Abstract
A seminal plasma protein, semenogelin I (SgI), contributes to sperm clotting, upon binding to Zn2+, and can be proteolyzed by prostate-specific antigen (PSA), resulting in release of the trapped spermatozoa after ejaculation. In contrast, the role of SgI in the development and progression of any types of malignancies remains largely unknown. We previously demonstrated that SgI was overexpressed in prostate cancer tissues and its expression was enhanced by zinc treatment in LNCaP cells. In the current study, using cell lines stably expressing SgI, we investigated its biological functions, in conjunction with zinc, androgen, and androgen receptor (AR), in prostate cancer. Zinc, without SgI, inhibited cell growth of both AR-positive and AR-negative lines. Co-expression of SgI prevented zinc inhibiting dihydrotestosterone-mediated proliferation of AR-positive cells, whereas SgI and/or dihydrotestosterone showed marginal effects in AR-negative cells. Similar effects of SgI overexpression in LNCaP on dihydrotestosterone-induced cell invasion, such as its significant enhancement with zinc, were seen. Overexpression of SgI in LNCaP and CWR22Rv1 cells also augmented dihydrotestosterone-mediated PSA expression (mRNA, protein) in the presence of zinc. However, culture in the conditioned medium containing secreted forms of SgI failed to significantly increase cell viability with or without zinc. In luciferase reporter gene assays, SgI showed even slight inhibitory effects (8% and 15% decreases in PC3 and CWR22Rv1, respectively) at 0 μM zinc and significant stimulatory effects (2.1- and 3.2-fold) at 100 μM zinc on dihydrotestosterone-enhanced AR transactivation. Co-immunoprecipitation then demonstrated dihydrotestosterone-induced physical interactions between AR and SgI. These results suggest that intracellular SgI, together with zinc, functions as an AR coactivator and thereby promotes androgen-mediated prostate cancer progression.
Keywords: Androgen receptor, prostate cancer, prostate-specific antigen, semenogelin, zinc
Introduction
The signaling pathway of androgen receptor (AR), a member of the nuclear receptor superfamily, plays a critical role in the growth of not only androgen-sensitive prostate cancer cells but also most cells from clinically defined androgen-independent prostate cancer. In particular, co-regulatory proteins that mediate receptor transcriptional activation or repression have been suggested to modulate the events of tumor progression. Various nuclear receptor coregulators as well as selective coactivators that enhance AR-mediated transcriptional activity have indeed been isolated [1-4].
The prostate accumulates the highest level of zinc (3,000-4,500 μM in normal peripheral zone) in the body and secretes high amounts of zinc in the prostatic fluid (8,000-10,000 μM) [5]. A significant decrease in zinc levels is seen in prostate cancer tissue, yet the concentrations (400-800 μM) remain relatively high, compared with those in other soft tissue (200-400 μM) or blood plasma (15 μM) [5,6]. Of note, however, zinc (e.g. 100 μM in PC3 culture) has been shown to considerably inhibit the proliferation of prostate cancer cells [7-10]. To our knowledge, there is no definitive molecular evidence explaining the enigma of high concentrations of cytotoxic zinc in prostate cancer tissue. Furthermore, there are controversial epidemiological data on the relationship between zinc intake and the risk of prostate cancer [5,11].
Semenogelins, mainly expressed and secreted by the seminal vesicle, are the major structural proteins in human semen containing a high concentration of Zn2+, and their physiological functions have been well characterized. Specifically, semenogelins, upon binding to Zn2+, play an important role in gel-like formation of the semen [12]. After ejaculation, these proteins are degraded into smaller fragments by prostate-specific antigen (PSA), resulting in clotted gel liquefaction and release of the encased spermatozoa [13]. Semenogelins have also been shown to inhibit the protease activity of PSA [14]. Semenogelins are expressed in other male genital organs, such as the vas deferens, epididymis, and prostate, as well as in non-genital organs, suggesting their physiological role as modulators of zinc-dependent proteases throughout the body [15,16]. Semenogelin I (SgI) expression has been detected in an androgen-sensitive prostate cancer line LNCaP, which is enhanced by zinc treatment, but not in other prostate cancer lines such as CWR22Rv1, DU145, and PC3 [15,17]. We additionally demonstrated significantly higher levels of nuclear SgI expression in prostatic carcinoma than in non-neoplastic prostatic epithelium or high-grade prostatic intraepithelial neoplasia (PIN), which could also predict biochemical recurrence after radical prostatectomy [17,18]. However, no functional analyses of semenogelins in pathological conditions have been reported and their roles in prostate cancer growth remain uncertain. In the current study, we aim to determine the biological significance of SgI, in conjunction with zinc, androgen, and AR, in prostate cancer cells.
Materials and methods
Plasmids
The entire coding region of SgI amplified using Phusion-High Fidelity DNA polymerase (Thermo Fisher Scientific) was subcloned into pSG5 [17] and lentivirus pWPI vector [19]. pSG5-AR, pGL3-MMTV-luciferase, and pRL-TK have been used in our previous studies [20,21].
Antibodies and chemicals
Anti-AR (N-20), anti-SgI (E-15), and anti-β-actin (R-22) antibodies were purchased from Santa Cruz Biotechnology. An anti-PSA antibody (A0562) was purchased from Dako. Dihydrotestosterone (DHT) and ZnCl2 were from Sigma-Aldrich and Alfa Aesar, respectively.
Cell lines
CWR22Rv1, LNCaP, PC3, and DU145 cell lines originally obtained from the American Type Culture Collection and recently authenticated by the institutional core facility were maintained with RPMI 1640 (Mediatech) supplemented with 10% fetal bovine serum (FBS). To generate cell lines stably expressing SgI, pWPI-SgI, along with GFP expressing vector, was co-transfected, using GeneJuice transfection reagent (Novagen), and GFP expressing cells were selected.
MTT assay
Cell viability was assessed, using methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. Cells (1-3 × 103/well) seeded in 96-well tissue culture plates were incubated in the presence or absence of zinc and DHT. The media were refreshed every 48 hours. After 96 hours of treatment, 10 μL MTT stock solution (5 mg/mL; Sigma) was added to each well with 100 μL of medium for 4 hours at 37°C. The medium was replaced with 100 μL dimethyl sulfoxide, followed by incubation for 5 minutes at room temperature. The absorbance at a wavelength of 570 nm with background subtraction at 655 nm was then measured.
Transwell invasion assay
Cell invasiveness was determined, using Matrigel-coated transwell chambers (Costar), as described previously [21]. Briefly, cells (5 × 104) in 100 l of serum-free medium were added to the upper chamber of the transwell, while 600 l of medium containing 5% FBS was added to the lower chamber. The media in both chambers contained ethanol, zinc, and/or DHT. After incubation for 36 hours at 37°C in a CO2 incubator, invaded cells were fixed, stained with 0.1% crystal violet, and counted.
Reverse transcription (RT)-polymerase chain reaction (PCR)
Total RNA (0.5 μg) was isolated from the cultured cells, using TRIzol (Invitrogen), and reverse transcribed with oligo (dT) primers and Omniscript reverse transcriptase (Qiagen), as described previously [19,21,22]. Real-time PCR was then performed, using RT2 SYBR® Green FAST Mastermix (Qiagen) for iCycler (Invitrogen). The following primer pairs were used for RT-PCR: PSA (forward, 5’-GCAGTCTGCGGCGGTGTTCT-3’; reverse, 5’-GCGGGTGTGGGAAGGTGTGG-3’), and GAPDH (forward, 5’-CTCCTCCACCTTTGACGCTG-3’; reverse, 5’-CATACCAGGAAATGAGCTTGACAA-3’).
Western blot
Protein extraction and western blotting were performed, as described previously [19-22] with minor modifications. Briefly, equal amounts of protein obtained from cell extracts were subjected to SDS-PAGE, transferred to polyvinylidene difluoride membranes electronically, blocked, and incubated with a specific primary antibody. The membrane was then incubated with a HRP-conjugated secondary antibody, and specific signals were detected, using chemiluminescent substrate kit (Thermo Fisher Scientific).
Luciferase assay
Cells were transfected with an androgen response element-reporter (MMTV-Luc), pSG5 or pSG5-SgI, and a control reporter (pRL-TK), using GeneJuice. pSG5-AR was also transfected into PC3 cells. Then, the cells were treated with zinc and/or DHT for 24 hours, and the luciferase activity was determined in the cell lysates, using a Dual-Luciferase Reporter Assay kit (Promega) and luminometer (FLUOstar Omega, BMG Labtech).
Co-immunoprecipitation
The cell lysates (500 g) were incubated with 2 g anti-AR antibody or normal rabbit IgG for 16 hours at 4°C with agitation. Protein A/G-agarose beads were then added, and binding proteins were eluted. The eluted proteins were analyzed by western blot with an anti-AR or anti-SgI antibody.
Statistical analysis
Student’s t-test was used to analyze differences in variables with a continuous distribution. P values less than 0.05 were considered statistically significant.
Results
Expression of SgI in prostate cancer cells and conditioned media
Using a lentivirus vector, we generated prostate cancer cell lines stably expressing SgI. Overexpression of SgI protein in these stable cell lines and relatively weak expression of endogenous SgI in LNCaP were confirmed (Figure 1A). To detect a secreted form of SgI, western blot was also performed in acetone-precipitated medium where each stable line was cultured under serum-free conditions for 24 hours. No signal was detected in conditioned medium after culturing SgI-weakly positive (i.e. no additional zinc; RPMI 1640 with 10% FBS contains approximately 3.8 μM zinc [23]) LNCaP-Vector (V) as well as three SgI-negative control lines (Figure 1B). In contrast, SgI was found to be secreted in the supernates where SgI-overexpressing cells were cultured. These results suggest that, in accordance with our immunohistochemistry data in radical prostatectomy specimens [18], prostate cancer cells do not normally secrete detectable amounts of SgI.
Figure 1.
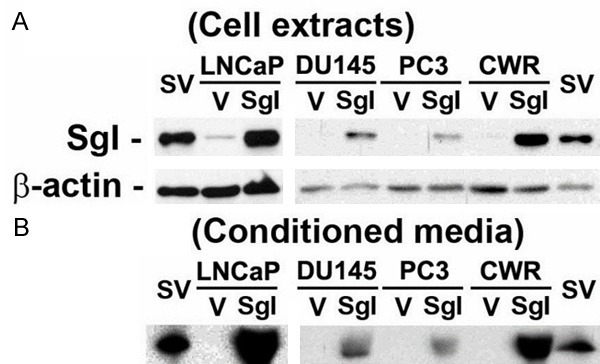
SgI protein expression and secretion in prostate cancer lines stably expressing SgI. Cell extracts (A) or acetone-precipitated proteins in conditioned media (serum-free, no additional zinc) (B) from LNCaP-V/SgI, DU145-V/SgI, PC3-V/SgI, and CWR22Rv1-V/SgI were analyzed on western blots, using an antibody to SgI (52 kDa) or β-actin (42 kDa). Fresh human seminal vesicle (SV) tissue was used as a positive control.
Induction of prostate cancer progression by SgI with zinc
To see if SgI affects prostate cancer cell proliferation, we performed MTT assay in the stable cells. Each line was cultured for 4 days in the presence or absence of DHT (1 nM) and zinc (100 μM). As expected, zinc treatment significantly inhibited the growth of all control lines (Figure 2; 21-45% decrease; lanes 1 vs. 2) except LNCaP-V. In AR-positive CWR22Rv1-derived cells (Figure 2A), DHT increased the growth by 12-13% without zinc treatment (lanes 1 vs. 3 and 5 vs. 7). In the presence of zinc, DHT showed a similar induction rate in CWR22Rv1-V (14% increase; lanes 2 vs. 4), whereas overexpression of SgI resulted in a statistically significant increase in the growth rate (27%; lanes 6 vs. 8; p = 0.034). Thus, zinc only marginally decreased cell growth of CWR22Rv1-SgI (lanes 5 vs. 6 and 7 vs. 8). In LNCaP cells with endogenous SgI (LNCaP-V; Figure 2B), zinc treatment did not decrease, rather marginally increased, the growth in the absence (lanes 1 vs. 2) or presence (lanes 3 vs. 4) of DHT. DHT increased the growth of LNCaP-V without (62%; lanes 1 vs. 3; p = 0.009) or with (52%; lanes 2 vs. 4; p = 0.014) zinc as well as that of LNCaP-SgI without (66%; lanes 5 vs. 7; p = 0.036) or with (82%; lanes 6 vs. 8; p = 0.018) zinc. Thus, co-expression of SgI in the presence of zinc appeared to induce androgen-mediated proliferation of AR-positive prostate cancer cells and, more importantly, protected the cells from cytotoxic effects of zinc. In AR-negative PC3-derived (Figure 2C) and DU145-derived (Figure 2D) cells, DHT treatment and SgI overexpression showed only marginal effects on their growth (< 10% changes). Because semenogelins are secreted proteins [12], we further tested whether secreted forms of SgI induced prostate cancer cell proliferation. MTT assay was again performed in CWR22Rv1 (Figure 2E) and DU145 (Figure 2F) cells incubated in the conditioned medium derived from CWR22Rv1-V/SgI culture. In these parental lines, the secreted form of SgI did not significantly affect cell viability in the absence (lanes 1 vs. 3) or presence (lanes 2 vs. 4) of zinc.
Figure 2.
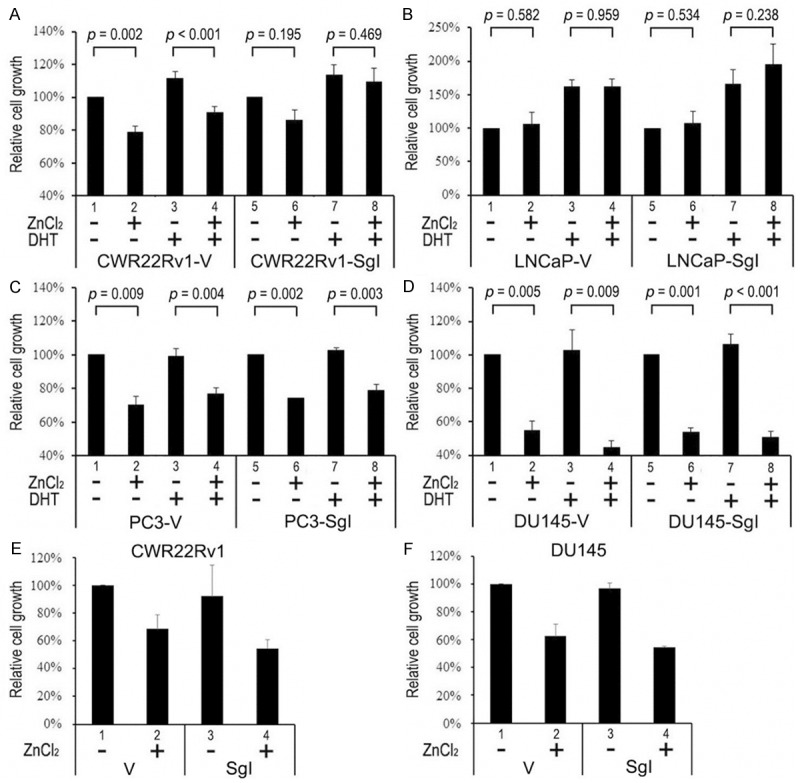
Cell viability of prostate cancer lines stably expressing SgI. CWR22Rv1-V/SgI (A), LNCaP-V/SgI (B), PC3-V/SgI (C), and DU145-V/SgI (D) were cultured in phenol red-free medium supplemented with 5% charcoal-stripped FBS in the presence or absence of 100 μM zinc and 1 nM DHT for 96 hours. CWR22Rv1 (E) and DU145 (F) were cultured in conditioned medium (containing 10% normal FBS) derived from CWR22Rv1-V/SgI culture in the presence or absence of 100 μM zinc for 96 hours. Proliferation was assayed with MTT, and growth rates are presented relative to cell number in respective lines with mock treatment [lanes 1 (A-F) and 5 (A-D); set as 100%]. Each value represents the mean + SD of at least three determinations.
To investigate whether SgI promotes tumor invasion, a transwell invasion assay was performed in the stable LNCaP lines (Figure 3A). DHT similarly induced cell invasion of LNCaP-V without (35% increase; lanes 1 vs. 2; p = 0.042) or with (48% increase; lanes 3 vs. 4; p = 0.009) zinc or LNCaP-SgI without zinc (48% increase; lanes 5 vs. 6; p = 0.026). In contrast, in LNCaP-SgI with zinc, the invasiveness was more significantly increased by DHT (2.8-fold over mock treatment; lanes 7 vs. 8; p = 0.006). Thus, significant induction of the DHT-mediated invasive properties by endogenous SgI (lanes 2 vs. 4; 19% increase) or exogenous SgI overexpression (lanes 6 vs. 8; 88% increase) with versus without addition of zinc was seen.
Figure 3.
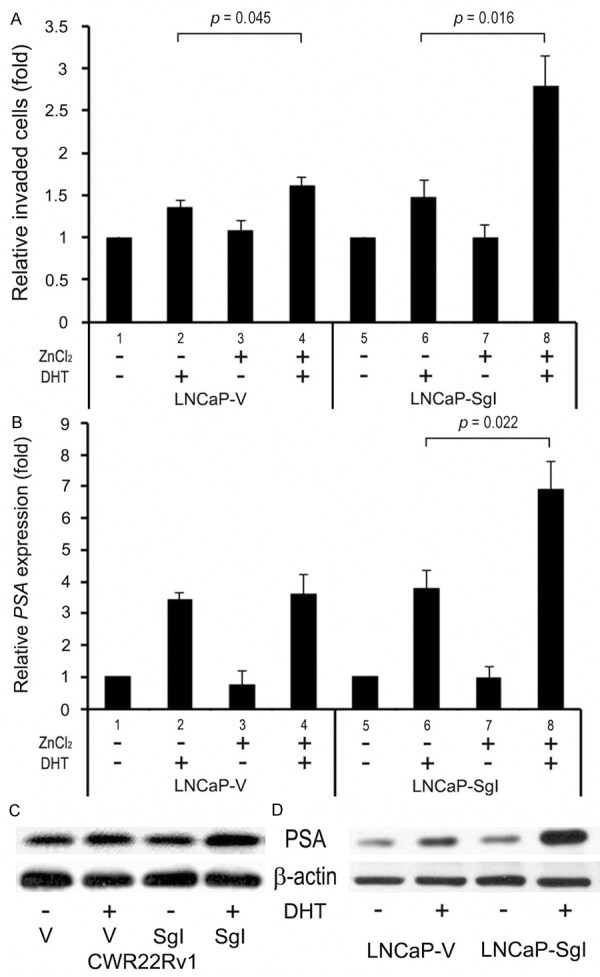
Progression of prostate cancer lines stably expressing SgI. (A) LNCaP-V/SgI cells cultured in the Matrigel-coated transwell chamber for 36 hours in the presence or absence of 300 μM zinc and 1 nM DHT were used for transwell assay. The number of invaded cells in five random fields was counted under a light microscope, using a 40x objective. Invasion ability is presented relative to that in each cell line with mock treatment (lane 1 or 5; set as 1-fold). Each value represents the mean + SD of at least three independent experiments. (B) LNCaP-V/SgI cells cultured in phenol red-free medium supplemented with 5% charcoal-stripped FBS in the presence or absence of 300 μM zinc and 1 nM DHT for 48 hours were subjected to a quantitative RT-PCR. Expression of PSA gene was normalized to that of GAPDH. Transcription amount is presented relative to that of mock treatment in each cell line (lane 1 or 5; set as 1-fold). Each value represents the mean + SD from at least three independent experiments. CWR22Rv1 cells (C) transiently transfected with pSG5 or pSG5-SgI were cultured in phenol red-free medium supplemented with 5% charcoal-stripped FBS in the presence or absence of 100 μM zinc and 1 nM DHT for 48 hours, and LNCaP-V/SgI cells (D) were similarly cultured with 300 μM zinc ± 1 nM DHT for 48 hours, as indicated. Cell extracts were then analyzed on western blots, using an antibody to PSA (33 kDa) or β-actin.
We next determined whether SgI regulated the expression of PSA, an androgen-inducible AR target and also known to proteolyze SgI in semen [12,13], in prostate cancer cells. A quantitative RT-PCR showed that DHT treatment, in the absence of additional zinc, increased endogenous PSA expression over mock treatment by 3.4-fold (lanes 1 vs. 2; p < 0.001)/3.8-fold (lanes 5 vs. 6; p = 0.009) in LNCaP-V/SgI (Figure 3B), respectively. In the presence of 300 μM zinc, DHT increased PSA expression by 4.7-fold (lanes 3 vs. 4; p = 0.004)/7.1-fold (lanes 7 vs. 8; p = 0.003) in LNCaP-V/SgI, respectively. The difference in DHT-mediated PSA expression in LNCaP-SgI with versus without zinc was also statistically significant (lanes 6 vs. 8; 1.8-fold). Similarly, western blots in CWR22Rv1 cells cultured with 100 μM zinc (Figure 3C) and LNCaP stable cells cultured with 300 μM zinc (Figure 3D) showed that overexpression of SgI resulted in considerable increases in DHT-mediated PSA expression. However, no significant additive effects of SgI on PSA protein expression were seen in these cell lines when cultured without additional zinc (figure not shown).
Enhancement of AR transcriptional activity by SgI
To assess the effect of SgI on androgen-mediated AR transactivation, luciferase activity was determined in PC3 cells transfected with AR, SgI, and an androgen response element-reporter plasmid, and treated with different concentrations of zinc and 1 nM DHT. DHT increased AR transcription by 17-fold (0 μM zinc; Figure 4A), 12-fold (15 μM zinc; Figure 4B), and 10-fold (100 μM zinc; Figure 4C), as compared with respective mock treatments. Thus, zinc reduced androgen-enhanced AR transactivation in a dose-dependent manner. SgI showed a slight inhibitory effect (15% decrease at 0 μM zinc; Figure 4A) or a slight stimulatory effect (31% increase at 15 μM zinc; Figure 4B) on DHT-induced AR transcription. In contrast, in the presence of 100 μM zinc, SgI further induced DHT-mediated AR transcription by 3.2-fold (Figure 4C). Induction of zinc/DHT-mediated AR transcription by SgI (2.1-fold) was confirmed in CWR22Rv1, while SgI did not significantly affect AR transactivation without additional zinc (8% decrease) (Figure 4D, 4E). These results suggest that SgI functions as an AR coactivator in the presence of zinc in prostate cancer cells.
Figure 4.
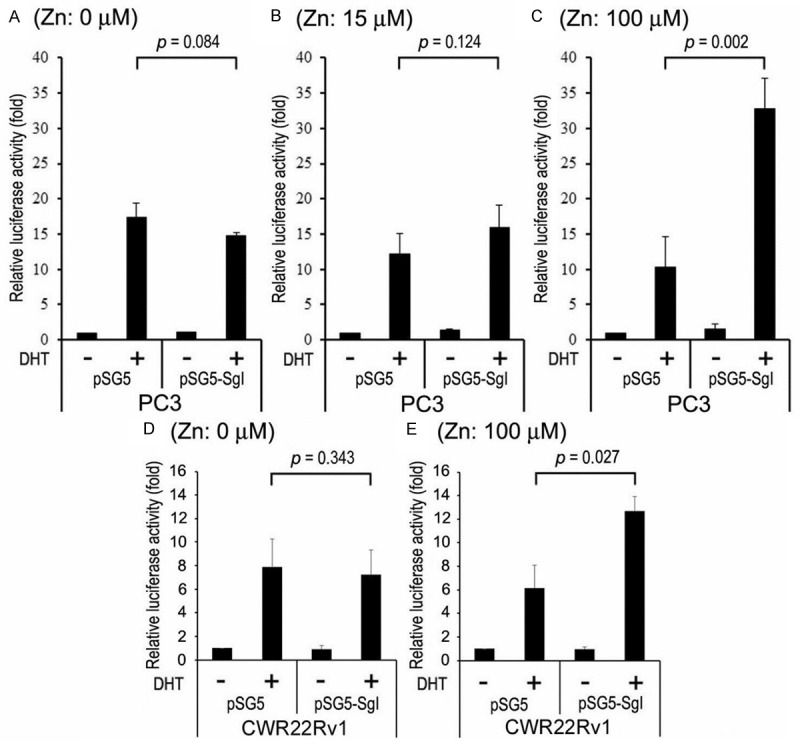
The effects of SgI on AR transcriptional activity in prostate cancer cells. PC3 cells were co-transfected with pSG5-AR, MMTV-Luc, pRL-TK, and either pSG5 or pSG5-SgI (AR:SgI = 1:5), and cultured in phenol red-free medium supplemented with 5% charcoal-stripped FBS along with mock (ethanol), zinc [(A) 0 μM; (B) 15 μM; (C) 100 μM], and/or 1 nM DHT for 24 hours. Similarly, CWR22Rv1 cells were co-transfected with MMTV-Luc, pRL-TK, and either pSG5 or pSG5-SgI, and treated with mock (ethanol) or 1 nM DHT in the absence (D) or presence (E) of 100 μM zinc for 24 hours. The luciferase activity is presented relative to that of mock treatment (first lanes; set as 1-fold). Each value represents the mean + SD of at least three determinations.
Interaction between AR and SgI
AR coregulators modulate AR-mediated transcriptional activity by interacting with AR [1-3]. To verify the interaction between AR and SgI, co-immunoprecipitation assay, using cell lysates with (293T) or without (LNCaP) transfection of AR and SgI, was performed. Using an anti-AR antibody, we precipitated the AR binding protein complex in the protein lysate. We then proved that AR-SgI form a complex, especially in the presence of DHT, in 293T (Figure 5A) and LNCaP (Figure 5B) cells.
Figure 5.
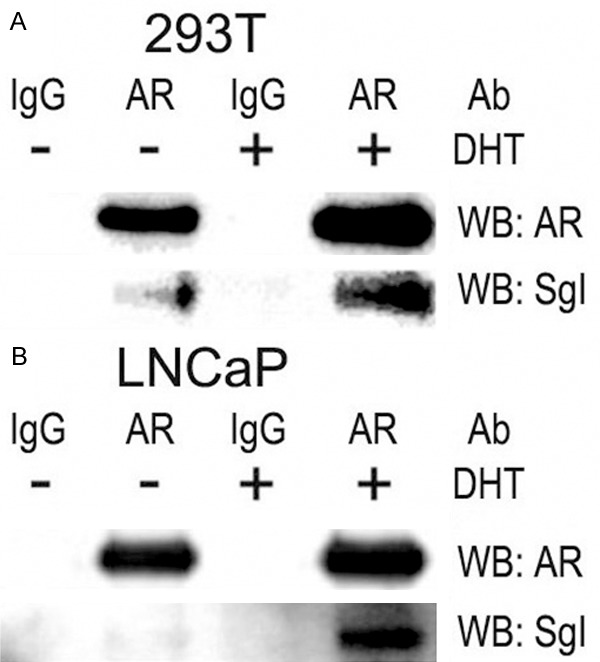
Co-precipitation of AR and SgI. Cell lysates from 293T transfected with pSG5-AR and pSG5-SgI (A) or LNCaP (B) treated with mock (ethanol) or 1 nM DHT were incubated with an anti-AR polyclonal antibody or normal rabbit IgG and then with A/G-agarose beads. The complex was resolved on a 10% SDS-polyacrylamide gel and blotted with an anti-AR or anti-SgI antibody.
Discussion
While functions of semenogelins have been thoroughly characterized in physiological environment especially in the male reproductive system, little is known about their roles in human malignancies. Our previous immunohistochemical studies showed that both SgI and semenogelin II (SgII) were overexpressed in prostate cancer tissue specimens and that patients with SgI-positive tumor, but not SgII-positive or SgII-negative tumor, had a significantly higher risk of recurrence following radical prostatectomy [17,18]. Furthermore, transient transfection of SgI, but not SgII, into AR-positive/semenogelin-negative CWR22Rv1 resulted in an increase in cell proliferation in the presence of a high level of zinc [17]. Based on these findings, we hypothesized that SgI, in conjunction with zinc, androgen, and AR, promoted prostate cancer progression. In the present study, we tested our hypothesis in prostate cancer cell lines.
Experimental evidence indicates an inhibitory role of zinc in the development and progression of prostate cancer. However, it remains controversial whether zinc supplements reduce the risk of prostate cancer [5,11]. In addition, the molecular basis for why prostate cancer tissue contains relatively high concentrations of cytotoxic zinc is poorly understood, although alterations of zinc transporters in prostate cancer cells have been suggested to prevent zinc accumulation [24,25]. Previous in vitro studies have shown that higher concentrations of zinc are required to inhibit cell proliferation of LNCaP (250-1000 μM), compared with PC3 (100 μM) [7,8]. We confirmed these findings and further demonstrated that 100 μM zinc could inhibit cell growth of other SgI-negative prostate cancer lines. Thus, endogenous SgI in LNCaP may protect the cells against inhibitory effects of zinc. Interestingly, co-expression of SgI only in AR-positive CWR22Rv1 cells resulted in prevention from zinc cytotoxicity. SgI also induced androgen-mediated prostate cancer cell invasion and PSA expression only in the presence of zinc. These results suggest that SgI may require not only zinc, as in the case of its physiological action [12-16], but also AR to function as a modulator of prostate cancer outgrowth. Moreover, the presence of SgI in prostate cancer cells can be a reason for zinc accumulation in tumors.
It is well documented that co-regulatory proteins modulate nuclear receptor-mediated transcriptional activity by interacting with the receptor [1-4]. We here showed that SgI interacted with AR and enhanced androgen-induced AR transactivation in prostate cancer cells, indicating that SgI is an AR coactivator. Again, a high level of zinc was most likely required for this newly recognized function of SgI. Although a variety of general or specific AR coactivators have been identified, physiological functions of these coactivators are largely unknown and their characterization has not yet led to the development of new therapeutic options in patients with prostate cancer [26,27]. It has been expected that suppression of coactivator actions or interruption of AR-coactivator interactions results in prostate cancer regression at any stages because castration-resistant tumors usually remain AR-dependent for their growth. Importantly, as aforementioned, physiological roles of semenogelins as seminal plasma proteins have been extensively studied. SgI was also shown to be highly expressed in prostate cancer cells [15,17,18]. In addition, because PSA is known to physiologically degrade semenogelins [13], elevated SgI may result in a further increase in PSA levels to attempt to target semenogelins. As a result, down-regulation of SgI expression, compared with other AR coactivators, may more effectively inhibit prostate cancer progression that can be facilitated by PSA itself via enhancing an AR coactivator ARA70-regulated AR transactivation [28]. The cytotoxic activity of zinc may also become distinct with SgI down-regulation. Further analyses of SgI in prostate cancer are necessary to credential a new therapeutic target.
The current results suggest that cellular SgI, but not its secreted forms, plays an important role in prostate cancer outgrowth. However, semenogelins are essentially secreted proteins, mainly derived from the seminal vesicle. Indeed, we detected SgI signals in secreted materials, in addition to cellular immunoreactivity, in prostatectomy specimens [18]. Although moderate to strong SgI signals were seen in the majority of benign (97%) or PIN (98%) glands where the secretions were present, intraluminal secretions in carcinoma glands were uncommonly (13%) immunoreactive and their signals, if present, were mostly weak. These findings suggested that, in contrast to benign or PIN cells, carcinoma cells did not generally secrete a large amount of SgI. We confirmed this by demonstrating the failure to detect SgI signals in the conditioned medium after culturing control LNCaP with endogenous SgI and other SgI-negative prostate cancer cell lines in our western blotting. Instead, increased levels of serum semenogelins were detected in 4 of 13 patients with lung cancer, although their functions in lung carcinogenesis and tumor progression were not studied [29]. Again, in our assays, a secreted form of SgI present in the conditioned medium where CWR22Rv1-SgI was cultured failed to induce the proliferation of parental CWR22Rv1 cells even in the presence of zinc. It is still possible that SgI secreted by benign prostate or PIN cells as well as tissues other than the prostate exerts an influence on prostate cancer growth with or without involving zinc and AR.
In conclusion, our current data indicating that intracellular SgI in the presence of zinc functions as an AR coactivator and promotes the growth of prostate cancer cells provide its novel role in tumor progression. Particularly, SgI protects the cells against zinc cytotoxicity, which may explain why prostate cancer tissue contains high levels of zinc.
Acknowledgements
This study was supported by Department of Defense Prostate Cancer Research Program (W81XWH-13-1-0412 to H. M.).
Disclosure of conflict of interest
None.
References
- 1.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–271. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Rahman M, Miyamoto H, Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10:2208–2219. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- 3.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 4.Lonard DM, O’Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaichick VY, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 7.Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untergasser G, Rumpold H, Plas E, Witkowski M, Pfister G, Berger P. High levels of zinc ions induce loss of mitochondrial potential and degradation of antiapoptotic Bcl-2 protein in in vitro cultivated human prostate epithelial cells. Biochem Biophys Res Commun. 2000;279:607–614. doi: 10.1006/bbrc.2000.3975. [DOI] [PubMed] [Google Scholar]
- 9.Golovine K, Uzzo RG, Makhov P, Crispen PL, Kunkle D, Kolenko VM. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-κB-dependent pathway. Prostate. 2008;68:1443–1449. doi: 10.1002/pros.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banudevi S, Senthilkumar K, Sharmila G, Arunkumar R, Viiavababu MR, Arunakaran J. Effect of zinc on regulation of insulin-like growth factor signaling in human androgen-independent prostate cancer cells. Clin Chim Acta. 2010;411:172–178. doi: 10.1016/j.cca.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson M, Lundwall Å, Malm J. The semenogelins: proteins with functions beyond reproduction? Cell Mol Life Sci. 2006;63:2886–2888. doi: 10.1007/s00018-006-6287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilja H, Oldbring J, Rannevik G, Laurell CB. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987;80:281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson M, Linse S, Frohm B, Lundwall Å, Malm J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem J. 2005;387:447–453. doi: 10.1042/BJ20041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundwall Å, Bjartell A, Olsson AY, Malm J. Semenogelin I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol Hum Reprod. 2002;8:805–810. doi: 10.1093/molehr/8.9.805. [DOI] [PubMed] [Google Scholar]
- 16.Bonilha VL, Rayborn ME, Shadrach K, Lundwall Å, Malm J, Bhattacharya SK, Crabb JW, Hollyfield JG. Characterization of semenogelin proteins in the human retina. Exp Eye Res. 2006;83:120–127. doi: 10.1016/j.exer.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Canacci AM, Izumi K, Zheng Y, Gordetsky J, Yao JL, Miyamoto H. Expression of semenogelins I and II and its prognostic significance in human prostate cancer. Prostate. 2011;71:1108–1114. doi: 10.1002/pros.21323. [DOI] [PubMed] [Google Scholar]
- 18.Izumi K, Li Y, Zheng Y, Gordetsky J, Yao JL, Miyamoto H. Seminal plasma proteins in prostatic carcinoma: increased nuclear semenogelin I expression is a predictor of biochemical recurrence after radical prostatectomy. Hum Pathol. 2012;43:1991–2000. doi: 10.1016/j.humpath.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zheng Y, Izumi K, Ishiguro H, Ye B, Li F, Miyamoto H. Androgen activates β-catenin signaling in bladder cancer cells. Endocr Relat Cancer. 2013;20:293–304. doi: 10.1530/ERC-12-0328. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto H, Marwah P, Marwah A, Yang Z, Chung CY, Altuwaijri S, Chang C, Lardy H. Identification of steroid derivatives that function as potent antiandrogens. Int J Cancer. 2005;117:866–872. doi: 10.1002/ijc.21217. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Izumi K, Li Y, Ishiguro H, Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Mol Cancer Ther. 2012;11:2621–2632. doi: 10.1158/1535-7163.MCT-12-0621. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Ishiguro H, Kawahara T, Miyamoto Y, Izumi K, Miyamoto H. GATA3 in the urinary bladder: suppression of neoplastic transformation and down-regulation by androgens. Am J Cancer Res. 2014;4:461–473. [PMC free article] [PubMed] [Google Scholar]
- 23.Bao B, Prasad AS, Beck FWJ, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285:E1095–E1102. doi: 10.1152/ajpendo.00545.2002. [DOI] [PubMed] [Google Scholar]
- 24.Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG, Quinn DI, Turner JJ, Delprado W, Lee CS, Golovsky D, Brenner PC, O’Neill GF, Kooner R, Stricker PD, Grygiel JJ, Mack DH, Sutherland RL. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene. 2003;22:6005–6012. doi: 10.1038/sj.onc.1206797. [DOI] [PubMed] [Google Scholar]
- 25.Makhov P, Golovine K, Uzzo RG, Wuestefeld T, Scoll BJ, Kolenko VM. Transcriptional regulation of the major zinc uptake protein hZip1 in prostate cancer cells. Gene. 2009;431:39–46. doi: 10.1016/j.gene.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiota M, Yokomizo A, Fujimoto N, Naito S. Androgen receptor cofactors in prostate cancer: Potential therapeutic targets of castration-resistant prostate cancer. Curr Cancer Drug Targets. 2011;11:870–881. doi: 10.2174/156800911796798904. [DOI] [PubMed] [Google Scholar]
- 27.Kawahara T, Miyamoto H. Androgen receptor antagonists in the treatment of prostate cancer. Clin Immunol Endocr Metab Drugs. 2014;1:11–19. [Google Scholar]
- 28.Niu Y, Yeh S, Miyamoto H, Li G, Altuwaijri S, Yuan J, Han R, Ma T, Kuo HC, Chang C. Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ARA70-regulated androgen receptor transactivation. Cancer Res. 2008;68:7110–7119. doi: 10.1158/0008-5472.CAN-07-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berti A, Virgili A, D’Errico G, Vespi G, Lago G, Cavazzana A. Expression of seminal vesicle-specific antigen in serum of lung tumor patients. J Forensic Sci. 2005;50:1114–1115. [PubMed] [Google Scholar]


