Abstract
Background: Chemotherapy resistance is a common problem faced by patients diagnosed with epithelial ovarian cancer (EOC). Currently there are no specific or sensitive clinical biomarkers that maybe implemented to identify chemotherapy resistance and give insight to prognosis. The aim of this study is to investigate the roles of Lewis y antigen and the markers associated with cell-adhesion-mediated drug resistance (CAM-DR) in patients with EOC. Methods: 92 EOC patients who were treated with systemic chemotherapy after cytoreductive surgery were included in this analysis. Patients were divided into two groups, chemotherapy sensitive (n = 56) and resistant (n = 36). Immunohistochemical (IHC) staining for Lewis y and CAM-DR-related cell surface proteins including CD44, CD147, HE4 (Human epididymis protein 4), integrin α5, β1, αv and β3 were conducted on tissues collected during primary debulking surgery. Using multivariate logistic regressions, IHC results were compared to clinical variables and chemotherapy resistance to determine possible correlations. The relationships between IHC expression and progression-free survival (PFS) and overall survival (OS) were analyzed using Kaplan–Meier method and Cox regression analysis. Results: Membranous expression of Lewis y and all these CAM-DR-related markers were significantly higher in the resistant group than that of the sensitive group (all P < 0.01). Multivariate regression analysis revealed that high expression of Lewis y, CD44, HE4, integrin α5 and β1 as well as advanced FIGO stage were independent risk factors for chemotherapy resistance (all P < 0.05). Advanced FIGO stage, lymph node metastasis and high expression of Lewis y, CD44, CD147, HE4, integrin α5, β1 were associated with a shorter PFS and OS (all P < 0.05). Moreover, multivariate COX analysis demonstrated that the following variates were independent predictors of worse PFS and OS survival: late FIGO stage (P = 0.013, 0.049), high expressions of Lewis y (P = 0.010, 0.036), HE4 (P = 0.006, 0.013) and integrin β1 (PFS, P = 0.003), integrin α5 (OS, P = 0.019). Conclusion: Membranous expression of Lewis y and CAM-DR-related markers including CD44, CD147, HE4, integrin α5, β1, αv and β3 are associated with the development of chemotherapy resistance. High expression of Lewis y antigen and CAM-DR-related markers including CD44, CD147, HE4, integrin α5 and β1 are independent markers for PFS and OS, in which Lewis y and HE4 are the most significant.
Keywords: Epithelial ovarian cancer, chemotherapy resistance, prognosis, CAM-DR, Lewis y, HE4
Introduction
Ovarian cancer is the eighth most common cancer and the seventh cause of death from cancer in women worldwide, it’s the second cause of death among female reproductive malignancies and claims 140,200 lives each year [1], the anticipated incidence and number of deaths in the United States is 21,980 and 14,270 respectively for the year 2014 [2]. Because of its innocuous symptoms of abdominal distension and discomfort at the onset, most of the ovarian cancer hence are often diagnosed at an advanced stage, with 60-70% having stage III-IV disease at the onset. The current standard treatment for advanced ovarian cancer is surgical debulking followed by platinum-based chemotherapy. This standard treatment results in > 80% response rates and 40-60% complete responses, however, the majority of patients with advanced disease (stages III-IV) will eventually relapse, even with initial disease response. Median progression-free survival ranges from 16 to 21 months and median overall survival ranges from 24 to 60 months [3]. After repeated cycles of chemotherapy, recurrent ovarian cancer eventually develops resistance to many available cytotoxic agents. As a result, researches into the mechanisms of drug-resistance, biomarkers for drug resistance, and the development of new-targeted therapies have been the subject of many ovarian cancer studies [3,4].
In recent years, a new drug-resistance mechanism in tumors, cell-adhesion-mediated drug resistance (CAM-DR), has drawn wide attention [5,6]. Tumor cells have greater survival potential and a greater capacity to resist apoptosis when they adhere to their surrounding environment. Cell-extracellular matrix (ECM) adhesion complexes are stabilized by actin cytoskeleton or intermediate filaments, but dynamically rearranged under some circumstances, such as cell migration and cancer metastasis [7,8]. Studies with metastatic hematopoietic, colon adenocarcinoma and breast cancer cells show that tumor-ECM interactions indeed determine a state of quiescence associated with CAM-DR [5].
Glycosyl antigen, an important component of glycoproteins and glycolipids, is widely expressed in the cell membrane. Changes in the antigen are significantly associated with several biological processes, such as cell canceration, invasion, and migration [9]. In particular, changes in glycosyl type II chain are mainly observed in ovarian cancer. Lewis y, a type of glycosyl antigen, is overexpressed in more than 75% of ovarian epithelial neoplasm, and high levels of expression are associated with poor prognosis [10,11]. Our previous studies demonstrated that Lewis y, as part of various crucial molecules on the cell surface (e.g., integrin α5β1 [12], αvβ3 [12,13], CD44 [14], CD147 [15], HE4 [16]), enhances cellular malignant biological behaviors, such as proliferation [17], adhesion [12] and multiple drug resistance [18].
Through the use of immunohistochemistry we have studied the expression of Lewis y antigen and CAM-DR related immune markers: CD44, CD147, HE4, integrin α5, β1, αv and β3 in tissue specimens from patients who harbor chemotherapy resistant or sensitive epithelial ovarian cancer (EOC). We also analyze how the expression of these molecules correlates with chemotherapy resistance and the resulting clinical significance including prognosis.
Materials and methods
Patients and specimens
With research approval from the Ethical Committee of Shengjing Hospital affiliated to China Medical University (number of approval: 2010PS84K), ninety-two paraffin samples were obtained from primary debulking operations done from 2006 to 2010 by the department of Gynecology in Shengjing Hospital. After cytoreductive surgery and 6-8 cycles of systemic chemotherapy (Paclitaxel + Carboplatin, TC regimen), each patient was followed clinically for at least 4 years. Clinical information was abstracted from the medical record including age at the time of operation, International Federation of Gynecology and Obstetrics (FIGO) stage, tumor differentiation, pathological subtype, lymphatic metastasis and residual tumor size (Table 1).
Table 1.
Comparison of demographic and clinical characteristics between chemotherapy resistant and sensitive patients of 92 cases epithelial ovarian cancer
| Characteristics | N | Sensitive Group | Resistant Group | P-value |
|---|---|---|---|---|
|
| ||||
| n = 56 | n = 36 | |||
| Age, years | ||||
| Mean ± SD | 54.15 ± 9.48 | 52.70 ± 9.28 | 56.42 ± 9.48 | 0.066† |
| Age group, n (%) | ||||
| ≤ 60 | 74 | 47 (83.9) | 27 (75.0) | 0.292‡ |
| > 60 | 18 | 9 (16.1) | 9 (25.0) | |
| FIGO Stage, n (%) | ||||
| I-II | 31 | 27 (48.2) | 4 (11.1) | < 0.001*,‡ |
| III-IV | 61 | 29 (51.8) | 32 (88.9) | |
| Differentiation, n (%) | ||||
| Well | 14 | 10 (17.9) | 4 (11.1) | 0.318‡ |
| Moderate | 43 | 28 (50.0) | 15 (41.7) | |
| Poor | 35 | 18 (32.1) | 17 (47.2) | |
| Pathological Subtype, n (%) | ||||
| Serous carcinoma | 60 | 36 (64.3) | 24 (66.7) | 0.815‡ |
| Non-serous carcinoma | 32 | 20 (35.7) | 12 (33.3) | |
| Lymph node metastasis, n (%) | ||||
| No | 63 | 43 (76.8) | 20 (55.6) | 0.032*,‡ |
| Yes | 29 | 13 (23.2) | 16 (44.4) | |
| Residual tumor size, n (%) | ||||
| ≤ 1 cm | 53 | 41 (73.2) | 12 (33.3) | < 0.001*,‡ |
| > 1 cm | 39 | 15 (26.8) | 24 (66.7) | |
P < 0.05;
Independent t-test;
Chi-square test.
Patients were assigned to groups according to criteria set forth in the 2012 NCCN (National Comprehensive Cancer Network) guidelines. The chemotherapy resistant group included patients who had a clinical response to initial paclitaxel and carboplatin (TC) chemotherapy, but experienced subsequent relapse either in the late stage of chemotherapy or within 6 months after completion of chemotherapy. The partially chemotherapy-sensitive group included patients who experienced ovarian cancer relapse within 6-12 months after completion of chemotherapy with TC. The chemotherapy- sensitive group included patients who maintained a clinical response for ≥ 12 months. Factors considered diagnostic for ovarian cancer relapse included continuously increasing CA125 levels, new fixed/solid lesions identified by examination, tumors visualized through imaging studies and/or accumulation of ascites. In accordance with the NCCN 2012 guidelines described above, ovarian cancer patients were assigned to either the chemotherapy resistant group (36 cases) or sensitive group (56 cases). There were 2 partially sensitive patients in this study, for ease of analysis they were included in the sensitive group.
Immunohistochemical analysis
Immunohistochemistry (IHC) was used to analyze the expression of Lewis y antigen, CD44, CD147, HE4, integrin α5, β1, αv and β3. The staining procedure was performed as described in the manuals for the SABC (Streptavidin-Biotin Complex) and SP (streptavidin-peroxidase) kits. Briefly, tissue sections were deparaffinized in xylene and rehydrated with graded ethanol. Antigen retrieval was carried out in citrate buffer (pH = 6.0, 12 min, microwave oven). Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide for 12 min. Non-specific binding sites were blocked with 5% normal horse serum in TBS-Tween (Wash buffer, Dako, Glostrup, Denmark) for 30 minutes. Sections were incubated with primary antibodies overnight at 4°C. All the sources, working dilutions of the first antibodies are given in Table 2. All sections were visualized using the Liquid DAB Substrate Chromogen System for peroxidase (DakoCytomation) and were counterstained with hematoxylin, dehydrated and mounted. Negative controls were performed by omission of the primary antibody or incubation with an isotype control antibody. Positive controls were performed as follows: a colon cancer sample for Lewis y antigen, a human kidney carcinoma sample for CD44, a human liver cancer sample for CD147, a normal epididymis tissue sample for HE4 and breast cancer samples for integrins α5, β1, αv and β3.
Table 2.
Sources and working dilution of the primary antibodies
| Antibodies | Dilution | Description | Source |
|---|---|---|---|
| Lewis y | 1:100 | Mouse monoclonal anti-Lewis y antibody (clone A 70-C/C8) | Abcam Company (Cambridge, UK) |
| CD44 | 1:200 | Mouse anti-CD44 monoclonal antibody (clone F-4) | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) |
| CD147 | 1:100 | Rabbit polyclonal anti-CD147 antibody | Abcam Company (Cambridge, UK) |
| HE4 | 1:50 | Rabbit polyclonal anti-HE4 antibody | Abcam Company (Cambridge, UK) |
| Integrin α5 | 1:200 | Rabbit polyclonal anti-α5 and anti-β1 antibodies | Boshide Biotech (Wuhan, China) |
| Integrin β1 | 1:300 | Rabbit polyclonal anti-α5 and anti-β1 antibodies | Boshide Biotech (Wuhan, China) |
| Integrin αv | 1:100 | Rabbit polyclonal anti-αv and anti-β3 antibodies | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) |
| Integrin β3 | 1:160 | Rabbit polyclonal anti-αv and anti-β3 antibodies | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) |
Abbreviation: HE4, Human epididymis protein 4.
Quantification of immunohistochemical staining
Two observers (LZ and JG) evaluated the samples independently and were blinded to patient outcomes. The proportion score, which represented the estimated percentage of tumor cells that stained positive for the protein (range: 0-100), was assigned. The intensity score, which estimated the average staining intensity of the positive tumor cells (4-value scoring system: 0 = below the level of detection, 1 = weak, 2 = moderate, and 3 = strong), was also assigned. A final score (0-300) was determined by multiplying proportion score and intensity score for each tumor associated protein. The median value of all scores in each marker was chosen as the cutoff point for low and high expression as previously described [19]. The cutoff point for Lewis y, CD44, CD147, HE4, as well as integrins α5, β1, αv and β3 were 134, 100, 134, 113, 105, 106, 98 and 100, respectively. Disagreements in independent histologic interpretations were resolved through simultaneous review by 3 observers (LZ, JG and ZH).
Statistical analysis
Immunohistochemistry scores and clinicopathological parameters for each group were compared using chi-square (χ2) analysis. The correlation coefficient R of CD44, CD147, HE4, as well as integrins α5, β1, αv and β3 with Lewis y were calculated by Spearman correlation analysis. Independent risk factors for chemotherapeutic resistant reaction were examined using a binary logistic regression analysis. The parameters identified to be significant in the univariate analysis were analyzed further through multivariate analysis (method: Forward: LR).
Survival analysis was analyzed using Kaplan-Meier curves, and significant differences between groups and among different immunomarkers were tested using the log-rank test. Multivariate Cox proportional hazards regression models were used to control for confounding variables [20]. Multivariate Cox regression models initially included age at operation, FIGO stage, tumor differentiation, pathological subtype, lymphatic metastasis, residual tumor size and the expression of Lewis y, CD44, CD147, HE4, and integrins α5, β1, αv, β3. Only those variables with P-value < 0.05 in the univariate analysis were included in the multivariate analysis. Follow-up time was calculated from the date of surgery to the date of progression, death, and last visit or contact with the patient. Overall survival (OS) was defined as the time interval between the date of surgery and the date of death; progression-free survival (PFS) was defined as the time interval between the date of surgery and the date of identification of progressive disease (disease not treatable with curative intent). For all three endpoints the last date of follow-up was used for censored subjects. Statistical analyses were performed using SPSS program (Version 22 for Mac; SPSS Inc., Chicago, IL, USA) and the Kaplan-Meier curve graphs were completed using Graph Pad Prism 5 (Graph Pad Prism Software Inc. San Diego, CA). A P-value < 0.05 was considered statistically significant.
Results
Clinicopathological variables of patients
Demographic, pathological and clinical variables were collected as below. It contained 92 patients, in which 74 patients were no more than 60-year-old. The age of patients at the time of diagnosis were ranging from 24 to 78-year-old, the median was 53-year-old, and mean was 54.15-year-old. Among 92 patients, 56 patients were included in the group considered sensitive to chemotherapy (including 2 patients who were partially sensitive to chemotherapy) and 36 patients were included in the resistant group. The age in these two groups were 52.70 ± 9.28 years’ old and 56.42 ± 9.48 years’ old, respectively. All the patients had undergone cytoreductive surgery of EOC. According to the 2010 International Federation of Obstetricians and Gynaecologists (FIGO) Staging System for Ovarian Cancer, there were 18 patients in stage I, 13 patients in stage II, 59 patients in stage III, 2 patients in stage IV. 35 cases were poor-differentiated, 43 were moderate-differentiated, and 14 were well-differentiated. By histological analyses [21], 60 patients were Serous carcinoma, 8 were mucinous carcinoma, 6 were endometrioid adenocarcinoma, 7 were clear cell carcinoma, 9 were poorly differentiated adenocarcinoma and 2 were undifferentiated. There were 29 patients who had lymph node metastasis, and 53 patients whose residual tumor size were no more than 1 cm and 22 patients 1-2 cm and 17 patients more than 2 cm. General clinical and pathological information of patients were shown in Table 1.
IHC expression in different ovarian cancer groups
Lewis y antigen was expressed in the cell membrane and cytoplasm, mainly on membrane and rarely in the nucleus. Similar to Lewis y, the expressions of CD44, CD147, HE4, integrin α5, β1, αv and β3 were mainly on membrane (Figure 1). Patients were dichotomised into high and low by the median final score of each marker expression. For all the markers, there are significant difference in low and high expression between sensitive group and resistant group (all P < 0.01, Table 3). Spearman correlation analysis revealed that the expressions of CD44, CD147, HE4, integrins α5, β1, αv and β3 were positive linear related with the Lewis y (r = 0.327, 0.239, 0.240, 0.240, 0.238, 0.239 and 0.217, respectively, all P < 0.05, Table 3). No significant association was found between IHC expression and clinicopathological features of the patients (Table 4). However, high Lewis y and CD44 expressions were significantly associated with higher possibilities of residual tumor size > 1 cm (P = 0.010, 0.024, respectively).
Figure 1.
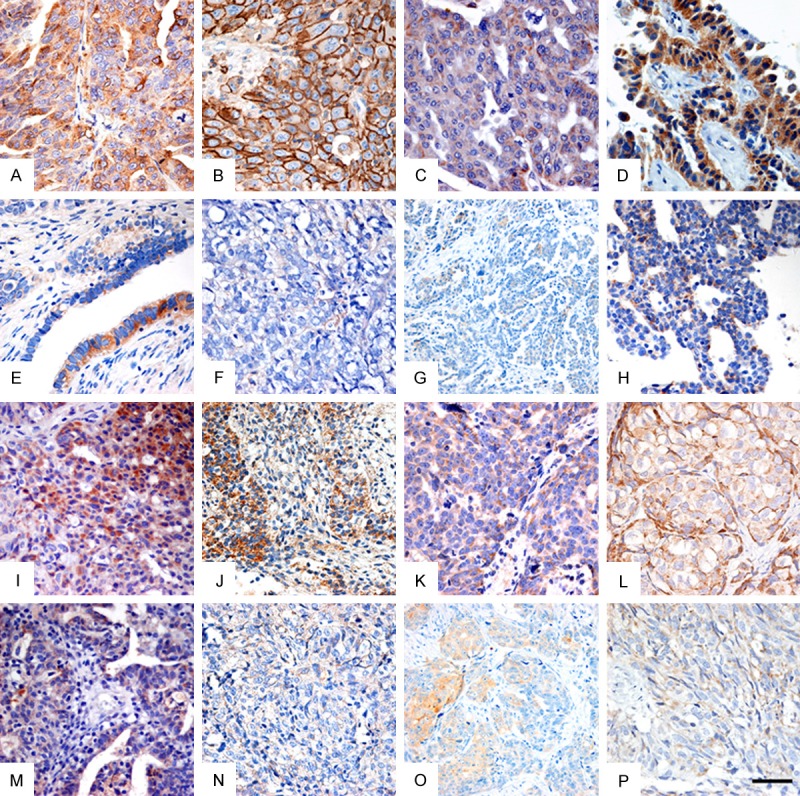
The expression of Lewis y, CD44, CD147, Human epididymis protein 4 (HE4), integrin α5, β1, αv and β3 in chemotherapy resistant group and chemotherapy sensitive group of EOC samples. Representative immunostaining for (A, E) Lewis y, (B, F) CD44, (C, G) CD147, (D, H) HE4, (I, M) integrin α5, (J, N) integrin β1, (K, O) integrin αv and (L, P) integrin β3 in (A-D, I-L) chemotherapy resistant ovarian cancer group and (E-H, M-P) chemotherapy sensitive EOC tissues. All of these immune markers are predominantly found on the membrane of tumor cells. Scale bar: 50 μm.
Table 3.
Expression of Lewis y, CD44, CD147, Integrin α5, β1, αv and β3 in chemotherapy sensitive group and resistant group of 92 cases epithelial ovarian cancer
| Marker (IHC score) | Sensitive Group | Resistant Group | P1 | rs * | P*,2 |
|---|---|---|---|---|---|
|
| |||||
| n = 56 | n = 36 | ||||
| Lewis y | |||||
| Low | 39 | 6 | < 0.001 | - | - |
| High | 17 | 30 | |||
| CD44 | |||||
| Low | 40 | 8 | < 0.001 | 0.327 | 0.001 |
| High | 16 | 28 | |||
| CD147 | |||||
| Low | 36 | 10 | 0.001 | 0.239 | 0.022 |
| High | 20 | 26 | |||
| HE4 | |||||
| Low | 39 | 9 | < 0.001 | 0.240 | 0.021 |
| High | 17 | 27 | |||
| Integrin α5 | |||||
| Low | 39 | 9 | < 0.001 | 0.240 | 0.021 |
| High | 17 | 27 | |||
| Integrin β1 | |||||
| Low | 35 | 9 | < 0.001 | 0.238 | 0.022 |
| High | 21 | 27 | |||
| Integrin αv | |||||
| Low | 35 | 11 | 0.003 | 0.239 | 0.022 |
| High | 21 | 25 | |||
| Integrin β3 | |||||
| Low | 33 | 12 | 0.017 | 0.217 | 0.038 |
| High | 23 | 24 | |||
P value of Chi-square;
P value of Spearman correlation compared with the expression of Lewis y.
Correlated with the expression of Lewis y.
Abbreviation: HE4, Human epididymis protein 4.
Table 4.
Clinical and pathologic characteristics of 92 epithelial ovarian cancer patients and their association to Lewis y, CD44, CD147, HE4, integrin α5, β1, αv, β3 protein expression
| Variable | N | Lewis y | CD44 | CD147 | HE4 | Integrin α5 | Integrin β1 | Integrin αv | Integrin β3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||||
| L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | ||
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| 45 | 47 | 48 | 44 | 46 | 46 | 48 | 44 | 48 | 44 | 44 | 48 | 46 | 46 | 45 | 47 | ||||||||||
| Age at diagnosis | 0.672 | 0.464 | 1.000 | 0.058 | 0.837 | 0.397 | 0.293 | 0.918 | |||||||||||||||||
| ≤ 60 | 74 | 37 | 37 | 40 | 34 | 37 | 37 | 35 | 39 | 39 | 35 | 37 | 37 | 39 | 35 | 36 | 38 | ||||||||
| > 60 | 18 | 8 | 10 | 8 | 10 | 9 | 9 | 13 | 5 | 9 | 9 | 7 | 11 | 7 | 11 | 9 | 9 | ||||||||
| FIGO Stage | 0.090 | 0.212 | 0.825 | 0.420 | 0.420 | 0.715 | 0.123 | 0.418 | |||||||||||||||||
| I-II | 31 | 19 | 12 | 19 | 12 | 16 | 15 | 18 | 13 | 18 | 13 | 14 | 17 | 19 | 12 | 17 | 14 | ||||||||
| III-IV | 61 | 26 | 35 | 29 | 32 | 30 | 31 | 30 | 31 | 30 | 31 | 30 | 31 | 27 | 34 | 28 | 33 | ||||||||
| Differentiation | 0.770 | 0.322 | 0.974 | 0.839 | 0.487 | 0.754 | 0.281 | 0.214 | |||||||||||||||||
| Well | 14 | 8 | 6 | 9 | 5 | 7 | 7 | 8 | 6 | 8 | 5 | 7 | 7 | 5 | 9 | 5 | 9 | ||||||||
| Moderate | 43 | 21 | 22 | 24 | 19 | 22 | 21 | 23 | 20 | 23 | 20 | 22 | 21 | 25 | 18 | 19 | 24 | ||||||||
| Poor | 35 | 16 | 19 | 15 | 35 | 17 | 18 | 17 | 18 | 16 | 19 | 15 | 20 | 16 | 19 | 21 | 14 | ||||||||
| Pathological Subtype | 0.555 | 0.894 | 0.662 | 0.313 | 0.760 | 0.148 | 0.662 | 0.304 | |||||||||||||||||
| Serous carcinoma | 60 | 28 | 32 | 31 | 29 | 31 | 29 | 29 | 31 | 32 | 28 | 32 | 28 | 29 | 31 | 27 | 33 | ||||||||
| Non-serous carcinoma | 32 | 17 | 15 | 17 | 15 | 15 | 17 | 19 | 13 | 16 | 16 | 12 | 20 | 17 | 15 | 18 | 14 | ||||||||
| Lymph node metastasis | 0.060 | 0.160 | 0.116 | 0.160 | 0.953 | 0.082 | 0.822 | 0.595 | |||||||||||||||||
| No | 63 | 35 | 28 | 36 | 27 | 35 | 28 | 36 | 27 | 33 | 30 | 34 | 29 | 32 | 31 | 32 | 31 | ||||||||
| Yes | 29 | 10 | 19 | 12 | 17 | 11 | 18 | 12 | 17 | 15 | 14 | 10 | 19 | 14 | 15 | 13 | 16 | ||||||||
| Residual tumor size | 0.010* | 0.024* | 0.291 | 0.157 | 0.066 | 0.569 | 0.058 | 0.381 | |||||||||||||||||
| ≤ 1 cm | 53 | 32 | 21 | 33 | 20 | 29 | 24 | 31 | 22 | 32 | 21 | 24 | 29 | 31 | 22 | 28 | 25 | ||||||||
| > 1 cm | 39 | 13 | 26 | 15 | 24 | 17 | 22 | 17 | 22 | 16 | 23 | 20 | 19 | 15 | 24 | 17 | 22 | ||||||||
P< 0.05.
Abbreviation: HE4, Human epididymis protein 4; L, low expression; H, high expression. The bold entries place emphasis on statistically significant P-values.
Independent risk factors for chemotherapeutic resistant reaction in EOC patients
The independent risk factors analysis for all of the clinicopathological variables and the IHC expression associated with chemotherapeutic resistant reaction was performed (Table 5). Regarding these variables, FIGO Stage III-IV, lymph node metastasis and residual tumor size > 1 cm (OR, 7.448, 2.646 and 5.467, respectively, all P < 0.05) and high expression of Lewis y, CD44, CD147, HE4, integrin α5, β1, αv and β3 (OR, 11.471, 8.750, 4.680, 6.882, 6.882, 5.000, 3.788 and 2.870, respectively, all P < 0.01) were found to be statistically significant predictors of chemotherapeutic reaction. The results of a multivariate analysis showed that FIGO Stage III-IV, high expression of Lewis y, CD44, HE4, integrin α5 and β1 (OR, 36.480, 16.663, 5.426, 26.721, 12.060 and 20.317, respectively, all P < 0.05) were independent risk factors for chemotherapeutic resistant reaction.
Table 5.
The results of the univariate and multivariate analyses for clinicopathologic variables and the IHC expression associated with chemotherapy resistance in 92 patients of EOC
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (> 60-year-old) | 1.741 (0.616-4.916) | 0.295 | ||
| FIGO Stage (III-IV) | 7.448 (2.325-23.857) | 0.001 | 36.480 (4.029-330.290) | 0.001 |
| Differentiation (poor) | 1.596 (0.852-2.990) | 0.144 | ||
| Pathological Subtype (others) | 0.900 (0.372-2.175) | 0.815 | ||
| Lymph node metastasis (Yes) | 2.646 (1.072-6.534) | 0.035 | ||
| Residual tumor size (> 1 cm) | 5.467 (2.198-13.595) | < 0.001 | ||
| Lewis y expression (high) | 11.471 (4.033-32.627) | < 0.001 | 16.663 (2.273-122.137) | 0.006 |
| CD44 expression (high) | 8.750 (3.296-23.232) | < 0.001 | 5.426 (1.072-27.460) | 0.041 |
| CD147 expression (high) | 4.680 (1.881-11.643) | 0.001 | ||
| HE4 expression (high) | 6.882 (2.674-17.712) | < 0.001 | 26.721 (3.423-208.576) | 0.002 |
| Integrin α5 expression (high) | 6.882 (2.674-17.712) | < 0.001 | 12.060 (1.668-87.177) | 0.014 |
| Integrin β1 expression (high) | 5.000 (1.976-12.651) | 0.001 | 20.317 (2.220-185.962) | 0.008 |
| Integrin αv expression (high) | 3.788 (1.552-9.242) | 0.003 | ||
| Integrin β3 expression (high) | 2.870 (1.198-6.876) | 0.018 | ||
Variables with P < 0.05 in the univariate analysis were included in the multivariate analysis. Abbreviation: HE4, Human epididymis protein 4.
Follow-up visit and prognostic factors analysis
During the time of follow up, 50 patients (54.3%) were dead, 14 patients (15.2%) were alive with disease, 24 patients (26.1%) were alive without evidence of disease, and 4 patients (4.4%) were lost. The median follow-up was 62.5 months (range, 49.1 to 103.6 months), the 5-year OS was 49.9% (Figure 2A) and median survival time was 56.0 months (95%CI, 48.9-63.1), the 5-year PFS was 37.5% (Figure 2C) and median survival time was 48.4 months (95% CI, 40.8-56.0). The chemotherapeutic sensitive patients had better outcomes than did the resistant patients in terms of PFS and OS, which are as follows: median PFS, 60.5 (95% CI, 57.2-63.8) vs 24.0 (95% CI, 18.6-29.4) months, P < 0.001; median OS, 70.0 (95% CI, 59.5-80.5) vs 24.0 (95% CI, 15.1-32.9) months, P < 0.001 (Figure 2B, 2D).
Figure 2.
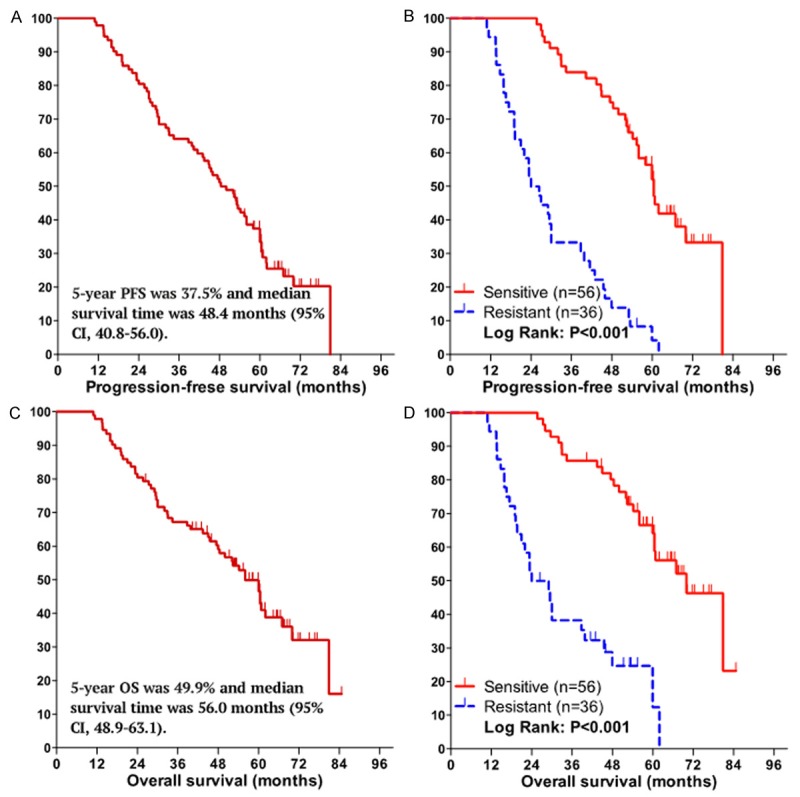
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) of the 92 patients with EOC. PFS curve (A) and OS curve (C) for all the patients, the 5-year survival rate and median survival time for PFS and OS are shown in figures. Patients in the chemotherapy sensitive group had a prolonged PFS (B) and OS (D) compared to the chemotherapy resistant group: median PFS, 60.5 (95% CI, 57.2-63.8) vs 24.0 (95% CI, 18.6-29.4) months, P < 0.001; median OS, 70.0 (95% CI, 59.5-80.5) vs 24.0 (95% CI, 15.1-32.9) months, P < 0.001, respecatively.
We further conducted univariate and multivariate analyses of prognostic factors for PFS and OS (Table 6). Among various prognostic factors as to PFS, FIGO Stage III-IV, poor differentiation, lymph node metastasis, residual tumor size > 1 cm, high expression of Lewis y, CD44, CD147, HE4 and integrin α5, β1 were found to be significant in the univariate analysis (OR, 2.001, 1.450, 1.701, 1.656, 2.276, 1.815, 1.869, 2.247, 1.917 and 2.310, respectively, all P < 0.05). Among those significant factors, the following multivariate analysis demonstrated that FIGO stage III-IV, and high expression of Lewis y, HE4, integrin β1 remained to be significant and independent factors (OR, 1.996, 1.931, 2.012 and 2.175, respectively, all P < 0.05). As to OS, FIGO Stage III-IV, lymph node metastasis, high expression of Lewis y, CD44, CD147, HE4, integrin α5, β1 and αv were found to be significant in the univariate analysis (OR, 2.106, 1.836, 2.338, 1.827, 2.386, 2.424, 2.342, 2.057 and 1.841, respectively, all P < 0.05). Among those significant factors, the following multivariate analysis demonstrated that FIGO stage III-IV, high expression of Lewis y, HE4 and integrin α5 remained to be significant and independent factors (OR, 1.901, 1.878, 2.071 and 1.982, respectively, all P < 0.05; Table 6, Figure 3).
Table 6.
Cox-proportional hazard model analysis of factors affecting patient’s progression-free survival or overall survival for clinicopathologic variables and the IHC expression in 92 patients of EOC
| Variables | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (> 60-year-old) | 1.777 (0.997-3.169) | 0.051 | 1.844 (0.950-3.579) | 0.071 | ||||
| FIGO Stage (III-IV) | 2.001 (1.162-3.445) | 0.012 | 1.996 (1.154-3.454) | 0.013 | 2.106 (1.120-3.958) | 0.021 | 1.901 (1.002-3.605) | 0.049 |
| Differentiation (poor) | 1.450 (1.016-2.071) | 0.041 | 1.365 (0.915-2.037) | 0.128 | ||||
| Pathological Subtype (others) | 1.250 (0.759-2.058) | 0.380 | 1.193 (0.677-2.103) | 0.541 | ||||
| Lymph node metastasis (Yes) | 1.701 (1.031-2.807) | 0.038 | 1.836 (1.048-3.218) | 0.034 | ||||
| Residual tumor size (> 1 cm) | 1.656 (1.024-2.679) | 0.040 | 1.357 (0.784-2.348) | 0.276 | ||||
| Lewis y expression (high) | 2.276 (1.394-3.716) | 0.001 | 1.931 (1.171-3.185) | 0.010 | 2.338 (1.333-4.100) | 0.003 | 1.878 (1.040-3.389) | 0.036 |
| CD44 expression (high) | 1.815 (1.120-2.941) | 0.016 | 1.827 (1.055-3.161) | 0.031 | ||||
| CD147 expression (high) | 1.869 (1.133-3.018) | 0.014 | 2.386 (1.360-4.185) | 0.002 | ||||
| HE4 expression (high) | 2.247 (1.378-3.663) | 0.001 | 2.012 (1.221-3.315) | 0.006 | 2.424 (1.393-4.219) | 0.002 | 2.071 (1.169-3.671) | 0.013 |
| Integrin α5 expression (high) | 1.917 (1.180-3.112) | 0.009 | 2.342 (1.340-4.093) | 0.003 | 1.982 (1.117-3.517) | 0.019 | ||
| Integrin β1 expression (high) | 2.310 (1.399-3.815) | 0.001 | 2.175 (1.312-3.603) | 0.003 | 2.057 (1.168-3.621) | 0.012 | ||
| Integrin αv expression (high) | 1.611 (0.993-2.616) | 0.054 | 1.841 (1.057-3.208) | 0.031 | ||||
| Integrin β3 expression (high) | 0.966 (0.594-1.570) | 0.889 | 1.022 (0.589-1.772) | 0.939 | ||||
Variables with P < 0.05 in the univariate analysis were included in the multivariate analysis. Abbreviation: PFS, progression-free survival; OS, overall survival; CI, confidence interval; HE4, Human epididymis protein 4.
Figure 3.
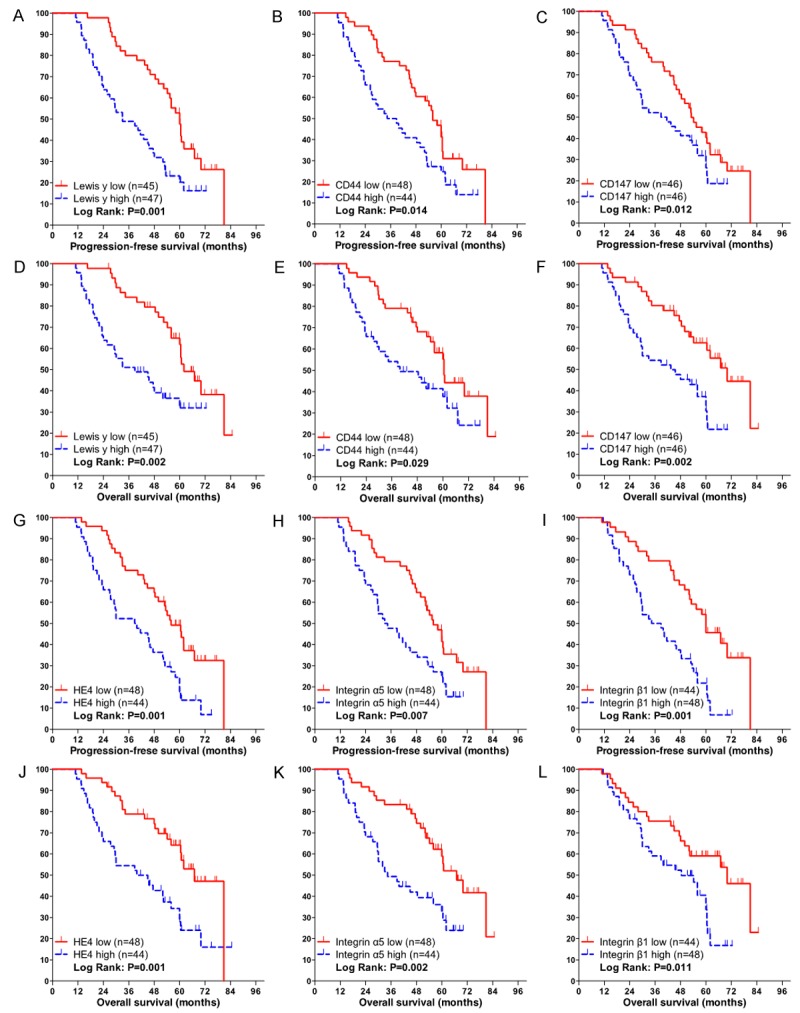
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) according to IHC markers. PFS and OS according to the low and high expression of (A, D) Lewis y, (B, E) CD44, (C, F) CD147, (G, J) Human epididymis protein 4 (HE4), (H, K) integrin α5, (I, L) integrin β1.
Discussion
Resistance of tumors to chemotherapeutic drugs remains a major clinical challenge for ovarian cancer treatment. The limitations of clinical chemotherapy have been ascribed primarily to mechanisms that mediate drug resistance at the cellular level. Evidence suggests that tumor cells have the ability to regulate genes that help to export, decrease uptake, or increase the metabolism of chemotherapeutic drugs. Newer data also suggest that interactions between tumor cells and the surrounding microenvironment allow for increased resistance of tumor cells to chemotherapy [22]. The complex interactions between tumor cells, the extracellular matrix and proteins secreted in the interstitial milieu that may lead to chemotherapeutic resistance are being elucidated. It is clear that cell adhesion to the extracellular matrix is critical for cancer survival, proliferation, and metastasis [5,23]. Currently available data has lead to the proposal that cell adhesion mediated drug resistance (CAM-DR) [24] receptor expression may play a key role in cancer cells developing drug resistance. Through the increased expression of these receptors, tumor cells have enhanced survival and decreased activation of apoptotic pathways [5].
The complex interactions of CAM-DR are still being elucidated. Currently available data suggests that CAM-DRs play a critical role in cell adhesion and signaling especially through CD44 and human epididymis protein 4 (HE4). Through interactions with hylauronan and formation of co-receptor complexes, CD44 activation leads to intracellular signal transduction [25,26]. Glycosylation or glycosaminoglycan modification of CD44 is key in the regulation of these interactions especially with hyaluronan [14]. Previous studies suggested that di-fucosylated Lewis y antigen is an integral component of CD44 and that high levels of Lewis y are associated with increased ovarian cancer cell adhesion and migration attributable to CD44 [14]. Furthermore, high levels of Lewis y and CD44 expression are correlated with increased chemotherapeutic drug resistance [9]. Lewis y antigen modification has also been seen in HE4 [16]. HE4 is highly overexpressed in epithelial ovarian cancer [27] and is thought to response to tumor microenvironment constituents, interact with a number of tumor associated pathways including EGFR, IGF1R and the transcription factor hypoxia induced factor 1a (HIF1a), all of which have been associated with ovarian tumor proliferation and chemotherapeutic resistance [28].
Expressions of CAM-DR-related markers are also thought to provide an increased capability for chemotherapy resistant tumors to metastasize and invade adjacent tissues [29]. CD147 has been shown to induce matrix metalloproteinases and angiogenesis factors in tumor cells and surrounding stroma [30]. Among its other functions CD147 has been suggested to interact with CD44 and other signaling molecules to increase activation of the RAS-ERK pathway leading to increased proliferation and tumor growth [31]. Data also suggest that CD147 can interact with drug resistance proteins causing an increased resistance to chemotherapy [32].
Like CD147, integrin receptors α and β have been implicated in the metastatic potential and development of resistance to chemotherapeutic agents. Furthermore, previous studies have shown that Lewis y antigen is a part of integrins α5β1 and αvβ3 [12,13]. When these integrins are expressed at high levels along with the modification of Lewis y antigen they have increased binding to the ECM ligands, fibronectin and vitronecticon, which may contribute to increased tumor resistance to platinum based chemotherapy. The data presented in this study support these previous conclusions.
The data presented in this study represent an analysis of the expression of Lewis y and CAM-DR related markers including CD44, CD147, HE4, as well as integrins α5, β1, αv, and β3 by immunohistochemistry in 92 samples of epithelial ovarian cancer (36 resistant and 56 sensitive). Data revealed that high expression of the CAM-DR related markers were significantly correlated with Lewis y antigen (P < 0.05). High expression of both Lewis y and CAM-DR related markers also correlated with chemotherapy resistance. These data suggest that there maybe a relationship between chemotherapy resistance and fucosylation of CAM-DR related markers. Multivariate regression analysis further confirmed that increased expression of Lewis y, CD44, HE4, as well as integrins α5 and β1 to be independently correlated with resistance to chemotherapy. These data are consistent with previous reports and provide further support to their clinical relevance.
Clinical practice currently relies on treating patients with chemotherapy and determining response to therapy based upon clinical examination, imaging and tumor markers. There are no available histologic methods to predict how ovarian cancers will respond to platinum based chemotherapy and disease prognosis. The data presented here that besides the late FIGO stage, lymph node metastasis and > 1 cm residual tumor size (for PFS), high expression of Lewis y and nearly all the CAM-DR-related markers (except integrins αvβ3 for PFS and integrin β3 for both of PFS and OS) were independent risk factors affecting the prognosis of ovarian cancer patients both in PFS and OS. And multivariate COX analysis further confirmed that high expression of Lewis y and HE4, integrin β1 were independent factors for PFS, Lewis y and HE4, integrin α5 were independent factors for OS. Data further suggest that high levels of expression of Lewis y antigen and HE4 are of great significance in predicting resistance to chemotherapy and in the prognosis of ovarian cancer patients. Obtaining this information in clinical practice may allow for improved outcomes through earlier alterations in chemotherapeutic management and potentially employing chemotherapeutic sensitivity assays to assess which chemotherapy would be most active. Information regarding the level of expression of these markers would also allow for better insight into patient prognosis.
In summary, results from immunohistochemical analyses of tumors from chemotherapy sensitive and resistant patients demonstrate that high expressions of CAM-DR-related markers including CD44, CD147, HE4, integrin α5, β1, αv, β3 are independent markers for chemotherapy resistance in patients with epithelial ovarian cancer. In addition, high expression of Lewis y antigen and CAM-DR-related markers including CD44, CD147, HE4, integrin α5 and β1 are independent markers for PFS and OS, in which Lewis y and HE4 are the most significant. An increased understanding of CAM-DR-related markers and the signal transduction pathway involved in the chemotherapeutic drug resistance induced by their glycosylations should provide the foundation for chemosensitization strategies and the development of new chemotherapeutic methods.
Acknowledgements
This work is supported by grants from The National Natural Science Foundation of China (81072118, 81172491, 81101527), item of Educational Department Doctor Startup Fund (20112104110016, 20112104120019) and Shengjing Freedom researchers’ plan (200807). The funding body had no role in the design or conduct of the study.
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Pliarchopoulou K, Pectasides D. Epithelial ovarian cancer: focus on targeted therapy. Crit Rev Oncol Hematol. 2011;79:17–23. doi: 10.1016/j.critrevonc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 5.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Kawauchi T. Cell Adhesion and Its Endocytic Regulation in Cell Migration during Neural Development and Cancer Metastasis. Int J Mol Sci. 2012;13:4564–4590. doi: 10.3390/ijms13044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamori M, Tanaka K, Kubushiro K, Lin B, Kiguchi K, Ishiwata I, Tsukazaki K, Nozawa S. Alterations in the glycolipid composition and cellular properties of ovarian carcinoma-derived RMG-1 cells on transfection of the alpha1,2-fucosyltransferase gene. Cancer Sci. 2005;96:26–30. doi: 10.1111/j.1349-7006.2005.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriwaki K, Miyoshi E. Fucosylation and gastrointestinal cancer. World J Hepatol. 2010;2:151–161. doi: 10.4254/wjh.v2.i4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhieng DC, Rodriguez-Burford C, Talley LI, Sviglin H, Stockard CR, Kleinberg MJ, Barnes MN, Partridge EE, Khazaeli MB, Grizzle WE. Expression of CEA, Tag-72, and Lewis-Y antigen in primary and metastatic lesions of ovarian carcinoma. Hum Pathol. 2003;34:1016–1021. doi: 10.1053/s0046-8177(03)00355-1. [DOI] [PubMed] [Google Scholar]
- 12.Yan LM, Lin B, Zhu LC, Hao YY, Qi Y, Wang CZ, Gao S, Liu SC, Zhang SL, Iwamori M. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin alpha5beta1 with the Lewis Y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochimie. 2010;92:852–857. doi: 10.1016/j.biochi.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu J, Lin B, Wang C, Li Q, Liu S, Yan L, Zhang S, Iwamori M. Study on the Expression and Clinical Significances of Lewis y Antigen and Integrin alphav, beta3 in Epithelial Ovarian Tumors. Int J Mol Sci. 2011;12:3409–3421. doi: 10.3390/ijms12063409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Yan L, Lin B, Gao J, Liang X, Wang Y, Liu J, Zhang S, Iwamori M. Enhancive effects of Lewis y antigen on CD44-mediated adhesion and spreading of human ovarian cancer cell line RMG-I. J Exp Clin Cancer Res. 2011;30:15. doi: 10.1186/1756-9966-30-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, Chen Q, Yang JM, Xu ZD, Liu XP. Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy. 2008;54:291–301. doi: 10.1159/000151225. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS One. 2013;8:e68994. doi: 10.1371/journal.pone.0068994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JJ, Lin B, Hao YY, Li FF, Liu DW, Qi Y, Zhu LC, Zhang SL, Iwamori M. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol Rep. 2010;23:833–841. [PubMed] [Google Scholar]
- 18.Gao S, Liu Q, Wang X, Lin B, Zhang S. Effects of Lewis Y antigen on the gene expression of multiple drug resistance-associated proteins in human ovarian cancer RMG-I-H cells. Med Oncol. 2010;27:960–967. doi: 10.1007/s12032-009-9317-6. [DOI] [PubMed] [Google Scholar]
- 19.Pajares MJ, Agorreta J, Salvo E, Behrens C, Wistuba II, Montuenga LM, Pio R, Rouzaut A. TGFBI expression is an independent predictor of survival in adjuvant-treated lung squamous cell carcinoma patients. Br J Cancer. 2014;110:1545–1551. doi: 10.1038/bjc.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 21.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, Nakano H. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36:9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 22.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 23.Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets. 2002;2:37–43. doi: 10.2174/1568009023334033. [DOI] [PubMed] [Google Scholar]
- 24.Shain KH, Dalton WS. Cell adhesion is a key determinant in de novo multidrug resistance (MDR): new targets for the prevention of acquired MDR. Mol Cancer Ther. 2001;1:69–78. [PubMed] [Google Scholar]
- 25.Slomiany MG, Dai L, Tolliver LB, Grass GD, Zeng Y, Toole BP. Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin Cancer Res. 2009;15:7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SJ, Bourguignon LY. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am J Pathol. 2011;178:956–963. doi: 10.1016/j.ajpath.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, Setala M, Harkki P, Jalkanen J, Fraser J, Makinen J, Auranen A, Poutanen M, Perheentupa A. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315–1319. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore RG, Hill EK, Horan T, Yano N, Kim K, MacLaughlan S, Lambert-Messerlian G, Tseng YD, Padbury JF, Miller MC, Lange TS, Singh RK. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep. 2014;4:3574. doi: 10.1038/srep03574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, Chen ZS, Kanekura T. RNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276:189–195. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Gardner MJ, Jones LM, Catterall JB, Turner GA. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett. 1995;91:229–234. doi: 10.1016/0304-3835(95)03743-g. [DOI] [PubMed] [Google Scholar]
- 31.Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, Kearsley JH, Li Y. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One. 2012;7:e40716. doi: 10.1371/journal.pone.0040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM, Xu ZD. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007;98:1064–1069. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


