Abstract
While 18F-Fluorodeoxyglucose (18F-FDG) Positron-Emission Tomography (PET) has limited value in prostate cancer (PCa), it may be useful for specific subgroups of PCa patients with hormone-resistant poorly differentiated cell types. 18F-Fluorocholine (18F-FCH) PET/CT has been increasingly used in primary and recurrent PCa and has been shown to add valuable information. Although there is a correlation between the foci of activity and the areas of malignancy in the prostate gland, the clinical value of 18F-FCH is still controversial for detection of the malignant focus in the prostate. For the T-staging of PCa at diagnosis the value of 18F-FCH is limited. This is probably due to limited resolution of PET system and positive findings in benign prostate diseases. Conversely, 18F-FCH PET/CT is a promising imaging modality for the delineation of local and distant nodal recurrence and bone metastases and is poised to have an impact on therapy management. In this review, recent studies of 18F-FCH PET/CT in PCa are summarized.
Keywords: Prostate cancer, PET/CT, 18F-Fluorocholine
Introduction
Prostate cancer (PCa) is the second most common cancer (skin cancer is the first), and is one of the most common causes of cancer death in men. Due to both aging of the population and the availability of Prostate Specific Antigen (PSA) as a serum prostate biomarker, the incidence and prevalence of PCa have significantly increased [1]. Accurate diagnosis, staging, and restaging of PCa are essential for optimal therapeutic management. In this regard, diagnostic imaging has various important and challenging roles.
Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) have been used for diagnosis and staging of PCa with modest accuracy [2]. MRI is especially helpful for evaluating capsular invasion and seminal vesicle involvement. Conventional nuclear medicine examinations such as bone scans with 99mTc-MDP have specific indications and limitations. There has been a growing tendency towards multi-parametric MRI and molecular imaging with Positron-Emission Tomography (PET) radiotracers in PCa. Hybrid PET/CT and PET/MR scanners with more robust attenuation correction allow images of tumor-specific function acquired with the PET component to be matched to anatomic locations on CT or MR to differentiate between physiologic activity and pathologic uptake.
18F-Fluorodeoxyglucose (18F-FDG), a glucose analog, is the most common radiotracer used in oncology. Although 18F-FDG PET/CT has proven useful in a variety of tumors, the results in PCa have not been promising because of the relatively low metabolic activity of prostate cancer cells and the close proximity of the bladder and the high urinary excretion of 18F [3,4]. 18F-FDG will most likely be useful in selected PCa patients with hormone-resistant poorly differentiated cell types [5-7].
The limited usefulness of 18F-FDG led to the development of other radiotracers for PCa. Choline derivatives are one class of PET tracers that have been extensively evaluated during the last decade. Choline is a component of prostate cell membrane phospholipid. Many radiotracers have been synthesized to image choline, including 11C-choline, and 18F-fluorocholine (18F-FCH). Although 11C compounds have the advantage of less urinary excretion, the shorter half-life of 11C (20 minutes) has limited its use in clinical practice. In this article, we review clinical studies of 18F-FCH in PCa, evaluating its performance in delineation of PCa in the prostate gland and detection of nodal involvement and distant metastases.
Mechanism of uptake and normal biodistribution of 18F-FCH
Choline is the precursor for the biosynthesis of phospholipids in the cell membrane and enters the cell through choline transporters. Choline is used for synthesising phosphatidylcholine via the Kennedy pathway [8]. The first step of this pathway is the rate-limiting step, in which choline kinase catalyzes the phosphorylation of choline into phosphocholine [9]. Choline kinase is overexpressed in PCa, resulting in the elevated levels of phosphocholine needed to support malignant transformation. The endogenous synthesis of choline also seems to be up-regulated in cancer cells. In 1998, Hara et al. described the usefulness of 11C-choline in PCa imaging [10]. In 2001, DeGrado et al. reported their experience in synthesizing 18F-FCH and other radiolabeled choline derivatives and showed that in vitro phosphorylation of 18F-FCH by choline kinase was similar to that of choline [11].
The optimal imaging protocol has not been established. Typically, the acquisition starts 1 min after intravenous injection of 18F-FCH (4.07 MBq/kg of body weight) with dynamic PET images of the pelvis acquired during the first 8-10 min (1 min/frame) to avoid the effect of urinary bladder activity, followed by a static semi-whole-body acquisition [12-14]. Delayed acquisition has also been described in the literature [15-18]. Unenhanced CT is performed for localization and attenuation correction. The effective dose of 18F-FCH has been estimated as 0.03 mSv/MBq by DeGrado et al. [19]. The critical organs are the kidneys (0.16 mSv/MBq). The bladder wall receives 0.06 mSv/MBq [19].
After injection of 18F-FCH, the radiotracer is rapidly cleared from the blood in 4-5 minutes. The liver and lung uptake plateau is reached by 10 minutes. Normal biodistribution of 18F-FCH is in the salivary glands, liver, spleen, pancreas; with variable activity in the bowel, in addition to the uptake in the kidneys and bladder from excretion. There is faint uptake in the cerebral cortex. Moderate uptake is seen in the choroid plexus, cavernous sinus, and extraocular and masticatory muscles. Variable uptake occurs in the lacrimal glands and nasal mucosa [20-24]. Diffuse or focal activity in the lungs may indicate pathology, although curvilinear-shaped mild physiologic activity in the dependent areas of the lungs can be seen, likely due to the supine position during the injection and uptake period. In the majority of cases, the adrenal glands do not show any uptake; there can be occasional uptake, however, in one or both glands [25]. The normal prostate may show faint activity; diffuse or focal increased uptake can be seen in prostatitis, benign prostate hyperplasia, or malignancy.
There may also be increased uptake of 18F-FCH in sites of inflammation such as the paranasal sinuses, thyroid, middle ear/mastoid and bone fractures [25,26]. This may lead to false-positive results [23]. Mild activity may be seen in mediastinal, hilar, axillary, and inguinal lymph nodes in the setting of a nearby inflammatory process (Figure 1).
Figure 1.
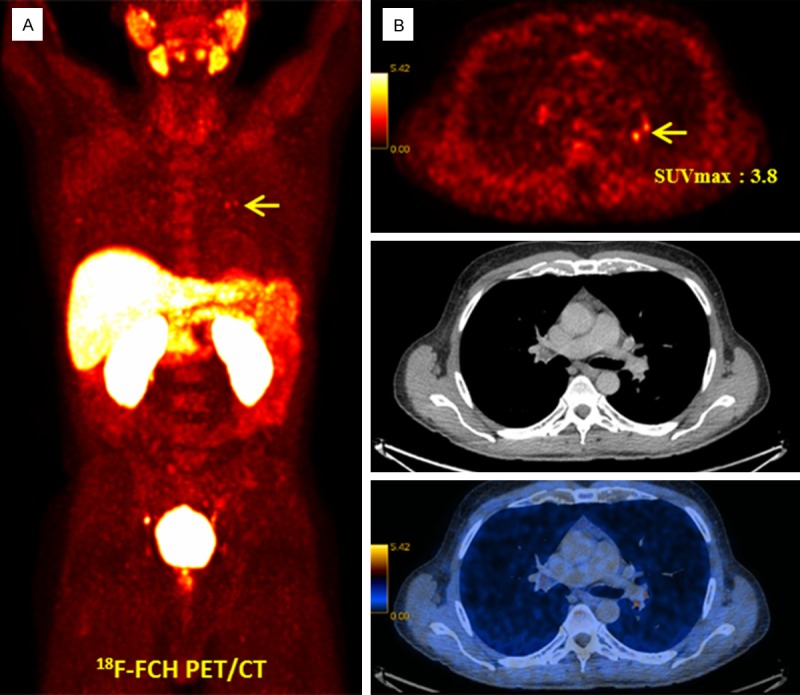
18F-FCH PET/CT in a 58-year-old prostate cancer patient, Gleason score 7, PSA 22.3 ng/mL, increasing PSA under anti-androgen treatment after prostatectomy and radiotherapy (biochemical recurrence). A: 18F-FCH PET MIP. B: Transaxial PET (upper row), CT (middle row) and PET/CT fusion (lower row). Mildly increased tracer uptake is visible in the left hilum arrow, SUVmax: 3.8)-suggestive of reactive lymph nodes verified as benign lesion in the follow-up clinical and imaging evaluation.
Local disease evaluation (imaging the prostate gland and T staging)
The diagnosis of a malignant focus in the prostate gland is usually based on ultrasound-guided biopsy when there is an elevated PSA level. However, it is not unusual to have a false-negative biopsy, which may lead to repeat biopsies. A noninvasive imaging modality to detect a malignant focus in the prostate gland would be helpful to guide biopsy and also to evaluate the size, extent, and multiplicity of the lesions inside the prostate gland [27]. Although 18F-FCH PET or PET/CT is more sensitive than US or CT, and is probably comparable with MRI for evaluating focal malignancy in PCa, the reported sensitivity and specificity of 18F-FCH for detection of malignancy in the prostate gland is variable, ranging from 64 to 100% for sensitivity and 47 to 90% for specificity [17,18,28,29]. The limited sensitivity is probably due to the limited spatial resolution of PET systems. The limited specificity is probably due to 18F-FCH uptake by benign prostatic hyperplasia and prostatitis.
Some investigators have reported poor correlation between foci of increased 18F-FCH activity in the prostate and malignancy. In a study by Igerc et al. in 20 patients with elevated PSA and negative biopsy, uptake in the prostate was categorized into focal, multifocal, or inhomogeneous patterns. A repeat biopsy was performed after the PET study, and in cases of focal uptake, the biopsy was guided by the PET images. Focal uptake was noted in 13 out of 20 patients. Malignancy was confirmed in five patients on repeat biopsy. None of the patients with multifocal or inhomogeneous 18F-FCH uptake had a malignancy found on repeat biopsy. They concluded that semi-quantitative values such as SUVmax were not helpful to differentiate benign prostate disease from malignancy [18]. Similarly, Schmid et al. studied the use of 18F-FCH in 19 patients with PCa. In nine patients who were evaluated at initial diagnosis, histologic findings of the resected prostate were compared to 18F-FCH uptake. Only in one patient did 18F-FCH correctly detect the focus of malignancy [30].
Other investigators have reported a closer correlation between foci of increased 18F-FCH uptake and malignancy in the prostate gland. Kwee et al. studied the value of 18F-FCH PET for sextant localization of malignant prostate tumors in 15 patients prior to radical prostatectomy. Histopathologic analysis of step-sectioned whole-mounted prostate specimens was used as a gold standard and compared with the SUVmax values in corresponding image sections. Histopathology demonstrated malignant involvement in 61 of 90 prostate sextants. Mean SUVmax was 6.0 ± 2.0 in malignant sextants and 3.8 ± 1.4 in benign sextants (P < 0.0001). They reported an area under the receiver operating characteristics (ROC) curve (AUC) of 0.82 for 18F-FCH uptake by means of SUVmax in predicting the presence of malignancy on a sextant basis in the prostate. However, tumor diameter directly correlated with sextant SUVmax in malignant sextants (r = 0.54, P < 0.05), and 18F-FCH PET failed to detect smaller foci of malignancy [29].
In a larger series (n = 130) of intermediate-to-high-risk patients prior to prostatectomy, the authors found a significant correlation (r = 0.68; P = 0.0001) between sections with the highest 18F-FCH uptake and sextants with the largest tumor burden on radical prostatectomy (Figure 2). However, we did not find good correlation between SUVmax and serum PSA levels (P = 0.10) or Gleason scores (P = 0.28) [15]. The key findings of studies on 18F-FCH uptake in malignant foci of prostate cancer are summarized in Table 1.
Figure 2.
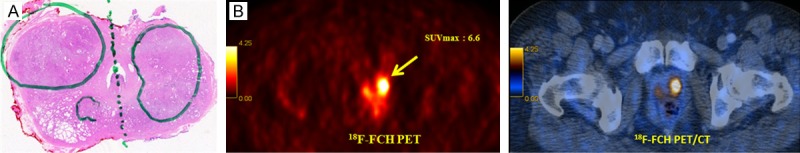
18F-FCH PET/CT staging in a 67-year-old prostate cancer patient, Gleason score 7, PSA 22.7 ng/mL [15]. A: Histopathology results: prostate adenocarcinoma in both lobes (marked). B: 18F-FCH PET/CT: left: transaxial PET image, right: transaxial PET/CT fusion image. 18F-FCH PET shows focal tracer uptake (SUVmax: 6.5) in both prostate lobes that correlate with histopathology findings (yellow arrow).
Table 1.
18F-FCH PET/CT for local disease (detection of malignancy in prostate gland)
| Article | Authors | Year | No. of patients | Findings |
|---|---|---|---|---|
| 1 [28] | Kwee et al. | 2005 | 17 | Prostate sextants positive for malignancy showed higher SUV-max than biopsy negative sextants |
| 2 [30] | Schmid et al. | 2005 | 19 | Only 1 out of 9 patients with 18F-FCH uptake proved to be malignant |
| 3 [17] | Husarik et al. | 2008 | 43 | Pathologic uptake was noted in 42 out of 43 patients with histologic proven PCa |
| 4 [18] | Igerc et al. | 2008 | 20 | Five out of 13 positive focal 18F-FCH uptake were malignant on pathology |
| 5 [29] | Kwee et al. | 2008 | 15 | Sixty one out of 90 prostate sextants with 18F-FCH uptake were malignant on pathology |
| 6 [15] | Beheshti et al. | 2010 | 130 | Good correlation between sections with the highest 18F-FCH uptake and malignancy |
Abbreviations: PCa, prostate cancer; 18F-FCH. [18F]fluoromethyl-dimethyl-2-hydroxyethyl-ammonium; SUV, standardized uptake value.
In summary, 18F-FCH has limited value for imaging and diagnosis of primary prostate gland malignancies. It may be helpful in cases with elevated PSA levels and negative biopsy to suggest the site for repeat biopsy. Based on currently available data, endorectal coil dynamic contrast-enhanced MRI/magnetic resonance spectroscopy (MRS) has better sensitivity than 18F-FCH [31,32]. However, this method is not widely available and the accuracy may be affected if it is done too soon after biopsy [33]. Integrated PET/MRI may eliminate the limitations of each modality alone and may have more indications in PCa [34,35].
Lymph node metastases
In a meta-analysis by Hovels et al. the pooled sensitivity and specificity of CT and MRI for detecting pelvic lymph node metastases from prostate cancer were approximately 39 and 80%, respectively [36]. The reported accuracy of 18F-FCH PET/CT for detecting regional lymph node metastases ranges from 10 to 100% for sensitivity, with a specificity of more than 90% (Table 2) [12,15,17,37,38]. This variable sensitivity is mostly due to the selected patient population (low-risk versus intermediate/high risk patients), size of the involved nodes, and the number of subjects in each study. In general, 18F-FCH PET/CT has a low to modest sensitivity and a high specificity for detecting involved nodes in the pelvic region.
Table 2.
18F-FCH PET/CT for detecting lymph node metastases in PCa at initial diagnosis
| Article [Reference] | Authors | Year | Patient’s Number | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 1 [12] | Hacker et al. | 2006 | 20 | 10% | 80% |
| 2 [17] | Husarik et al. | 2008 | 43 | 20% | |
| 3 [15] | Beheshti et al. | 2010 | 130 | 66%** | 96% |
| 4 [38] | Poulsen et al. | 2010 | 25* | 100% | 95% |
| 5 [37] | Beauregard et al. | 2010 | 15 | 63%*** | |
| 100%**** |
Abbreviations: PCa, prostate cancer; 18F-FCH. [18F]fluoromethyl-dimethyl-2-hydroxyethyl-ammonium; LNs, lymph nodes.
Patient population was intermediate to high risk patients with PCa.
For detecting lymph nodes greater than or equal to 5 millimeters.
For regional LNs.
For extra-pelvic LNs.
In a study by Hacker et al. on 20 men with intermediate-risk PCa, 18F-FCH PET/CT showed a low sensitivity (10%) and good specificity (80%) for the detection of lymph node metastases. Laparoscopic radioisotope-guided sentinel lymph node biopsy was more sensitive than 18F-FCH PET/CT [12]. A low sensitivity (20%) was also reported by Husarik et al. in 43 PCa patients undergoing preoperative PET/CT [17].
Conversely, Beauregard et al. reported a sensitivity of 63% for regional pelvic lymph node metastases and a sensitivity of 100% for extrapelvic lymph node metastases with 18F-FCH PET/CT among 16 patients with intermediate-to-high-risk PCa [37]. Poulsen reported a sensitivity of 100% and specificity of 95% in 25 intermediate-to-high risk PCa patients undergoing 18F-FCH PET/CT [38].
The authors also described the performance of 18F-FCH PET/CT in detecting lymph node metastases in 130 high-risk PCa patients. In our study the sensitivity, specificity, positive, and negative predictive values of 18F-FCH PET/CT for lymph node metastases ≥ 5 mm were 66, 96, 82, and 92%, respectively [15].
In summary, 18F-FCH PET/CT has a fair sensitivity and high specificity for detecting lymph node metastases ≥ 5 mm in intermediate-to-high-risk PCa patients (Figures 3 and 4). Radioisotope-guided sentinel lymph node biopsy/dissection is more sensitive for smaller lymph nodes [12].
Figure 3.
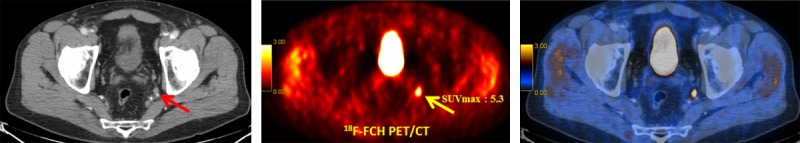
18F-FCH PET/CT staging in a 67-year-old prostate cancer patient, Gleason score 7, PSA 21.1 ng/mL. left: transaxial CT, middle: transaxial PET image, right: transaxial PET/CT fusion image. 18F-FCH PET/CT shows markedly increased tracer uptake (SUVmax: 5.3) in a small lymph node in the left internal iliac chain (arrows), verified as lymph node metastasis by histopathology [15].
Figure 4.
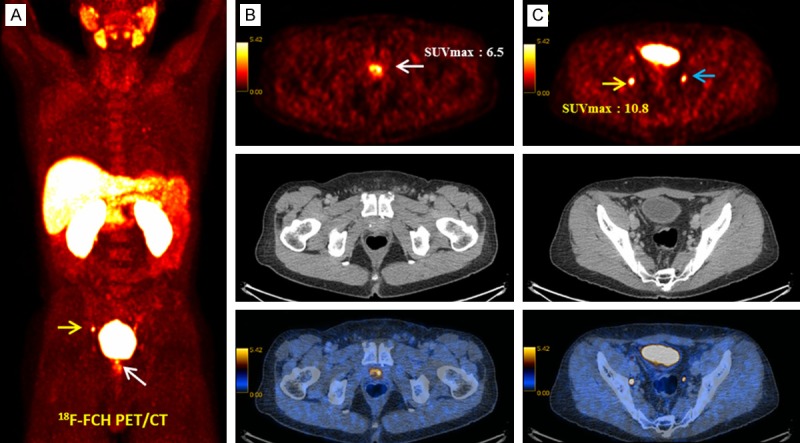
18F-FCH PET/CT in a 58-year-old prostate cancer patient, Gleason score 7, PSA 22.3 ng/mL, with increasing PSA under anti androgen treatment after prostatectomy and radiotherapy (Biochemical recurrence) [15]. A: 18F-FCH PET MIP. B: Transaxial PET (upper row), CT (middle row) and PET/CT fusion (lower row). Focally-increased tracer uptake in the prostate bed (arrow, SUVmax: 6.5) is suggestive of local recurrence verified in the clinical and imaging follow-up. C: Transaxial PET (upper row), CT (middle row) and PET/CT fusion (lower row). Focally-increased tracer uptake in a small right external iliac chain (yellow arrow, SUVmax: 10.8), proved as lymph node metastasis in the clinical and imaging follow-up. Incidental focal tracer accumulation is noticed in the left ureter (blue arrow) [47].
Bone metastases
Bone metastases are detected in approximately 65-75% of patients with PCa and often alter the prognosis [39,40]. Early detection of bone metastases is necessary for appropriate treatment management and to avoid complications such as fractures and spinal cord compression [41]. Whole body bone scan with 99mTc-MDP and single photon-emission tomography (SPECT) technique is still the most common examination for evaluating bone metastases with a good sensitivity but a low specificity. However, studies have revealed higher sensitivity with 18F-fluoride PET, likely due to better resolution of the PET system compared with gamma cameras and higher target-to-background ratios for 18F-fluoride than 99mTc-phosphonates because of its biological kinetics [42,43]. Both 99mTc-MDP bone scans and 18F-fluoride PET are nonspecific in their action; they show foci of bone with elevated bone turnover but do not specifically bind to malignancies. Choline derivatives have the advantage of binding to the actual malignant foci in the skeleton as well as to lymph node metastases.
Different studies have shown the use of 18F-FCH for detecting bone metastases in PCa (Table 3). Beauregard et al. reported a higher sensitivity for 18F-FCH PET/CT than conventional imaging modalities (100 vs 67%) for detection of bone metastases in 16 patients with PCa [37]. In another study, McCarthy et al. evaluated the usefulness of 18F-FCH PET compared with standard bone scans and CT in 26 patients with castration-resistant prostate carcinoma. The lesions in each modality were recorded and classified as concordant or discordant for the presence or absence of prostate cancer metastases. Discordant bone or soft tissue lesions were followed up for 2 years or until a definitive diagnosis of the discordant lesion could be made. Overall, 183 lesions were detected with 149 being concordant and 34 (19%) being discordant. Based on follow-up, 18F-FCH PET correctly identified the presence or absence of disease in 27 of 34 lesions. In 14 cases, FCH-positive lesions not identified on initial imaging were confirmed as disease on follow-up. The sensitivity, specificity, positive predictive and negative predictive values for lesion detection by 18F-FCH PET were 96, 96, 99 and 81%, respectively [44].
Table 3.
18F-FCH PET/CT for evaluating bone metastases in PCa
| Article | Authors | Year | No. of patients | Sensitivity | Specificity | Comment |
|---|---|---|---|---|---|---|
| 1 [47] | Beheshti et al. | 2008 | 70 (210 lesions) | 79% | 97% | (1) |
| 2 [37] | Beauregard et al. | 2010 | 16 | 100% | 67% for bone scan | |
| 3 [15] | Beheshti et al. | 2010 | 130 (43 lesions) | Change the therapy in 15% | ||
| 4 [44] | McCarthy et al. | 2011 | 26 (183 lesions) | 96% | 96% | |
| 5 [46] | Langsteger et al. | 2011 | 17 | (2) | ||
| 6 [45] | Kjolhede et al. | 2012 | 90* | (3) |
Abbreviations: PCa, prostate cancer; 18F-FCH. [18F]fluoromethyl-dimethyl-2-hydroxyethyl-ammonium. (1): 24% of bone metastases didn’t show any abnormality on CT scan. There was an inverse relationship between the intensity of 18F-FCH uptake and the degree of lesion sclerosis by means of Hounsfield Units. (2): A comparative study with 18F-Fluoride showed similar sensitivity. However, the specificity was slightly higher for 18F-FCH. (3) In 50 out of the 90 included patients (56%) one or both PET/CT scans indicated metastases. 18F-FCH PET/CT indicated lymph node metastases and/or bone metastases in 35 patients (39%).
The study was performed with both 18F-Fluoride and 18F-FCH on patients with normal or equivocal bone scans.
Kjolhede et al. evaluated the added value of 18F-FCH PET/CT and 8F-fluoride PET/CT in 90 patients with high-risk prostate cancers (PSA levels between 20 and 99 ng/mL and/or Gleason score 8-10) and normal or equivocal results on bone scan with 99mTc-MDP and CT [45]. None of the patients had received hormonal therapy before the staging procedures were completed. In 50 out of the 90 patients (56%] one or both PET/CT scans indicated bone or nodal metastases. 18F-FCH PET/CT indicated lymph node metastases and/or bone metastases in 35 patients (39%). 18F-fluoride PET/CT was suggestive for bone metastases in 37 patients (41%). In 18 patients (20%) the PET/CT scans changed the management. They concluded that PET/CT scans with 18F-FCH PET/CT and 18F-fluoride commonly detect metastases in patients with high-risk prostate cancer, and a negative or inconclusive bone scan and may change the therapy management.
The authors also evaluated the performance of 18F-FCH PET/CT for evaluating bone metastases in 130 PCa patients [15]. Forty-three bone metastases were detected in 13 patients. In two patients, early bone marrow infiltration was detected only with 18F-FCH PET/CT. 18F-FCH PET/CT led to a change in therapy in 15% of all patients and 20% of high-risk patients. We also compared the performances of 18F-FCH PET/CT and 18F-fluoride PET/CT in 17 patients with newly-diagnosed prostate cancer and 23 patients with suspected recurrence with a history of bone pain. Both radiotracers showed good diagnostic performance on patient-based and lesion-based analyses. However, the lesion-based analysis showed a significantly better specificity for 18F-FCH PET/CT [46].
In another study, the authors noted a sensitivity, specificity, and accuracy of 79, 97, and 84%, respectively, of 18F-FCH PET/CT to identify bone metastases in 70 patients undergoing either initial staging or restaging (Figure 5). In that study, 262 lesions showed increased 18F-FCH uptake, of which 210 were interpreted as malignant on the basis of the pattern of 18F-FCH uptake and CT findings. Of those 210 lesions, 207 were true positives and three were false positives. Interestingly, 49 out of 207 (24%) proven malignant lesions that were positive on 18F-FCH PET/CT had no corresponding morphological changes on CT. We also found an inverse relationship between the intensity of 18F-FCH uptake and the degree of lesion sclerosis measured in Hounsfield Units (HU). The lesions with more sclerosis on CT (> 825 HU) correlated with normal 18F-FCH activity. These lesions were mainly observed in patients who were on androgen deprivation therapy [47].
Figure 5.
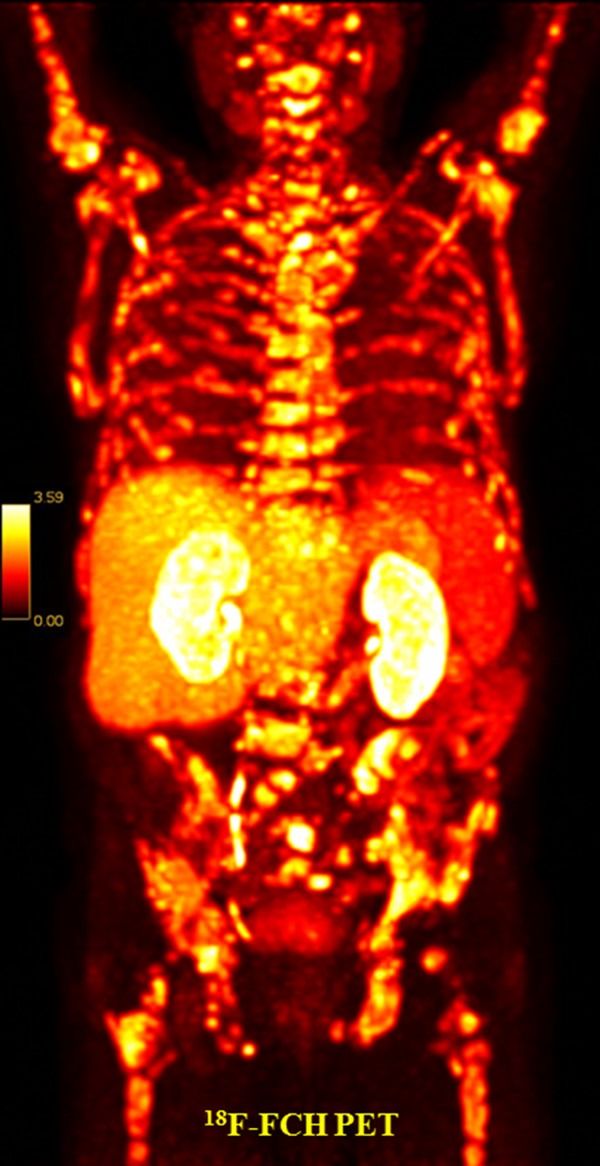
18F-FCH PET/CT: generalized bone metastases in the skeleton in a 74-year-old prostate cancer patient, Gleason score 9, PSA 53.22 ng/mL, status post radiotherapy to the prostate, regional lymph nodes, and lumbar spine with continued anti-androgen blockade. Planning for radionuclide treatment with 223radium.
In summary, the studies suggest better detection of bone metastases with 18F-FCH PET/CT and 18F-fluoride PET/CT compared with conventional bone scan. 18F-FCH PET/CT and 18F-fluoride PET/CT are taken up by bone metastases with different mechanisms and 18F-FCH PET/CT was slightly less sensitive. However, the specificity of 18F-FCH PET/CT was higher than that of bone scan or 18F-fluoride PET/CT.
Evaluation for recurrent disease
Approximately half of patients with PCa experience recurrence over the 10 years post initial therapy [48]. PSA is a sensitive indicator of recurrent disease. However, PSA is a biochemical marker of recurrence and does not show the site of recurrence or metastases, which is necessary for therapy planning. Conventional imaging modalities including CT, ultrasound, MRI, and bone scan have different limitations and a low-to-moderate sensitivity [49]. Transrectal ultrasound (TRUS) and MR with an endorectal coil are accurate modalities in evaluation for local recurrence [32].
18F-FCH PET/CT has been used to assess for local recurrence or metastases in PCa in the setting of biochemical recurrence (Table 4). Sensitivities from 42 to 96% have been reported in different studies [13,16,17,50-52]. The detection rate was higher in cases with higher PSA levels at the time of recurrence, and shorter PSA doubling time [50,53-56].
Table 4.
Some examples of 18F-FCH PET/CT studies in recurrent PCa
| Article | Authors | Year | No. of patients | PSA level | Sensitivity or detection rate | Comment |
|---|---|---|---|---|---|---|
| 1 [16] | Cimitan et al. | 2006 | 100 | > 0.1 ng/ml | 53% | (1) |
| 2 [32] | Vees et al. | 2007 | 22 | < 1 ng/ml | 55% | |
| 3 [51] | Pelosi et al. | 2008 | 56 | < 1, 1-5, > 5 ng/ml | 42% | (2) |
| 4 [17] | Husarik et al. | 2008 | 68 | Mean: 10.8 microg/L | 86% | |
| 5 [57] | Chondrogiannis et al. | 2013 | 46 | 1.1-49.4 ng/ml | 80.4% | (3) |
| 6 [55] | Beheshti et al. | 2013 | 250 | (4) | 74% | (4) |
Abbreviations: PCa, prostate cancer; 18F-FCH, [18F]. fluoromethyl-dimethyl-2-hydroxyethyl-ammonium. (1): 18F-FCH PET/CT is not likely to have a significant management impact on PCa patients with biochemical recurrence until PSA increases to above 4 ng/ml. However, in selected patients, 18F-FCH PET/CT helps to exclude distant metastases when salvage local treatment is intended. (2) Sensitivity was related to PSA levels, with 20%, 44% and 81.8% sensitivity values in the PSA < or = 1, 1 < PSA < or = 5 and PSA > 5 ng/ml subgroups, respectively. (3) 18F-CH PET/CT showed a high overall detection rate (80%), proportional to the trigger PSA (both for local and distant relapse) not influenced by androgen deprivation therapy. (4) 18F-FCH PET sensitivity was higher with increased trigger PSA levels (33, 77.5, 80.7, 85.2, and 92.8% for the trigger PSA levels of less than 0.3, more than 0.5, 1.0, 2.0, and 4.0 ng/mL, respectively).
Pelosi et al. reported a relatively low sensitivity (42%) for 18F-FCH PET/CT to detect lesions in post-prostatectomy patients with rising PSA. However, their detection rates increased with increasing PSA: 20% at PSA < 1 ng/mL; 44% at PSA = 1-5 ng/mL; 82% at PSA > 5 ng/mL) [51]. Vees et al. reported a 55% detection rate for 18F-FCH and 11C-acetate PET/CT in a small population (n = 22) of PCa patients with PSA levels < 1 ng/mL who were referred for adjuvant or salvage radiotherapy [32]. Prostate MRI was locally positive in 15 of 18 patients (83%). They concluded that since PET/CT studies correctly detected local residual or recurrent disease in only half of post-prostatectomy patients with PSA levels of < 1 ng/mL (Figure 4), the sensitivity and specificity were too low to use it as a standard diagnostic modality for early relapse or suspicion of subclinical minimally persistent disease, and that prostate MRI is probably more helpful, especially in patients with a low likelihood of distant metastases.
Studies reporting a higher positive detection rate (PDR) for 18F-FCH PET/CT for restaging include the report by Chondrogiannis et al., who found an 80.4% PDR in a study on 46 patients with radiotherapy-treated PCa with suspicion of relapse [57]. Similar to Pelosi et al.’s findings, they found that the PDR increased with increasing trigger PSA values. They also showed that the detection rate was not significantly influenced by androgen deprivation therapy (ADT).
In a study by the authors on 250 PCa patients with PSA relapse, 18F-FCH PET/CT correctly detected malignant lesions in 74% (185/250) of patients. The sensitivity of 18F-FCH PET was significantly higher (P = 0.001) in subgroups of patients with ongoing ADT (85%) compared with the patients who didn’t receive ADT (59.5%). Similar to the findings of Pelosi et al. and Chondrogiannis et al., 18F-FCH PET sensitivity was higher with increasing trigger PSA levels (77.5, 80.7, 85.2, and 92.8% for trigger PSA levels of > 0.5, 1.0, 2.0, and 4.0 ng/mL, respectively). The sensitivity was 33% in patients with a trigger PSA level < 0.3 ng/mL and 77% in patients with a trigger PSA level > 0.3 ng/mL. Using a binary logistic regression analysis model, we showed trigger PSA and ADT to be the only significant predictors of positive PET findings [55]. Other studies have suggest that ADT may decrease the detection rate of 18F-FCH PET/CT and should be withheld before the examination to reduce the risk of a false-negative study [17,58-60].
A recent meta-analysis by Evangelista et al. (19 studies were selected with a total of 1555 patients) for the role of 18F-FCH PET/CT for restaging in PCa recurrence was promising [61]. They calculated a pooled sensitivity of 85.6% and specificity of 92.6% for all sites of disease (prostatic fossa, lymph nodes, and bone), a pooled sensitivity of 75.4% and specificity of 82% for prostatic fossa recurrence, and a pooled sensitivity of 100% and specificity of 81.8% for lymph node metastases.
Despite a wide range of 18F-FCH PET/CT detection rates, a patient’s management may change based on the scan findings. Soyka et al. investigated the clinical value of 18F-FCH PET/CT in treatment decisions on PCa patients. They prepared questionnaires for 156 patients and sent them to their referring physicians 14-64 months after the studies. Questions included information regarding initial extent of disease, curative first-line therapy, treatment plan before and after 18F-FCH PET/CT, and also PSA values at diagnosis, after initial treatment, before 18F-FCH PET/CT, and at the end of follow-up. In 75 out of the 156 patients (48%) the management was changed based on the results of 18F-FCH PET/CT. They concluded that 18F-FCH PET/CT has an important impact on the therapeutic strategy in patients with PCa [62].
In summary, 18F-FCH PET/CT is a useful modality to detect recurrence or metastases in patients with PCa and rising PSA; the detection rate is higher with higher PSA levels; and patient management may change based on the 18F-FCH PET/CT findings.
Radiotherapy planning
Due to the limited lesion-based sensitivity in primary nodal staging with 18F-FCH PET/CT, radiation planning based on the choline PET/CT results is controversial. However, because of the high positive predictive value of choline PET/CT for diagnosing lymph node metastases in high-risk PCa, it is potentially useful to include the involved lymph nodes in the conventional irradiation field. Additionally, detection of unsuspected distant metastatic disease may change the management from radiation therapy to a systemic treatment [63].
18F-FCH PET/CT has been also investigated in PCa patients to select and delineate target volumes in the prostate gland or prostate fossa [64-66]. In particular, with intensity modulated radiotherapy (IMRT) and imaged guided radiotherapy (IGRT), it may be possible to select a specific site for radiotherapy and to minimize unnecessary irradiation of surrounding tissues. Wurschmidt et al. evaluated the usefulness of 18F-FCH PET/CT data in planning dose escalation to nodal sites of PCa in 26 patients [65]. The median dose to primary tumors was 75.6 Gy and to choline-positive recurrent nodal sites was 66.6 Gy. At 28 months the overall survival rate was 94%, and biochemical relapse-free survival was 83% for primary cancer and 49% for recurrent tumors. Distant disease-free survival was 100% and 75% for primary and recurrent tumors, respectively. Early and late side effects were mild in 85 and 84%, respectively. Similar findings were reported by Casamassima et al. in a study on 71 PCa patients with biochemical recurrence [67]. 18F-FCH or 11C choline PET/CT detected recurrences in 39 of 71 patients. Twenty-five patients with limited nodal recurrences received eradicative radiotherapy. At the 3-year follow-up, overall survival, disease-free survival and local control rates were 92, 17 and 90%, respectively.
Pinkawa et al. reported on the use of 18F-FCH PET/CT to delineate dominant intra-prostatic lesions (DILs) in radiation treatment planning for 66 patients [68]. They suggested using a relative SUVmax threshold of twice the background activity to identify the DILs. These DILs are potentially the best targets for focal dose escalation. However, since the sensitivity of 18F-FCH PET/CT is modest in PCa patients with biochemical recurrence, mathematical dose modeling by Niyazi et al. suggested that it would be of limited value [69].
Summary
18F-FCH PET/CT in prostate cancer has been widely investigated in the last decade. There is not enough data to support the usefulness of 18F-FCH PET/CT for diagnosis of primary prostate cancer. However, it may be useful in patients with increased PSA levels and negative repeated biopsies to guide repeat biopsy. The role of 18F-FCH PET/CT for evaluating a local tumor extent (T-staging) is also limited. Dynamic contrast-enhanced MRI/magnetic resonance spectroscopy (MRS) with an endorectal coil has better sensitivity for T-staging. The positive predictive value of 18F-FCH PET/CT for the detection of lymph node involvement and the sensitivity and specificity of 18F-FCH PET/CT for evaluating bone metastases is relatively high. 18F-FCH PET/CT is useful for distinction between locoregional recurrence and distant metastases in cases of biochemical recurrence, particularly in intermediate-to-high risk patients with certain criteria (e.g. elevated trigger PSA values and/or a short PSA doubling time and/or Gleason score > 7). 18F-FCH PET/CT may also play a role in radiotherapy dose escalation or salvage therapy.
Disclosure of conflict of interest
None declared.
References
- 1.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 2.Oehr P, Bouchelouche K. Imaging of prostate cancer. Curr Opin Oncol. 2007;19:259–264. doi: 10.1097/CCO.0b013e3280ad439b. [DOI] [PubMed] [Google Scholar]
- 3.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, Verbel D, Schwartz L, Larson SM, Scher HI. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 4.Sanz G, Robles JE, Gimenez M, Arocena J, Sanchez D, Rodriguez-Rubio F, Rosell D, Richter JA, Berian JM. Positron emission tomography with 18fluorine-labelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int. 1999;84:1028–1031. doi: 10.1046/j.1464-410x.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 5.Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, Shizukuishi K, Tateishi U, Kubota Y, Inoue T. The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–27. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 6.Shiiba M, Ishihara K, Kimura G, Kuwako T, Yoshihara H, Sato H, Kondo Y, Tsuchiya S, Kumita S. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Ann Nucl Med. 2012;26:138–145. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 7.Effert P, Beniers AJ, Tamimi Y, Handt S, Jakse G. Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res. 2004;24:3057–3063. [PubMed] [Google Scholar]
- 8.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 9.Kent C. Regulation of phosphatidylcholine biosynthesis. Prog Lipid Res. 1990;29:87–105. doi: 10.1016/0163-7827(90)90010-i. [DOI] [PubMed] [Google Scholar]
- 10.Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998;39:990–995. [PubMed] [Google Scholar]
- 11.DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, Reiman R, Price DT, Coleman RE. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–1814. [PubMed] [Google Scholar]
- 12.Hacker A, Jeschke S, Leeb K, Prammer K, Ziegerhofer J, Sega W, Langsteger W, Janetschek G. Detection of pelvic lymph node metastases in patients with clinically localized prostate cancer: comparison of [18F] fluorocholine positron emission tomography-computerized tomography and laparoscopic radioisotope guided sentinel lymph node dissection. J Urol. 2006;176:2014–2018. doi: 10.1016/j.juro.2006.07.037. discussion 2018-2019. [DOI] [PubMed] [Google Scholar]
- 13.Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, Langsteger W. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol. 2006;8:43–48. doi: 10.1007/s11307-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 14.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, Nader M, Gruy B, Janetschek G, Langsteger W. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254:925–933. doi: 10.1148/radiol.09090413. [DOI] [PubMed] [Google Scholar]
- 16.Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, Borsatti E, Drigo A, Trovo MG. [18F] fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33:1387–1398. doi: 10.1007/s00259-006-0150-2. [DOI] [PubMed] [Google Scholar]
- 17.Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, Cservenyak T, Hany TF. Evaluation of [(18)F] -choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253–263. doi: 10.1007/s00259-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 18.Igerc I, Kohlfurst S, Gallowitsch HJ, Matschnig S, Kresnik E, Gomez-Segovia I, Lind P. The value of 18F-choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:976–983. doi: 10.1007/s00259-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 19.DeGrado TR, Reiman RE, Price DT, Wang S, Coleman RE. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J Nucl Med. 2002;43:92–96. [PubMed] [Google Scholar]
- 20.Mertens K, Ham H, Deblaere K, Kalala JP, Van den Broecke C, Slaets D, De Vos F, Goethals I. Distribution patterns of 18F-labelled fluoromethylcholine in normal structures and tumors of the head: a PET/MRI evaluation. Clin Nucl Med. 2010;37:e196–203. doi: 10.1097/RLU.0b013e31824c5dd0. [DOI] [PubMed] [Google Scholar]
- 21.Gu J. Primary Liver Cancer: Challenges and Perspectives. Springer; 2012. [Google Scholar]
- 22.Terauchi T, Tateishi U, Maeda T, Kanou D, Daisaki H, Moriya Y, Moriyama N, Kakizoe T. A case of colon cancer detected by carbon-11 choline positron emission tomography/computed tomography: an initial report. Jpn J Clin Oncol. 2007;37:797–800. doi: 10.1093/jjco/hym102. [DOI] [PubMed] [Google Scholar]
- 23.Schillaci O, Calabria F, Tavolozza M, Ciccio C, Carlani M, Caracciolo CR, Danieli R, Orlacchio A, Simonetti G. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun. 2010;31:39–45. doi: 10.1097/mnm.0b013e328330adc5. [DOI] [PubMed] [Google Scholar]
- 24.Katz DS, Hines J, Math KR, Nardi PM, Mindelzun RE, Lane MJ. Using CT to reveal fat-containing abnormalities of the pancreas. AJR Am J Roentgenol. 1999;172:393–396. doi: 10.2214/ajr.172.2.9930790. [DOI] [PubMed] [Google Scholar]
- 25.Calabria F, Chiaravalloti A, Schillaci O. (18)F-choline PET/CT pitfalls in image interpretation: an update on 300 examined patients with prostate cancer. Clin Nucl Med. 2014;39:122–130. doi: 10.1097/RLU.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 26.Wyss MT, Weber B, Honer M, Spath N, Ametamey SM, Westera G, Bode B, Kaim AH, Buck A. 18F-choline in experimental soft tissue infection assessed with autoradiography and high-resolution PET. Eur J Nucl Med Mol Imaging. 2004;31:312–316. doi: 10.1007/s00259-003-1337-4. [DOI] [PubMed] [Google Scholar]
- 27.Kwee SA, DeGrado T. Prostate biopsy guided by 18F-fluorocholine PET in men with persistently elevated PSA levels. Eur J Nucl Med Mol Imaging. 2008;35:1567–1569. doi: 10.1007/s00259-008-0781-6. author reply 1570. [DOI] [PubMed] [Google Scholar]
- 28.Kwee SA, Coel MN, Lim J, Ko JP. Prostate cancer localization with 18fluorine fluorocholine positron emission tomography. J Urol. 2005;173:252–255. doi: 10.1097/01.ju.0000142099.80156.85. [DOI] [PubMed] [Google Scholar]
- 29.Kwee SA, Thibault GP, Stack RS, Coel MN, Furusato B, Sesterhenn IA. Use of step-section histopathology to evaluate 18F-fluorocholine PET sextant localization of prostate cancer. Mol Imaging. 2008;7:12–20. [PubMed] [Google Scholar]
- 30.Schmid DT, John H, Zweifel R, Cservenyak T, Westera G, Goerres GW, von Schulthess GK, Hany TF. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology. 2005;235:623–628. doi: 10.1148/radiol.2352040494. [DOI] [PubMed] [Google Scholar]
- 31.Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C, Gentile V, Laghi A, Passariello R. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F] choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP) Eur J Radiol. 2012;81:700–708. doi: 10.1016/j.ejrad.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 32.Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, Soloviev D, Hany TF, Miralbell R. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (< 1 ng/mL) after radical prostatectomy. BJU Int. 2007;99:1415–1420. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 33.Qayyum A, Coakley FV, Lu Y, Olpin JD, Wu L, Yeh BM, Carroll PR, Kurhanewicz J. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183:1079–1083. doi: 10.2214/ajr.183.4.1831079. [DOI] [PubMed] [Google Scholar]
- 34.Lord M, Ratib O, Vallee JP. (1)(8)F-Fluorocholine integrated PET/MRI for the initial staging of prostate cancer. Eur J Nucl Med Mol Imaging. 2011;38:2288. doi: 10.1007/s00259-011-1837-6. [DOI] [PubMed] [Google Scholar]
- 35.Wetter A, Lipponer C, Nensa F, Beiderwellen K, Olbricht T, Rubben H, Bockisch A, Schlosser T, Heusner TA, Lauenstein TC. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate: initial results. Invest Radiol. 2013;48:256–262. doi: 10.1097/RLI.0b013e318282c654. [DOI] [PubMed] [Google Scholar]
- 36.Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Beauregard JM, Williams SG, Degrado TR, Roselt P, Hicks RJ. Pilot comparison of F-fluorocholine and F-fluorodeoxyglucose PET/CT with conventional imaging in prostate cancer. J Med Imaging Radiat Oncol. 2010;54:325–332. doi: 10.1111/j.1754-9485.2010.02178.x. [DOI] [PubMed] [Google Scholar]
- 38.Poulsen MH, Bouchelouche K, Gerke O, Petersen H, Svolgaard B, Marcussen N, Svolgaard N, Ogren M, Vach W, Hoilund-Carlsen PF, Geertsen U, Walter S. [18F] -fluorocholine positron-emission/computed tomography for lymph node staging of patients with prostate cancer: preliminary results of a prospective study. BJU Int. 2010;106:639–643. doi: 10.1111/j.1464-410X.2009.09191.x. discussion 644. [DOI] [PubMed] [Google Scholar]
- 39.McMurtry CT, McMurtry JM. Metastatic prostate cancer: complications and treatment. J Am Geriatr Soc. 2003;51:1136–1142. doi: 10.1046/j.1532-5415.2003.51367.x. [DOI] [PubMed] [Google Scholar]
- 40.Yu KK, Hawkins RA. The prostate: diagnostic evaluation of metastatic disease. Radiol Clin North Am. 2000;38:139–157. ix. doi: 10.1016/s0033-8389(05)70153-6. [DOI] [PubMed] [Google Scholar]
- 41.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–2994. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, Satyamurthy N, Barrio JR, Phelps ME. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 44.McCarthy M, Siew T, Campbell A, Lenzo N, Spry N, Vivian J, Morandeau L. (1)(8)F-Fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: prospective comparison with standard imaging. Eur J Nucl Med Mol Imaging. 2011;38:14–22. doi: 10.1007/s00259-010-1579-x. [DOI] [PubMed] [Google Scholar]
- 45.Kjolhede H, Ahlgren G, Almquist H, Liedberg F, Lyttkens K, Ohlsson T, Bratt O. Combined 18F-fluorocholine and 18F-fluoride positron emission tomography/computed tomography imaging for staging of high-risk prostate cancer. BJU Int. 2012;110:1501–1506. doi: 10.1111/j.1464-410X.2012.11123.x. [DOI] [PubMed] [Google Scholar]
- 46.Langsteger W, Balogova S, Huchet V, Beheshti M, Paycha F, Egrot C, Janetschek G, Loidl W, Nataf V, Kerrou K, Pascal O, Cussenot O, Talbot JN. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging. 2011;55:448–457. [PubMed] [Google Scholar]
- 47.Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Loidl W, Broinger G, Stoiber F, Foglman I, Langsteger W. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766–1774. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]
- 48.Freedland SJ, Presti JC Jr, Amling CL, Kane CJ, Aronson WJ, Dorey F, Terris MK SEARCH Database Study Group. Time trends in biochemical recurrence after radical prostatectomy: results of the SEARCH database. Urology. 2003;61:736–741. doi: 10.1016/s0090-4295(02)02526-8. [DOI] [PubMed] [Google Scholar]
- 49.Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179:906–910. doi: 10.1016/j.juro.2007.10.059. discussion 910. [DOI] [PubMed] [Google Scholar]
- 50.Kwee SA, Coel MN, Lim J. Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med. 2012;26:501–507. doi: 10.1007/s12149-012-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelosi E, Arena V, Skanjeti A, Pirro V, Douroukas A, Pupi A, Mancini M. Role of whole-body 18F-choline PET/CT in disease detection in patients with biochemical relapse after radical treatment for prostate cancer. Radiol Med. 2008;113:895–904. doi: 10.1007/s11547-008-0263-8. [DOI] [PubMed] [Google Scholar]
- 52.Steiner C, Vees H, Zaidi H, Wissmeyer M, Berrebi O, Kossovsky MP, Khan HG, Miralbell R, Ratib O, Buchegger F. Three-phase 18F-fluorocholine PET/CT in the evaluation of prostate cancer recurrence. Nuklearmedizin. 2009;48:1–9. doi: 10.3413/nukmed-0194. quiz N2-3. [DOI] [PubMed] [Google Scholar]
- 53.Graute V, Jansen N, Ubleis C, Seitz M, Hartenbach M, Scherr MK, Thieme S, Cumming P, Klanke K, Tiling R, Bartenstein P, Hacker M. Relationship between PSA kinetics and [18F] fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39:271–282. doi: 10.1007/s00259-011-1970-2. [DOI] [PubMed] [Google Scholar]
- 54.Schillaci O, Calabria F, Tavolozza M, Caracciolo CR, Finazzi Agro E, Miano R, Orlacchio A, Danieli R, Simonetti G. Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39:589–596. doi: 10.1007/s00259-011-2030-7. [DOI] [PubMed] [Google Scholar]
- 55.Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, Nader M, Langsteger W, Loidl W. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54:833–840. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]
- 56.Marzola MC, Chondrogiannis S, Ferretti A, Grassetto G, Rampin L, Massaro A, Castellucci P, Picchio M, Al-Nahhas A, Colletti PM, Marcolongo A, Rubello D. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med. 2013;38:e26–32. doi: 10.1097/RLU.0b013e318266cc38. [DOI] [PubMed] [Google Scholar]
- 57.Chondrogiannis S, Marzola MC, Ferretti A, Maffione AM, Rampin L, Grassetto G, Nanni C, Colletti PM, Rubello D. Role of (1)(8)F-choline PET/CT in suspicion of relapse following definitive radiotherapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:1356–1364. doi: 10.1007/s00259-013-2433-8. [DOI] [PubMed] [Google Scholar]
- 58.Henninger B, Vesco P, Putzer D, Kendler D, Loizides A, Bale RJ, Virgolini IJ. [18F] choline positron emission tomography in prostate cancer patients with biochemical recurrence after radical prostatectomy: influence of antiandrogen therapy - a preliminary study. Nucl Med Commun. 2012;33:889–894. doi: 10.1097/MNM.0b013e328355990f. [DOI] [PubMed] [Google Scholar]
- 59.Reske SN, Blumstein NM, Glatting G. [11C] choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2008;35:9–17. doi: 10.1007/s00259-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 60.Rinnab L, Mottaghy FM, Blumstein NM, Reske SN, Hautmann RE, Hohl K, Moller P, Wiegel T, Kuefer R, Gschwend JE. Evaluation of [11C] -choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int. 2007;100:786–793. doi: 10.1111/j.1464-410X.2007.07083.x. [DOI] [PubMed] [Google Scholar]
- 61.Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate-to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2013;63:1040–1048. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 62.Soyka JD, Muster MA, Schmid DT, Seifert B, Schick U, Miralbell R, Jorcano S, Zaugg K, Seifert HH, Veit-Haibach P, Strobel K, Schaefer NG, Husarik DB, Hany TF. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39:936–943. doi: 10.1007/s00259-012-2083-2. [DOI] [PubMed] [Google Scholar]
- 63.Schwarzenbock SM, Kurth J, Gocke C, Kuhnt T, Hildebrandt G, Krause BJ. Role of choline PET/CT in guiding target volume delineation for irradiation of prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S28–35. doi: 10.1007/s00259-013-2404-0. [DOI] [PubMed] [Google Scholar]
- 64.Ciernik IF, Brown DW, Schmid D, Hany T, Egli P, Davis JB. 3D-segmentation of the 18F-choline PET signal for target volume definition in radiation therapy of the prostate. Technol Cancer Res Treat. 2007;6:23–30. doi: 10.1177/153303460700600104. [DOI] [PubMed] [Google Scholar]
- 65.Wurschmidt F, Petersen C, Wahl A, Dahle J, Kretschmer M. [18F] fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44. doi: 10.1186/1748-717X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber DC, Wang H, Cozzi L, Dipasquale G, Khan HG, Ratib O, Rouzaud M, Vees H, Zaidi H, Miralbell R. RapidArc, intensity modulated photon and proton techniques for recurrent prostate cancer in previously irradiated patients: a treatment planning comparison study. Radiat Oncol. 2009;4:34. doi: 10.1186/1748-717X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casamassima F, Masi L, Menichelli C, Bonucci I, Casamassima E, Lazzeri M, Gulisano M, Aterini S. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori. 2011;97:49–55. doi: 10.1177/030089161109700110. [DOI] [PubMed] [Google Scholar]
- 68.Pinkawa M, Holy R, Piroth MD, Klotz J, Nussen S, Krohn T, Mottaghy FM, Weibrecht M, Eble MJ. Intensity-modulated radiotherapy for prostate cancer implementing molecular imaging with 18F-choline PET-CT to define a simultaneous integrated boost. Strahlenther Onkol. 2010;186:600–606. doi: 10.1007/s00066-010-2122-5. [DOI] [PubMed] [Google Scholar]
- 69.Niyazi M, Bartenstein P, Belka C, Ganswindt U. Choline PET based dose-painting in prostate cancer--modelling of dose effects. Radiat Oncol. 2010;5:23. doi: 10.1186/1748-717X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]


