Abstract
Tamoxifen provided a successful treatment for ER-positive breast cancer for many years. However, HER2 overexpressing breast cancer cells respond poorly to tamoxifen therapy presumably by pass. The molecular mechanisms underlying development of tamoxifen resistance have not been well established. Recently, we reported that breast cancer cells with high levels of ER-α36, a variant of ER-α, were resistant to tamoxifen and knockdown of ER-α36 expression in tamoxifen resistant cells with the shRNA method restored tamoxifen sensitivity, indicating that gained ER-α36 expression is one of the underlying mechanisms of tamoxifen resistance. Here, we found that tamoxifen induced expression of ER-α36-EGFR/HER2 positive regulatory loops and tamoxifen resistant MCF7 cells (MCF7/TAM) expressed enhanced levels of the loops. Disruption of the ER-α36-EGFR/HER2 positive regulatory loops with the dual tyrosine kinase inhibitor Lapatinib or ER-α36 down-regulator Broussoflavonol B in tamoxifen resistant MCF7 cells restored tamoxifen sensitivity. In addition, we also found both Lapatinib and Broussoflavonol B increased the growth inhibitory activity of tamoxifen in tumorsphere cells derived from MCF7/TAM cells. Our results thus demonstrated that elevated expression of the ER-α36-EGFR/HER2 loops is one of the mechanisms by which ER-positive breast cancer cells escape tamoxifen therapy. Our results thus provided a rational to develop novel therapeutic approaches for tamoxifen resistant patients by targeting the ER-α36-EGFR/HER2 loops.
Keywords: ER-α36, EGFR, HER2, breast cancer, tamoxifen resistance
Introduction
Human epidermal growth factor receptor 2 (ERBB2/HER2) is amplified and/or overexpressed in approximately 15-20% of breast cancers [1,2]. HER2-overexpressing breast cancer patients exhibit a poor prognosis because of a high incidence of metastases, disease progression, and resistance to current endocrine therapy regimens in the tumors co-expressing estrogen receptor (ER) [1,3,4]. Clinically, the current therapy for HER2 expressing, early-stage and metastatic breast cancer patients employs a combination of HER2-targeted monoclonal antibody (Trastuzumab, Herceptin) treatment with chemotherapy (Docetaxel or Vinorelbine) [5]. Additionally, the dual inhibitor of EGFR (ERBB1)/HER2 (ERBB2) receptor, Laptinib, is also used as a combination treatment with Capecitabine for HER2-positive advanced breast cancer that has progressed after previous treatment with other chemotherapies or combinational therapies [5]. However, 60% of metastatic breast cancers that express HER2 fail to respond to current available anti-HER2 therapies [6-8]. Trastuzumab/Lapatinib combination treatment has provided significant benefits to patients; 50% of patients to the combination therapy whereas 32-43% with Trastuzumab alone, but there is still a large percentage (50%) of patients that do not respond [9]. Thus, novel therapeutic approaches are urgently needed for the patients with HER2 positive breast cancer but fail to respond to anti-HER2 therapies.
Estrogen signaling through its cognate receptor (ER) plays an important role in breast cancer tumorigenesis and biology. Antiestrogen therapy has been widely used for treatment of ER-positive breast cancer. However, in clinical practice, it has been observed that HER2 overexpressing tumors, even co-expressing ER, have reduced responsiveness to antiestrogen therapy [10,11]. Hence, enhanced HER2 expression stimulates growth factor signaling that can rescue estrogen-dependent breast cancer cells from the effects of estrogen deprivation.
The selective estrogen-receptor modulator tamoxifen is the most widely used antiestrogen in clinical practice. Previously, it has been reported that overexpression of HER2 in estrogen-dependent and tamoxifen sensitive cells resulted in tamoxifen resistance while a HER tyrosine kinase inhibitor restored tamoxifen sensitivity to these cells [12]. In preclinical studies, a combination of trastuzumab and tamoxifen treatment has been demonstrated to result in synergistic growth inhibition of HER2 expressing breast cancer cells [13-16]. The dual kinase inhibitor, Lapatinib, also has been shown to cooperate with tamoxifen to inhibit cell proliferation and estrogen dependent gene expression in antiestrogen-resistant breast cancer [17]. Thus, combination of HER2 and ER targeted therapies may provide a novel and effective approach to treatment of HER2-overexpressing, tamoxifen resistant breast cancer. The molecular mechanism underlying the synergistic interaction between tamoxifen and trastuzumab has not been well established, which might influence further development of this therapeutic approach.
Previously, our laboratory identified and cloned a variant of ER-α, ER-α36, which has a molecular weight of 36-kDa [18,19]. The transcript of ER-α36 is initiated from a previously unidentified promoter in the first intron of the ER-α gene [20]. This ER-α differs from the original 66 kDa ER-a (ER-α66) because it lacks both transcriptional activation domains (AF-1 and AF-2) but retains the DNA-binding and dimerization domains, and partial ligand-binding domain [19]. ER-α36 is mainly localized near the plasma membrane and mediates membrane-initiated estrogen signaling [19]. Previously, we reported the existence of a cross-regulatory loop between ER-α36 and HER2 [21]; ER-α36 positively regulates HER2 expression while HER2 up-regulates the promoter activity of ER-α36 through an Ap1 site located in the promoter region of ER-α36. We also found that the breast cancer patients with tumors expressing high levels of ER-α36 less benefited from tamoxifen therapy than those with low levels of ER-α36 expression and ER-α36 expression is well correlated with HER2 expression in tumor samples [22], suggesting that gained the ER-α36/HER2 positive regulatory loop is one of the underlying mechanisms of tamoxifen resistance. In addition, we also found a positive feedback loop between ER-α36 and EGFR [23]. ER-α36 is able to mediate agonist activity of tamoxifen such as activation of the MAPK/ERK and the PI3K/AKT signaling pathways [24,25] and is involved in development of tamoxifen resistance [26].
Based on these observations, we hypothesized that the positive regulatory loop between ER-α36 and HER2 is involved in tamoxifen resistance of HER2 overexpressing breast cancer cells. Thus, disruption of this loop may restore tamoxifen sensitivity in tamoxifen resistant cells. Using HER2 overexpressing ER-positive breast cancer BT474 cells and MCF7/HER2-18 cells as models, we investigated the effects of disruption of the ER-α36-HER2 positive regulatory loop in tamoxifen resistance.
Methods
Chemicals and antibodies
Tamoxifen was purchased from Sigma Chemical Co. (St. Louis, MO). Broussoflavonol B was obtained from Shenogen Pharma Group (Beijing, P.R. China). Anti-phospho-EGFR (Tyr1045) and -HER2/ErbB2 (Tyr1221/1222) as well as anti-EGFR and -HER2/ErbB2 (D8F12) antibodies were purchased from Cell Signaling Technology (Boston, MA). Antibodies of ER-α66 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-ER-α36 antibody was generated and characterized as described before [25].
Cell culture and establishment of stable cell lines
MCF7 and BT474 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA). MCF7/HER2-18 cells were kindly provided by Dr. Jian Huang at Medical College of Wisconsin. All cells were maintained at 37°C in a 10% CO22 atmosphere in IMEM without phenol red plus 10% fetal calf serum.
To examine cell growth in the presence or absence of tamoxifen, cells maintained for three days in phenol red-free DMEM plus 2.5% dextran-charcoal-stripped fetal calf serum (HyClone, Logan, UT) were treated with different concentrations of tamoxifen, or ethanol vehicle as a control. The cells were seeded at 1 × 104 cells per dish in 60 mm dishes and the cell numbers were determined using the ADAM automatic cell counter (Digital Bio., Korea) after seven days. Five dishes were used for each treatment and experiments were repeated more than three times.
Western blot analysis
For immunoblot analysis, cells washed with PBS were lysed with the lysis buffer (50 mM Tris-HCl pH8.0, 150 mM NaCl, 0.25 mM EDTA pH8.0, 0.1% SDS, 1% Triton X-100, 50 mM NaF) supplemented with protease and phosphatase inhibitors (Sigma). The protein amounts were measured using the DC protein assay kit (BIO-RAD Laboratories, Hercules, CA). The same amounts of the cell lysates were boiled for five minutes in loading buffer and separated on a SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane. The membranes were probed with various primary antibodies, HRP-conjugated secondary antibodies, and visualized with enhanced chemiluminescence (ECL) detection reagents (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Tumorsphere formation and flow cytometry analysis
To establish tumorspheres, cells were seeded onto Corning Ultra-Low Attachment 6-well plate (Corning Incorporated, CA) at 10,000 cells/ml and cultured seven days in the tumorsphere medium: phenol-red free DMEM/F12 medium (Invitrogen) supplemented with 1 X B-27 (Invitrogen), 20 ng/ml epidermal growth factor (Sigma-Aldrich) and 20 ng/ml basic fibroblast growth factor (ProSpec, NJ), 0.5 µg/mL hydrocortisone (Sigma). Tumorspheres were collected, washed with PBS, and incubated with Trypsin-EDTA (0.25%/0.5 mM) for two minutes at 37°C to dissociated cells. The number of tumorspheres and dissociated cells were counted using a Multisizer 3 Coulter Counter (Beckman Coulter, Brea, CA) and the ADAM automatic cell counter, respectively. For tamoxifen treatment assays, tumorspheres were treated with tamoxifen or vehicle (ethanol) as a control. Three dishes were used for each group and all experiments were repeated three times.
For CD44+/CD24- cell analysis, single cell suspension washed with cold PBS/1% BSA were incubated with PerCP-CyTM5.5 mouse anti-human CD44 and PE mouse anti-human CD24 in PBS/1% BSA for 30 minutes at 4°C. After incubation, the cells were washed twice in cold PBS/1% BSA and re-suspended in cold PBS/1% BSA for flow cytometry analysis.
Statistical analysis
Data were summarized as the mean ± standard error (SE) using the GraphPad InStat software program. Tukey-Kramer Multiple Comparisons Test was also used, and the significance was accepted for P < 0.05.
Results
Enhanced ER-α36 and EGFR expression in ER-positive breast cancer BT474 cells
BT474 is a human breast cancer cell line that is positive for ER and is estrogen dependent. BT474 highly expresses HER2 in association with gene amplification [26]. Previously, our laboratory identified and cloned a 36 kDa variant of ER-α, ER-α36 that functions differently from the 66 kDa full-length ER-α, ER-α66 [19]. Compared to ER-positive breast cancer MCF7 cells, we found that the steady state level of ER-α36 protein was increased in BT474 cells accompanied by upregulated HER2 and EGFR expression (Figure 1A). We also examined ER-α36 expression in the MCF7/HER2-18 cell line, a cell line generated by stable transfection of a HER2 expression vector [27] and found that ER-α36 and EGFR expression is also upregulated in MCF7/HER2-18 cells compared to MCF7 cells (Figure 1B). Our results suggested that the positive regulatory loops of ER-α36 and EGFR/HER2 observed previously also exist in HER2 expressing breast cancer cells.
Figure 1.
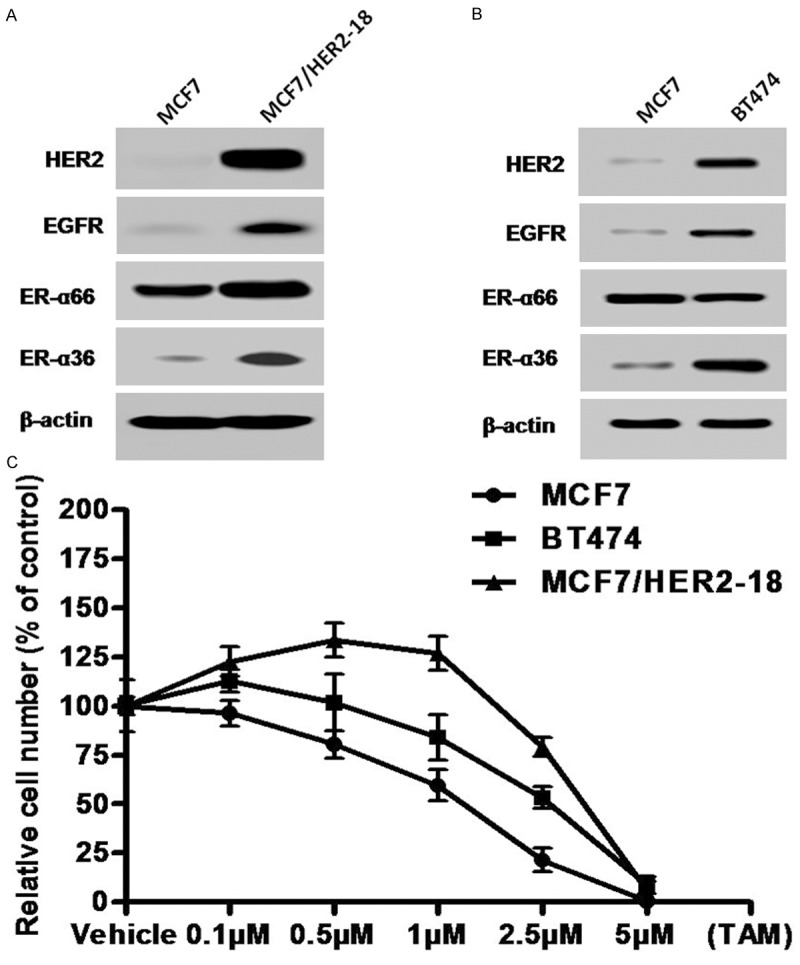
HER2 expressing breast cancer cells exhibit enhanced expression of ER-α36 and EGFR as well as tamoxifen resistance. A & B. Western blot analysis of the expression levels of ER-α66, ER-α36, HER2 and EGFR in ER-positive breast cancer MCF7, BT474 and MCF7HER2-18 cells. C. Cells were treated with indicated concentrations of tamoxifen for seven days and the numbers of survived cells were counted. Each point represents the means of three experiments.
We then examined the sensitivity of these HER2 expressing cells to tamoxifen. Cells were treated with different concentrations of tamoxifen for seven days and then the survived cells were counted. We found both BT474 and MCF7/HER2-18 cells are relatively more resistant to tamoxifen compared to MCF7 cells (Figure 1C), consistent with the concept that HER2 overexpression confers tamoxifen resistance in ER-positive breast cancer cells.
ER-α36 knock-down sensitizes HER2-expressing cells to tamoxifen
Previously, we reported ER-α36 is involved in tamoxifen resistance of ER-positive breast cancer cells [23,26]. To examine whether ER-α36 is also involved in tamoxifen insensitivity of HER2-expressing breast cancer cells, we transiently transfected HER2-expressing cells with an ER-α36 specific shRNA expression vector and found the shRNA expression vector efficiently knocked down ER-α36 expression in these HER2-expressing cells while had no effect on ER-α66 expression compared to the empty vector transfected cells (Figure 2A, 2C). We also observed that the expression levels of both HER2 and EGFR were also downregulated (Figure 2A, 2C), suggesting the existence of the positive regulatory loops between ER-α36 and HER2/EGFR we previously reported [22,28].
Figure 2.
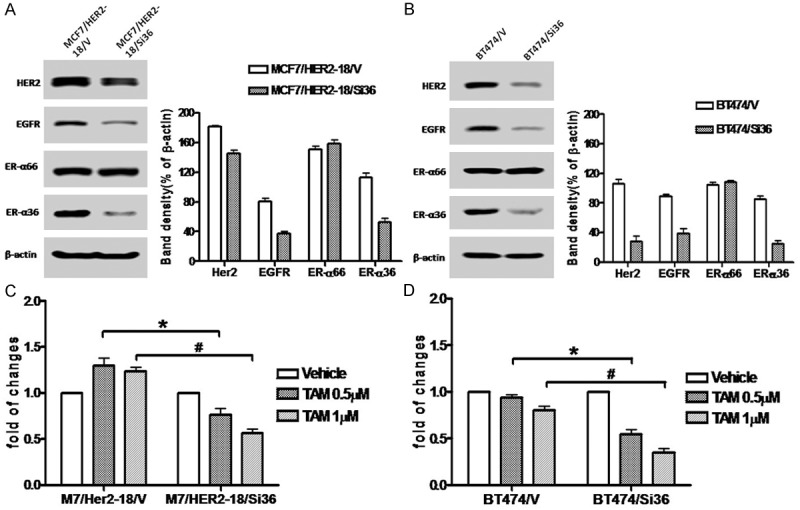
Knock-down of ER-α36 expression sensitizes HER2 expressing cells to tamoxifen. A. Western blot analysis of the expression levels of ER-α66, ER-α36, HER2 and EGFR in MCF7/HER2-18 cells transfected with an empty expression vector (MCF7/HER2-18/V) and with the ER-α36 shRNA expression vector (MCF7/HER2-18/Si36). B. Western blot analysis of the expression levels of ER-α66, ER-α36, HER2 and EGFR in BT474 cells transfected with an empty expression vector (BT474/V) and with the ER-α36 shRNA expression vector (BT474/Si36). C & D. Cells were treated with indicated concentrations of tamoxifen for seven days and the numbers of survived cells were counted. The columns represent the means of three experiments; bars, SE. * and #, P < 0.05 for control cells transfected with the empty vector vs the cells transfected with ER-α36 the shRNA expression vector, respectively.
We then examined the sensitivity of the cells with knocked-down levels of ER-α36 to tamoxifen and found that these cells were relatively more sensitive to tamoxifen compared o the vector transfected cells (Figure 2B, 2D), consistent with our previous report that increased level of ER-α36 expression is one of the underlying mechanisms of TAM resistance and knock-down of ER-α36 expression restored TAM sensitivity in MCF7/TAM cells [26]. Our results thus suggested that ER-α36 is also involved in tamoxifen resistance of HER2-expressing ER-positive breast cancer cells and disruption of the ER-α36 and EGFR/HER2 regulatory loops may restore tamoxifen sensitivity in these HER2-expressing cells.
Dual kinase inhibitor Lapatinib downregulates ER-α36 expression and sensitizes HER2-texpressing cells to tamoxifen
We then sought to examine whether disruption of the ER-α36 and EGFR/HER2 regulatory loops restores tamoxifen sensitivity in these HER2-expressing cells. We first treated MCF7/HER2-18 cells with different concentrations of Lapatinib and the level of ER-α36 expression was examined with Western blot analysis. We found that Lapatinib inhibited phosphorylation of both EGFR and HER2 effectively (Figure 3A, 3B) and also downregulated ER-α36 expression in MCF7/HER2-18 cells (Figure 3C). Lapatinib treatment significantly increased sensitivity to tamoxifen in MCF7/HER2-18 cells (Figure 3D). Similar results were also obtained in BT474 cells (Figure 3E, 3F). Taken together, these results demonstrated that the dual kinase inhibitor Lapatinib was able to disrupt the ER-α36-EGFR/HER2 positive regulatory loops and restored tamoxifen sensitivity in these HER2-expressing cells.
Figure 3.

Dual kinase inhibitor Lapatinib downregulates ER-α36 expression and sensitizes HER2 expressing cells to tamoxifen. A. Western blot analysis of the expression of phosphorylated EGFR and HER2 in MCF7/HER2-18 treated with indicated concentrations of Lapatinib for 12 hours. B. Western blot analysis of the expression of phosphorylated EGFR and HER2 in BT474 cells treated with indicated concentrations of Lapatinib for 12 hours. C & E. Western blot analysis of the expression of ER-α36 and ER-α66 as well as EGFR and HER2 in parental MCF7, MCF7/HER2-18 and BT474 cells treated with vehicle or indicated concentrations of Lapatinib (LAP) for 12 hours. D & F. Cells were treated with indicated concentrations of tamoxifen (TAM) together with vehicle or 1 μM of Lapatinib (LAP) for seven days and the numbers of survived cells were counted. The columns represent the means of three experiments; bars, SE. * and #, P < 0.05 for cells treated with vehicle vs cells treated with 0.5 and 1 μM of tamoxifen, respectively.
ER-α36 disruptor Broussoflavonol B also downregulates EGFR/HER2 expression and restores tamoxifen sensitivity
Previously, we found that the classic ER disruptor ICI 182, 780 failed to destabilize ER-α36 protein [28]. We later discovered a novel ER-α36 disruptor Broussoflavonol B (5, 7, 3’, 4’-Tetrahydroxy-3-methoxy-6,8-diprenylflavone) purified from the bark of Broussonetia papyrifera that specifically and effectively downregulated the steady state levels of ER-α36 protein [29,30]. We then examined whether the ER-α36 disruptor Broussoflavonol B (BB) is also able to disrupt the ER-α36-EGFR/HER2 loops and restores tamoxifen sensitivity in HER2-expressing cells. We treated HER2-expressing cells with different concentrations of BB and the expression levels of ER-α36, EGFR and HER2 were examined with Western blot analysis. We found that BB potently down-regulated ER-α36 expression but modestly downregulated expression levels of EGFR and HER2 proteins in MCF7/HER2-18 cells (Figure 4A). BB treatment, however, strongly down-regulated ER-α36, EGFR and HER2 expression in BT474 cells (Figure 4C). BB treatment significantly sensitized these HER2 expressing cells to tamoxifen (Figure 4B, 4D). Our results thus demonstrated that ER-α36 downregulator BB was also able to disrupt the ER-α36-EGFR/HER2 positive regulatory loops and restored tamoxifen sensitivity in HER2-overexpressing cells.
Figure 4.
ER-α36 disruptor Broussoflavonol B restores tamoxifen sensitivity in HER2 expressing cells. A & C. Western blot analysis of the expression of ER-α36, ER-α66, EGFR and HER2 in MCF7 and MCF7/HER2-18 and BT474 cells treated with indicated concentrations of Broussoflavonol B (BB) for 12 hours. B & D. Cells were treated with indicated concentrations of tamoxifen (TAM) together with vehicle or 1 μM of Broussoflavonol B (BB) for seven days and the numbers of survived cells were counted. The columns represent the means of three experiments; bars, SE. * and #, P < 0.05 for cells treated with vehicle vs cells treated with 1 μM of tamoxifen.
ER-α36 knock-down reduces the populations of breast cancer stem/progenitor cells in these HER2-expressing cells
Recently, we found that ER-positive breast cancer stem/progenitor cells express higher levels of ER-α36 and ER-α36 plays an important role in maintenance of breast cancer stem/progenitor cells [31,32]. In addition, tamoxifen resistant breast cancer cells contain high percentage of breast cancer stem/progenitor cells [33]. We sought to investigate the function of ER-α36 in these HER2-expressing breast cancer stem/progenitor cells. We transiently transfected HER2-expressing cells with the ER-α36 specific shRNA expression vector and examined the CD44+/CD24- phenotype cell populations. We found that ER-α36 knock-down significantly reduced the percentages of the CD44+/CD24- cells from these HER2 expressing cells (Figure 5A, 5B). We also tested the ability of these HER2-expressing cells to form tumorspheres and the cells were cultured in the tumorsphere media and under suspension condition to form tumorspheres. We found that the HER2 expressing cells formed more tumorspheres compared to MCF7 cells while ER-α36 knocked-down cells significantly decreased tumorsphere numbers (Figure 5C, 5D). Our results thus indicated that ER-α36 plays a critical role in maintenance of HER2-expressing breast cancer stem/progenitor cells.
Figure 5.
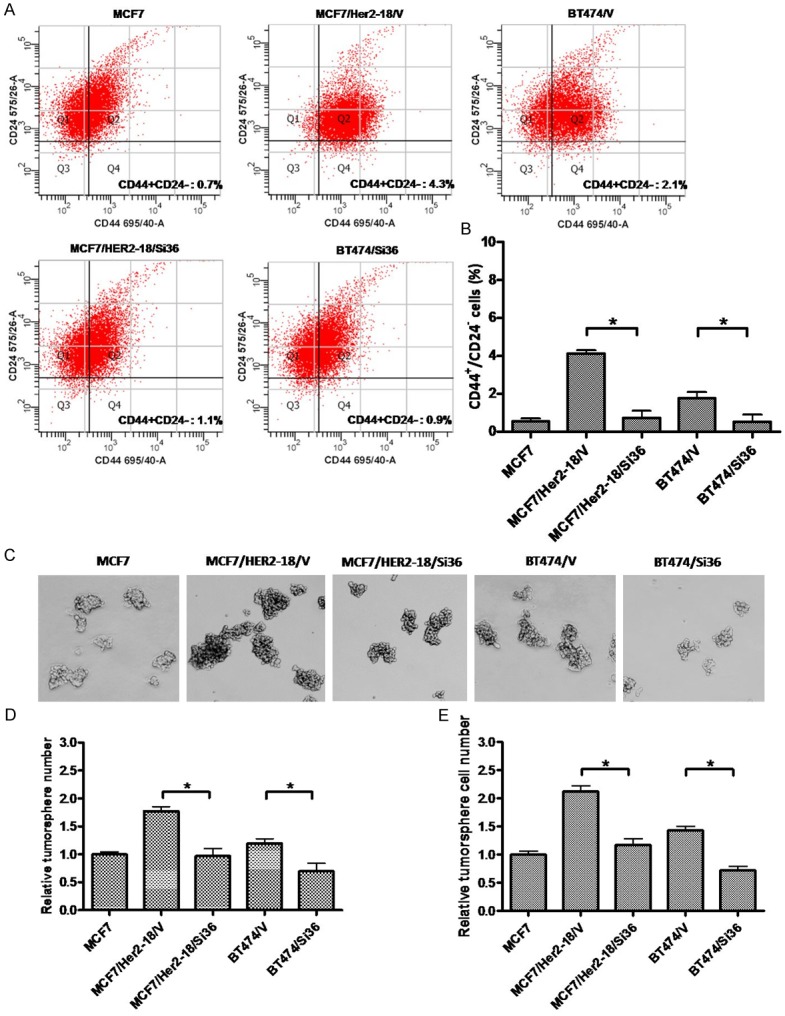
Knock-down of ER-α36 reduces populations of the breast cancer stem/progenitor cells from HER2 expressing cells. A & B. Knock-down of ER-α36 expression reduces the populations of CD44+/CD24- cells in BT474 and MCF/HER2-18 cells. The columns represent the means of three experiments; bars, SE. *, P < 0.05 for cells transfected with the empty expression vector vs cells transfected with the ER-α36 shRNA expression vector. C. Tumorsphere formation assay was used to assess the effects of ER-a36 knock-down on breast cancer stem/progenitor cells derived from MCF7, BT474 and MCF7/HER2-18 cells transfected with the empty expression vector (BT474/V and MCF7/HER2-18/V), or the ER-α36 shRNA expression vector (BT474/Si36 and MCF7/HER2-18/Si36). The representative results are shown. D. The numbers of tumorspheres formed by these cells. E. The number of cells from dissociated tumorspheres formed by these cells. The columns represent the means of three experiments; bars, SE. *, P < 0.05 for cells transfected with the empty expression vector vs cells transfected with the ER-α36 shRNA expression vector.
We also used Western blot analysis to assess expression of ER-α66, ER-α36, EGFR and HER2 in tumorsphere and attached cells. We found that ER-α36, EGFR and HER2 were all highly expressed in tumorsphere cells derived from MCF7 cells compared to attached bulk cells while ER-α66 expression was down-regulated (Figure 6A), consistent with our previous report [32]. HER2 expressing cells exhibited enhanced basal levels of ER-α36, EGFR and HER2 expression in bulk cells, which was modestly increased in tumorsphere cells derived from these cells (Figure 6A).
Figure 6.
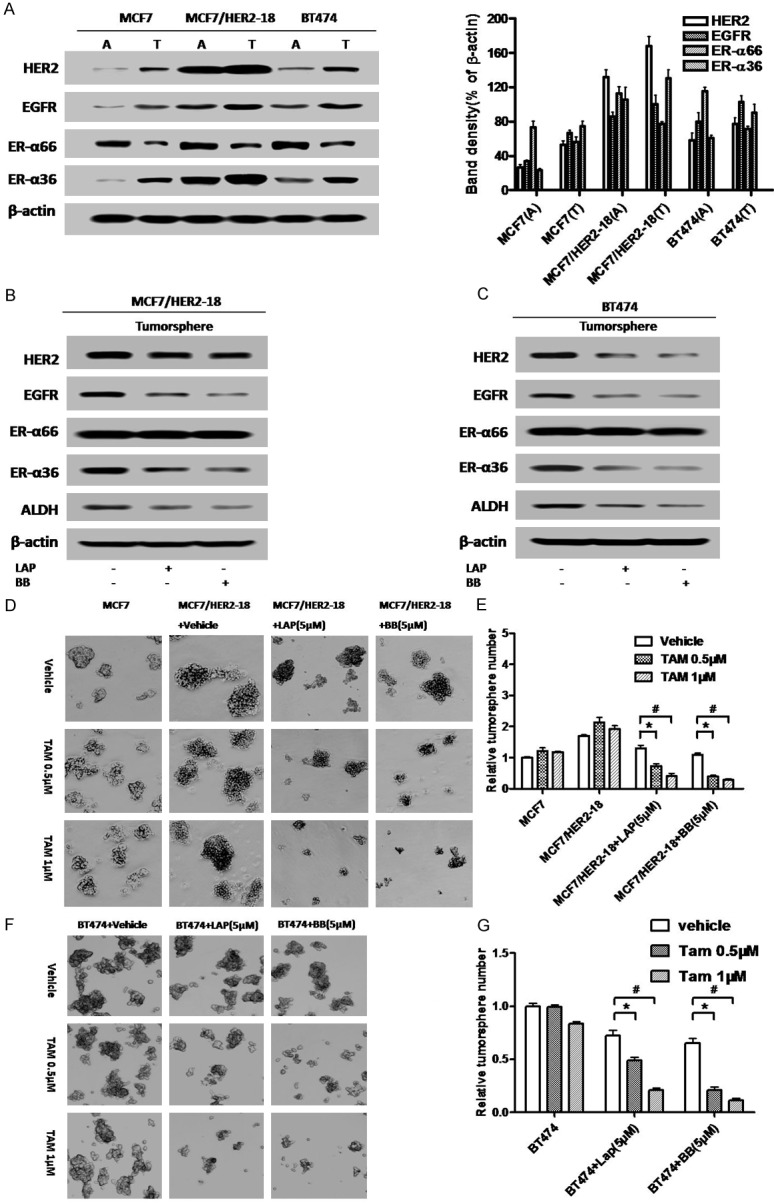
Disruption of the ER-α36-EGFR/HER2 positive regulatory loops sensitizes HER2 expressing breast cancer stem/progenitor cells to tamoxifen. (A) Western blot analysis of the expression of ER-α36, ER-α66, EGFR and HER2 in the monolayer cells grown on attachment dishes (A) and tumorsphere cells grown on low-attachment dishes (T). Band density (% of β-actin) is also shown. (B & C) Tumorsphere cells derived from BT474 and MCF7/HER2-18 cells were treated with 5 μM of Broussoflavonol B (BB) or Lapatinib (LAP) for five days. Western blot analysis of expression levels different proteins was performed. (D) Tumorsphere formation assay was used to assess the effects of tamoxifen alone or together with Lapatinib (LAP) or Broussoflavonol B (BB) on the breast cancer stem/progenitor cells derived from MCF7/HER2-18 cells. The representative results are shown. (E) The numbers of tumorspheres formed by the cells treated with tamoxifen alone or together with Lapatinib (LAP) or Broussoflavonol B (BB). (F) Tumorsphere formation assay was used to assess the effects of tamoxifen alone or together with Lapatinib (LAP) or Broussoflavonol B (BB) on the breast cancer stem/progenitor cells derived from BT474 cells. The representative results are shown. (G) The numbers of tumorspheres formed by the cells treated with tamoxifen alone or together with Lapatinib (LAP) or Broussoflavonol B (BB). The columns represent the means of three experiments; bars, SE. *&#, P < 0.05 for cells treated with vehicle vs cells treated with 0.5 and 1 μM of tamoxifen.
We then sought to examine whether Lapatinib and Broussoflavonol B are still able to disrupt the ER-α36-EGFR/HER2 regulatory loops in ER-positive breast cancer stem/progenitor cells. Lapatinib and BB treatment again downregulated EGFR and ER-α36 in both HER2-expressing cell lines while only slightly downregulated HER2 expression in MCF7/HER2-18 cells that were stably transfected with an HER2 expression vector. The results thus demonstrated that there exist ER-α36-EGFR/HER2 regulatory loops in ER-positive stem/progenitor cells derived from HER2 expressing breast cancer cells and both Lapatinib and Broussoflavonol B were able to disrupt the ER-α36-EGFR/HER2 regulatory loops in these breast cancer stem/progenitor cells.
Disruption of ER-α36-EGFR/HER2 positive regulatory loops sensitizes HER-expressing breast cancer stem/progenitor cells to tamoxifen
Next, we sought to examine whether disruption of the ER-α36-EGFR/HER2 positive regulatory loops will sensitize HER2-expressing breast cancer stem/progenitor cells to tamoxifen. We cultured these HER2-expressing cells under suspension conditions to form tumorspheres for five days and then different concentrations of tamoxifen together with Lapatinib (LAP, 5 μM) or Broussoflavonol B (BB, 5 μM) were added for another five days. We found that in the presence of Lapatinib or Broussoflavonol B, the tumorspheres formed by HER2 expressing cells became sensitive to tamoxifen; tamoxifen reduced the number of tumorspheres (Figure 6D-F).
Discussion
Endocrine therapy using antiestrogen tamoxifen is the most effective treatment for advanced ER-positive breast cancer for four decades. Tamoxifen acts through ER pathway, which has been proven to reduce relapse, death rates and risk of contralateral breast cancer. However, patients often develop resistance tamoxifen, which limit its effectiveness. Many researches were conducted to understand the molecular pathways involved in tamoxifen resistance and have revealed that multiple signaling molecules and pathways such as EGFR and HER2 are implicated in tamoxifen resistance [34,35]. All these pathways often bypass the requirement of estrogen signaling for growth of ER-positive breast cancer cells.
Both experimental and clinical evidence have indicated that the HER2 signaling pathway interacts with the estrogen-signaling pathway. Experimental evidence has shown that estrogen-dependent MCF7 cells that over express HER2 are rendered tamoxifen resistant and have reduced numbers of ER [34,35]. Hence the HER2 pathway has been investigated for its contribution towards development of tamoxifen resistance and HER2 has been proposed as a potential marker of tamoxifen sensitivity. Many clinical studies have found an association between HER2 overexpression and tamoxifen failure [36-44]. Thus, the combination therapy by targeting both HER2 and ER-a was hypothesized and tested in preclinical studies. The combination of tamoxifen and anti-HER2 antibody exhibits strong synergistic inhibition of growth in HER2-expressing ER-positive breast cancer cells [13,15]. Chu et al., also reported that the dual kinase inhibitor Laptinib cooperates with tamoxifen to inhibit cell proliferation in antiestrogen resistant breast cancer [17].
Previously, we reported that breast cancer patients with tumors expressing high levels of endogenous ER-α36 less benefited from tamoxifen therapy than those with low levels of ER-α36 expression [23], suggesting elevated expression of ER-α36 is a mechanism underlying acquired tamoxifen resistance. Recently, we confirmed that elevated ER-α36 expression is involved in tamoxifen resistance through mediating agonist activity of tamoxifen [26]. We also reported that ER-α36 expression is highly correlated with HER2 expression and there are positive regulatory loops between ER-α36 and EGFR as well as ER-α36 and HER2; EGFR signaling induces the promoter activity of ER-α36 via an Ap1-binding site and ER-α36 stabilizes the EGFR protein [21-23]. HER2 signaling also activates ER-α36 promoter activity and ER-α36-mediated estrogen signaling induces HER2 promoter activity [21]. Here, we showed that knock-down of ER-α36 expression downregulated both HER2 and EGFR expression in HER-overexpressing BT474 cells while only modestly downregulated HER2 expression in MCF7/HER2-18 cells that were stably transfected with a HER2 expression vector, consistent with our previous reports that ER-α36 modulates HER2 promoter activity [21]. In addition, an experiment with a proteasome inhibitor MG132 in MCF7/HER2-18 cells with knocked-down levels of ER-α36 expression showed that MG132 treatment restored the steady state levels of HER2 protein (see Supplementary data), suggesting ER-α36 also modulates the steady state levels of HER2 protein presumably through the proteasome system. Taken together, our results demonstrated that the ER-α36-EGFR/HER2 positive regulatory loops are involved in tamoxifen resistance of HER2 overexpressing ER-positive breast cancer cells. Enhanced expression of HER2 and EGFR render the cell bypass the requirement of estrogen for cell proliferation.
In the current study, we observed that inhibition of both EGFR and HER2 signaling pathways with the dual kinase inhibitor Lapatinib disrupted the positive regulatory loops, downregulated ER-α36 expression and restored tamoxifen sensitivity. Our results thus are in good agreement with the previous reports that Lapatinib restores antiestrogen sensitivity in breast cancer cells with acquired endocrine resistance [17,45]. Here, we also observed that Lapatinib downregulated EGFR, HER2 and ER-α36 in BT474 cells, indicating the existence of the positive regulatory loops. Our data thus provided a novel molecular mechanism to the function of the dual kinase inhibitor Lapatinib; disruption of the ER-α36-EGFR/HER2 positive regulatory loops, which restores tamoxifen sensitivity.
Previously, we found that the potent ER-α disruptor ICI 182, 780 failed to degrade ER-α36 due to the lacking of the critical Helix 12 in the C-terminal of ER-α36 protein [28]. Recently, we found that a falconoid, Broussoflavonol B (5, 7, 3’,4’-Tetrahydroxy-3-methoxy-6,8-diprenylflavone) purified from the bark of Broussonetia papyrifera was able to down-regulate ER-α36 expression and inhibits proliferation of ER-positive and -negative breast cancer cells [29,30]. Here we showed that Broussoflavonol B was also able to disrupt the ER-α36-EGFR/HER2 positive regulatory loops; downregulated ER-α36, HER2 and EGFR, which restored tamoxifen sensitivity in HER2 expressing cells. Thus, further development of chemical compounds like Broussoflavonol B may provide novel approaches to restore tamoxifen sensitivity in HER2 expressing cells.
Accumulating experimental and clinical evidence indicate that breast cancer arises from mammary stem/progenitor cell populations [46-48]. Although the possible involvement of breast cancer stem/progenitor cells in tamoxifen resistance has been proposed [49] and demonstrated [33], the exact function and the underlying mechanism of breast cancer stem/progenitor cells in TAM resistance remain largely unknown. Recently, we found that ER-positive breast cancer stem/progenitor cells express higher levels of ER-α36 and were more resistant to tamoxifen than the bulk cells [31]. Here, we showed that percentages of breast cancer stem-like cells (CD44+/CD24- cells and tumorsphere cells) from HER2 expressing cells were higher than those from MCF7 cells, consistent with the previous report that HER2 signaling positively regulate breast cancer stem/progenitor cells [50]. We also found that knock-down of ER-α36 expression decreased the populations of the stem/progenitor cells from these HER2-expressing cells, suggesting an important role of ER-α36 in maintenance of breast cancer stem/progenitor cells in these HER2-expressing cells. We further found that in the cells derived from the tumorspheres from these HER2-expressing cells express elevated levels of ER-α36, EGFR and HER2, suggesting there exist the ER-α36-EGFR/HER2 regulatory loops in the ER-positive breast cancer stem/progenitor cells. Again, disruption of these regulatory loops with Lapatinib or Broussoflavonol B was able to sensitize these breast cancer stem/progenitor cells to tamoxifen. Our results thus provided rationales to develop novel therapeutic approaches to treat Her2-expressing breast cancer via eliminating breast cancer stem/progenitor cells by targeting the ER-α36-EGFR/HER2 loops.
In summary, here we provided evidence to demonstrate that there exist ER-α36-EGFR/HER2 positive regulatory loops in HER2-expressing breast cancer cells and that disruption of these regulatory loops restored tamoxifen sensitivity in these cells. Our findings that elevated expression of the ER-α36-EGFR/HER2 regulatory loops is one of the mechanisms by which HER2-expressing and ER-positive breast cancer cells escape the hormonal therapy that was based on estrogen deprivation provided a rational to develop novel therapeutic approaches for antiestrogen resistant patients by targeting these regulatory loops.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31240031) and Natural Science Foundation of Shandong Province of China (ZR2012HQ016) to M Wang.International Science & Technology Cooperation Program of China (2013DFG32700) to Y Gong. This work was also supported by Department of Defense grant DAMD 11-1-0497 and Nebraska Tobacco Settlement Biomedical Research Program Awards (LB-595) to Z.Y. Wang. Technical assistance for flow cytometry was provided by Dr. Perry Greg.
Disclosure of conflict of interest
The authors declare that they have no conflicts of interest.
Abbreviations
- EGFR
Epidermal growth factor receptor
- HER2
Human epidermal growth factor receptor 2
Supporting Information
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, Huntsman D, Olivotto IA, Nielsen TO, Gelmon K. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J. Clin. Oncol. 2008;26:5697–704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 3.Wright C, Angus B, Nicholson S, Sainsbury JR, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CH. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087–90. [PubMed] [Google Scholar]
- 4.Pegram MD, Pauletti G, Slamon DJ. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/a:1006111117877. [DOI] [PubMed] [Google Scholar]
- 5.Hoeferlin LA, C EC, Park MA. Challenges in the Treatment of Triple Negative and HER2-Overexpressing Breast Cancer. J Surg Sci. 2013;1:3–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, Manola J, Younger J, Matulonis U, Bunnell CA, Partridge AH, Richardson PG, Clarke K, Shulman LN, Winer EP. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2001;19:2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 7.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J. Clin. Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 8.Murphy CG, Morris PG. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anticancer Drugs. 2012;23:765–76. doi: 10.1097/CAD.0b013e328352d292. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.DiGiovanna MP. Clinical significance of HER-2/neu overexpression: Part II. Cedar Knolls, NJ: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 11.DiGiovanna MP. Clinical significance of HER-2/neu overexpression: Part I. 9 ed. Cedar Knolls, NJ: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 12.Kurokawa H, Arteaga CL. Inhibition of erbB receptor (HER) tyrosine kinases as a strategy to abrogate antiestrogen resistance in human breast cancer. Clin Cancer Res. 2001;7:4436s–42s. discussion 11s-12s. [PubMed] [Google Scholar]
- 13.Argiris A, Wang CX, Whalen SG, DiGiovanna MP. Synergistic interactions between tamoxifen and trastuzumab (Herceptin) Clin Cancer Res. 2004;10:1409–20. doi: 10.1158/1078-0432.ccr-1060-02. [DOI] [PubMed] [Google Scholar]
- 14.Kunisue H, Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, Doihara H, Shimizu N, Sonoo H. Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer. 2000;82:46–51. doi: 10.1054/bjoc.1999.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CX, Koay DC, Edwards A, Lu Z, Mor G, Ocal IT, Digiovanna MP. In vitro and in vivo effects of combination of Trastuzumab (Herceptin) and Tamoxifen in breast cancer. Breast Cancer Res Treat. 2005;92:251–63. doi: 10.1007/s10549-005-3375-z. [DOI] [PubMed] [Google Scholar]
- 16.Witters LM, Kumar R, Chinchilli VM, Lipton A. Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat. 1997;42:1–5. doi: 10.1023/a:1005798224288. [DOI] [PubMed] [Google Scholar]
- 17.Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 18.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–8. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583:1368–74. doi: 10.1016/j.febslet.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang L, Guo Y, Zhang X, Meng J, Wang ZY. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127:262–8. doi: 10.1016/j.jsbmb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-alpha36/ EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–80. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Wang Z, Xie Y. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J. Clin. Oncol. 2009;27:3423–9. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J, Wang ZY, Sun QY. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5:e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Ding L, Kang L, Wang ZY. Estrogen receptor-alpha 36 mediates mitogenic antiestrogen signaling in ER-negative breast cancer cells. PLoS One. 2012;7:e30174. doi: 10.1371/journal.pone.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Wang ZY. Estrogen receptor-alpha variant, ER-alpha36, is involved in tamoxifen resistance and estrogen hypersensitivity. Endocrinology. 2013;154:1990–8. doi: 10.1210/en.2013-1116. [DOI] [PubMed] [Google Scholar]
- 27.Lasfargues EY, Coutinho WG, Redfield ES. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst. 1978;61:967–78. [PubMed] [Google Scholar]
- 28.Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. J Cell Mol Med. 2010;14:1485–93. doi: 10.1111/j.1582-4934.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo M, Wang M, Deng H, Zhang X, Wang ZY. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-alpha36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur J Pharmacol. 2013;714:56–64. doi: 10.1016/j.ejphar.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Guo M, Wang M, Zhang X, Deng H, Wang ZY. Broussoflavonol B restricts growth of ER-negative breast cancer stem-like cells. Anticancer Res. 2013;33:1873–9. [PubMed] [Google Scholar]
- 31.Deng H, Yin L, Zhang XT, Liu LJ, Wang ML, Wang ZY. ER-alpha variant ER-alpha36 mediates antiestrogen resistance in ER-positive breast cancer stem/progenitor cells. J Steroid Biochem Mol Biol. 2014;144PtB:417–26. doi: 10.1016/j.jsbmb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS One. 2014;9:e88034. doi: 10.1371/journal.pone.0088034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piva M, Domenici G, Iriondo O, Rábano M, Simões BM, Comaills V, Barredo I, López-Ruiz JA, Zabalza I, Kypta R, Vivanco MD. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6:66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F NCI-Naple Breast Cancer Group. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer. 2005;12:721–47. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 35.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, Budman DR, Henderson IC, Barcos M, Hayes D, Norton L. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J. Clin. Oncol. 2000;18:3471–9. doi: 10.1200/JCO.2000.18.20.3471. [DOI] [PubMed] [Google Scholar]
- 37.Borg A, Baldetorp B, Ferno M, Killander D, Olsson H, Ryden S, Sigurdsson H. ERBB2 amplification is associated with tamoxifen resistance in steroid-receptor positive breast cancer. Cancer Lett. 1994;81:137–44. doi: 10.1016/0304-3835(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 38.Carlomagno C, Perrone F, Gallo C, De Laurentiis M, Lauria R, Morabito A, Pettinato G, Panico L, D’Antonio A, Bianco AR, De Placido S. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J. Clin. Oncol. 1996;14:2702–8. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 39.Elledge RM, Green S, Ciocca D, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, O’Sullivan J, Martino S, Osborne CK. HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a Southwest Oncology Group Study. Clin Cancer Res. 1998;4:7–12. [PubMed] [Google Scholar]
- 40.Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J. Clin. Oncol. 1995;13:1129–35. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 41.McCann AH, Dervan PA, O’Regan M, Codd MB, Gullick WJ, Tobin BM, Carney DN. Prognostic significance of c-erbB-2 and estrogen receptor status in human breast cancer. Cancer Res. 1991;51:3296–303. [PubMed] [Google Scholar]
- 42.Yamauchi H, O’Neill A, Gelman R, Carney W, Tenney DY, Hosch S, Hayes DF. Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J. Clin. Oncol. 1997;15:2518–25. doi: 10.1200/JCO.1997.15.7.2518. [DOI] [PubMed] [Google Scholar]
- 43.Ravdin PM GS, Albain KS, Boucher V, Ingle J, Pritchard K, Shepard L, Davidson N, Hayes DF, Clark GM, Martino S, Osborne CK, Allred DC. Initial report of the SWOG biological correlative study of c-erbB-2 expression as a predictor of outcome in a trial comparing adjuvant CAF T with tamoxifen (T) alone [abstract] . Proc ASCO. 1998:17. [Google Scholar]
- 44.Bianco AR DLM, Carlomango C, Lauria R, Petrella G, Panico L, Pettinato G, Perrone F, Gallo C, Marinelli A, De Placido S. 20 year update of the Naples GUN trial of adjuvant breast cancer therapy: evidence of interaction between c-erb-B2 expression and tamoxifen efficacy (ABSTRACT) Program/Proc Am Soc Clin Oncol. 1998:17. [Google Scholar]
- 45.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16:1486–97. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 46.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira LR, Jeffrey SS, Ribeiro-Silva A. Stem cells in human breast cancer. Histol Histopathol. 2010;25:371–85. doi: 10.14670/HH-25.371. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien CS, Howell SJ, Farnie G, Clarke RB. Resistance to endocrine therapy: are breast cancer stem cells the culprits? J Mammary Gland Biol Neoplasia. 2009;14:45–54. doi: 10.1007/s10911-009-9115-y. [DOI] [PubMed] [Google Scholar]
- 50.Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7. doi: 10.1016/j.canlet.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



