eur J Nucl Med. 1998;25:1219–1223. doi: 10.1007/s002590050287. [DOI] [PubMed] [Google Scholar]
- 12.Even-Sapir E. Imaging of Malignant Bone Involvement by Morphologic, Scintigraphic, and Hybrid Modalities. J Nucl Med. 2005;46:1356–67. [PubMed] [Google Scholar]
- 13.Blau M, Nagler W, Bender MA. A new isotope for bone scanning. J Nucl Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 14.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, Satyamurthy N, Barrio JR, Phelps ME. Evaluation of skeletal kinetics of fluorine 18-fluoride ion and PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 15.Schiepers C, Nuytes J, Bormans G, Dequeker J, Bouillon R, Mortelmans L, Verbruggen A, De Roo M. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med. 1997;38:1970–1976. [PubMed] [Google Scholar]
- 16.Cook JR, Fogelman I. The role of positron emission tomography in the management of bone metastases. Cancer. 2000;88:2927–2933. doi: 10.1002/1097-0142(20000615)88:12+<2927::aid-cncr8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Al-Beyatti Y, Siddique M, Frost ML, Fogelman I, Blake GM. Precision of 18F-fluoride PET skeletal kinetic studies in the assessment of bone metabolism. Osteoporos Intl. 2012;23:2535–2541. doi: 10.1007/s00198-011-1889-2. [DOI] [PubMed] [Google Scholar]
- 18.Fischer RD. Musculoskeletal imaging using fluoride PET. Semin Nucl Med. 2013;43:427–433. doi: 10.1053/j.semnuclmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, Lievshitz G, Ron I, Mishani E. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004;45:272–278. [PubMed] [Google Scholar]
- 20.Even-Sapir E, Martin RH, Barnes DC, Pringle CR, Iles SE, Mitchell MJ. Role of SPECT in differentiating malignant from benign lesions in the lower thoracic and lumbar vertebrae. Radiology. 1993;187:193–198. doi: 10.1148/radiology.187.1.8451412. [DOI] [PubMed] [Google Scholar]
- 21.Georgy BA. Metastatic spinal lesions: State-of-the-art treatment options and furture trends. AJNR Am J Neuroradiol. 2008;29:1605–1611. doi: 10.3174/ajnr.A1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki J, Watanabe H, Shinozaki T, Takagishi K, Ishijima H, Oya N, Sato N, Inoue T, Endo K. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology. 2001;219:774–777. doi: 10.1148/radiology.219.3.r01ma08774. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Schiepers C, Lake R, Dadparvar S, Berenji GR. Clinical utility of (18)F-fluoride PET/CT in benign and malignant bone diseases. Bone. 2012;50:128–139. doi: 10.1016/j.bone.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 24.Weber WA. Positron emission tomography as an imaging biomarker. J. Clin. Oncol. 2006;24:3282–3292. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 25.Iagaru A, Young P, Mittra E, Dick DW, Herfkens R, Gambhir SS. Pilot prospective evaluation of 99mTc-MDP scintigraphy, 18F-NaF PET/CT, 18F FDG PET/CT and whole-body MRI for detection of skeletal metastases. Clin Nucl Med. 2013;38:e290–296. doi: 10.1097/RLU.0b013e3182815f64. [DOI] [PubMed] [Google Scholar]
- 26.Suenaga H, Yokoyama M, Yamaguchi K, Sasaki K. Residual ridge beneath the denture base of an RPD observed using NaF-PET/CT. J Prosthodont Res. 2012;56:42–46. doi: 10.1016/j.jpor.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Cook G Jr, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin) EJNMMI Res. 2011;1:4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser EP, Boerman OC, Oyen WJ. SUV: from silly useless value to smart uptake value. J Nucl Med. 2010;51:173–175. doi: 10.2967/jnumed.109.068411. [DOI] [PubMed] [Google Scholar]
- 29.Keyes JW Jr. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]


