Abstract
B-cell acute lymphoblastic leukemia (B-ALL) remains a challenging disease to treat in adults because of the high rates of relapse and refractory. Bortezomib, as a proteasome inhibitor, exerts obvious cytotoxicity against ALL cells and increases the sensitivity of ALL cells to conventional chemotherapeutic agents. We observed that bortezomib inhibited proliferation, induced apoptosis, arrested the cell cycle and induced autophagy in the Nalm-6 cell line and CD34+ primary cells. Additionally, we demonstrated that bortezomib promoted the disruption of the Bcl-2/Beclin-1 complex and increased the formation of the Beclin-1/PI3KC3 complex, leading to the initiation of autophagy. Autophagy inhibitors were employed in this study, and we found that autophagy inhibitors enhanced the anti-ALL activity of bortezomib. Taken together, these results revealed that autophagy protected B-ALL cells against the cytotoxicity of bortezomib and, in combination with autophagy inhibitors, can enhance the anticancer effects of bortezomib.
Keywords: Bortezomib, autophagy, autophagy inhibitors, anticancer effects, B-cell acute lymphoblastic leukemia
Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is a heterogeneous group of diseases characterized by malignant proliferation of precursor B lymphocytes in the bone marrow. Over the years, different protocols have been exploited to ameliorate the outcome, but the rates of relapse and refractory are high, overall survival is low, and B-ALL remains a challenging disease to treat in adults [1].
Bortezomib, a dipeptidyl boronic acid analog, is a proteasome inhibitor authorized for use in the treatment of myeloma and mantle cell lymphoma [2,3] that reversibly inhibits the 26S proteasome [4]. As reviewed elsewhere, the anticancer activity of proteasome inhibition involves several different mechanisms, including blocking cell cycle progression, inducing apoptosis, inhibiting cell growth, and anti-angiogenesis [5]. Previous studies have reported that bortezomib exerts marked cytotoxicity against ALL cells and increases the sensitivity of ALL cells to conventional chemotherapeutic agents, which is associated with the molecular mechanisms involved in Notch1, NF-κB, and PI3K/AKT signaling [6,7]. Although the mechanism of bortezomib’s anticancer activity is still not completely understood, it is a new treatment choice for patients with refractory or relapsed ALL, especially when treated in combination with conventional chemotherapy or targeted agents.
Autophagy is characterized by the formation of autophagosomes, which are double-membrane vesicles that swallow cytoplasmic material. Sequentially, autophagosomes fuse with lysosomes and lysosomal enzymes degrade their contents [8,9]. Recent studies suggest that autophagy may represent a novel therapeutic target for treating cancer. Bortezomib-induced autophagy has been reported in several types of cancer cells [10-12]; however, whether the exact molecular mechanism by which bortezomib acts against ALL, especially in B-ALL cells, is associated with autophagy has not been clearly defined.
In this study, we observed that bortezomib induced autophagy in the B-ALL cell line NALM-6 and primary cells from two patients, and we explored the effect of bortezomib on apoptosis and the cell cycle in the aforementioned cells. Furthermore, we also examined whether inhibition of autophagy would potentiate cell apoptosis when bortezomib was used.
Materials and methods
Cells and cell culture
The human precursor B cell lymphoblastic leukemia (B-ALL) cell line Nalm-6 from Leibniz institute DSMZ was cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) at 37°C in a humidified incubator with 5% CO2 and 95% air. Bone marrow mononuclear cells (BMMCs) from 2 B-ALL patients were isolated by Ficoll density gradient centrifugation. CD34 positive cells from BMMCs were isolated and purified with a CD34 selection kit (Miltenyi Biotec GmbH, Germany). B-ALL was diagnosed according to the MICM classification. The study was authorized by the institution’s review boards and ethics committees, and all patients gave written informed consent according to the Declaration of Helsinki. CD34+ cells were cultured in X-VIVO 15 (BioWhittaker, MD) containing a cytokine cocktail.
Drugs and antibodies
The antibodies to LC3 and P62 were obtained from Novus (Littleton, CO). The antibodies to Beclin-1, Bcl-2, Caspase-3, cleaved Caspase-3 and Bax were obtained from Cell Signaling Technology (Danvers, MA). The antibody to PI3KC3 was obtained from GeneTex (Irvine, CA). The antibody to cytochrome C was obtained from Santa Cruz Technology (Dallas, Texas). β-actin, MTT, monodansylcadaverine, E64D, leupeptin and 3-MA were purchased from Sigma-Aldrich (St. Louis, MO). SP600125, an inhibitor of JNK and Bortezomib were purchased from Selleck (Houston, TX). Horseradish peroxidase-conjugate secondary antibodies and FITC-labeled goat anti-rabbit IgG were purchased from KPL (Gaithersburg, MD). DAPI was obtained from Millipore (Billerica, MA). An apoptosis detection kit (KGA108) and cell cycle detection kit (KGA512) were purchased from KeyGEN (Nanjing, China). A mitochondria isolation kit for cultured cells (89874) was purchased from Thermo Scientific (Waltham, MA).
Cell viability and apoptosis assays
Cell viability was analyzed with MTT assays. Annexin V/propidium iodide (PI) staining assays were conducted according to the manufacturer’s instructions. Annexin-V positive cells were measured using a FACSCaliburTM flow cytometer (Becton Dickinson, San Jose, CA) and data were assessed using CellQuestTM software (BD Biosciences).
Cell cycle analysis
The cell cycle was assessed by propidium iodide (PI) staining and measured with a FACSCaliburTM flow cytometer. The cell distribution of each phase of the cell cycle was evaluated with ModFit LT software (BD Biosciences).
Western blot analysis
The protein concentrations of cell lysates were measured using the BCA Protein assay (Pierce, Rockford, IL). Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane and sequentially incubated overnight with primary antibodies at 4°C. After incubation with secondary antibodies, the signals were visualized by chemiluminescence using the SuperSignal reagent (Pierce, Rockford, IL).
Immunofluorescence
Nalm-6 and primary CD34+ cells were fixed and permeated and subsequently incubated overnight with an anti-LC3 antibody at 4°C, which was followed by staining with FITC-conjugated IgG and DAPI. After three 5-minute washes with PBS containing 0.2% BSA, cells were spread on glass slides by centrifugation at 1000 rpm for 5 min using a cytospin system (StatSpin, Westwood, MA). Fluorescence signals were analyzed using an Olympus BX50 microscope. The average percentage of LC3 puncta positive cells was assessed from at least 50 cells for each experiment.
Co-immunoprecipitation
Cells were lysed in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA). The protein concentrations in the supernatant were determined with the BCA assay. Before immunoprecipitation, samples were precleared by adding 20 µl of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Dallas, Texas) and 1 µg of an appropriate control IgG; they were then incubated with an anti-Beclin 1 antibody or control IgG in the presence of protein A/G PLUS-Agarose overnight at 4°C with gentle rotation. The agarose beads were collected and washed five times with PBS, and the proteins were eluted by boiling in 1 × SDS sample buffer before SDS-PAGE.
Statistical analysis
Results are expressed as the mean ± SD of three independent experiments. Two-group comparisons were performed using Student’s t-test. P values < 0.05 were defined as statistically significant. All data analyses were performed with GraphPad Prism 5.
Results
Bortezomib induces autophagy in B-ALL cells
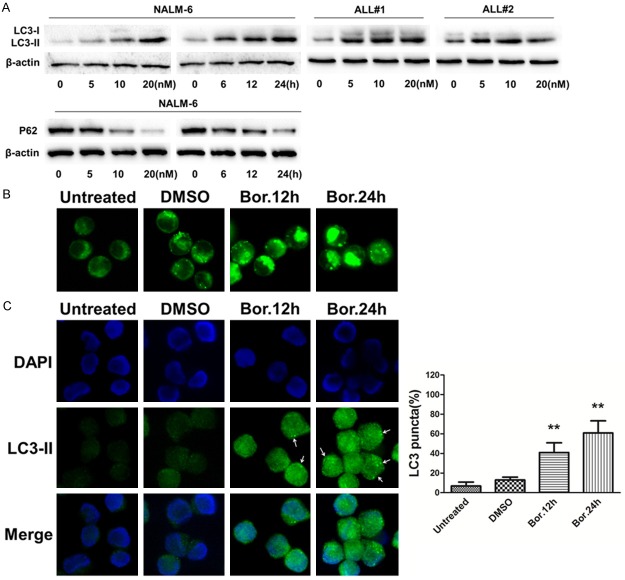
LC3 is widely used to monitor autophagy by analyzing the conversion of LC3 from LC3-I to LC3-II, which is situated to the autophagosomal membranes [13,14]. P62 is an autophagic receptor that interacts with LC3-II at the forming autophagosome, and it is degraded by autophagy, which makes P62 a useful marker of autophagy [15,16]. To determine whether bortezomib is a direct activator of autophagic flux, we monitored the autophagy markers, including LC3-II and P62, in the presence of bortezomib. Treatment with bortezomib induced a dose-dependent and time-dependent increase in the expression of LC3-II, while down-regulating the P62 expression in the Nalm-6 cell line (Figure 1A). Furthermore, we tested the levels of LC3 in CD34+ primary cells treated with various concentrations of bortezomib and found that bortezomib dose-dependently induced autophagy in CD34+ primary cells from two B-ALL patients (Figure 1A). We next used monodansylcadaverine (MDC), a dye that stains autophagolysosomes [12]. MDC staining increased at 12 h and 24 h in Nalm-6 cells (Figure 1B). Additionally, LC3 puncta, an indicator of autophagosome formation, were examined by immunofluorescent staining, as shown in Figure 1C where LC3 puncta were significantly increased after treatment at 12 h and 24 h in Nalm-6 cells, whereas minimal autophagosome formation was present in the control group.
Figure 1.
Bortezomib induces autophagy in B-ALL cells. A. Nalm-6 cells and CD34+ primary cells isolated from bone marrow mononuclear cells of B-ALL patients were exposed to increasing concentrations of bortezomib for 24 h or treated with 10 nM of bortezomib for 6, 12 or 24 h, and the LC3 and P62 levels were analyzed by Western blot. B and C. Nalm-6 cells were untreated or treated with 0.1% DMSO for 12 h or with 10 nM bortezomib for 12 or 24 h and stained with monodansylcadaverine (MDC, 50 µM) or DAPI (blue) and LC3 (green); the cells were then observed with an Olympus BX50 microscope. The formation of autophagic vacuoles was defined by the accumulation of LC3, and the arrows point to autophagic vacuoles. The LC3 puncta-positive cells were calculated as described in materials and methods. The columns represent the average percent of LC3 puncta-positive cells from 3 independent experiments and were shown as the mean ± SD (**, p < 0.01 versus untreated). Representative images are shown in the left panel.
Bortezomib-induced autophagy is dependent on the PI3KC3 signaling pathway and JNK activity
The Beclin 1/PI3KC3 complex is one of the most important regulators essential for autophagosome formation, and inhibition of PI3KC3 activity will block autophagy [17,18]. To determine whether bortezomib-induced autophagy in B-ALL cells is associated with the PI3KC3 signaling pathway, Nalm-6 or CD34+ primary cells were exposed to 3-methyladenine (3-MA), a specific inhibitor of PI3KC3, in the present or absent of bortezomib. We observed that the LC3-II level was increased for treatment with bortezomib alone and 3-MA down-regulated the bortezomib-induced LC3-II accumulation (Figure 2A and 2B). To further study this finding, autophagosome formation was assessed in Nalm-6 and CD34+ primary cells on a fluorescent microscope after treatment with bortezomib alone or in combination with 3-MA. Consistent with the immunoblotting results, fluorescent microscopic analyses showed that bortezomib obviously increased LC3 puncta, an indicator of autophagic vacuoles, but this was inhibited by 3-MA (Figure 2C). These results indicated that bortezomib induces autophagy via the PI3KC3 signaling pathway.
Figure 2.
Bortezomib-induced autophagy is blocked by an autophagy inhibitor. A and B. Nalm-6 cells and CD34+ primary cells from patients were treated with 10 nM bortezomib for 24 h in the presence or absence of 3-methyladenine (3-MA, 5 mM), lysosomal protease inhibitors leupeptin (Leu, 10 µM) and E64D (10 µM) or JNKi (JNK inhibitor, 10 µM), which was followed by LC3 analysis. C. Nalm-6 cells and CD34+ primary cells were treated with 10 nM bortezomib for 24 h with or without 3-MA (5 mM), Leu (10 µM) and E64D (10 µM) or JNKi (10 µM); cells were then stained with DAPI (blue) and LC3 (green) and then observed with an Olympus BX50 microscope. The arrows point at autophagic vacuoles. The columns represent the average percent of LC3 puncta-positive cells from 3 independent experiments and were shown as the mean ± SD (*, p < 0.05, **, p < 0.01, ***, p < 0.001 versus the Bor group). Representative images are shown in the left panel.
Previous reports have suggested that phosphorylation of Bcl-2, which liberates Beclin-1, may also be a crucial mechanism for initiating autophagy [19]. The kinase c-JUN N-terminal kinase (JNK) can phosphorylate Bcl-2, leading to dissociation of Beclin 1 from Bcl-2, liberating beclin-1 to activate the autophagy pathway [20,21]. Several studies have shown that bortezomib inhibits growth and induces apoptosis by activating the JNK enzyme [22,23]. We next evaluated whether bortezomib induces autophagy by activating JNK; cells were co-treated with bortezomib and SP600125, a JNK inhibitor (JNKi), and the LC3-II level was examined by immunoblotting and fluorescent microscopy, respectively. We found that the LC3-II level was decreased by co-treatment with bortezomib and JNKi compared to treatment with bortezomib alone (Figure 2), suggesting that JNKi blocked the autophagy process and bortezomib induced autophagy via activating JNK.
Furthermore, cells treated with bortezomib in combination with E64d/Leu (inhibitors of lysosomal proteases) had an increased level of LC3-II (Figure 2).
Taken together, these results demonstrated that PI3KC3 signaling and JNK are involved in bortezmib-mediated autophagy.
Bortezomib increases the formation of the Beclin-1/PI3KC3 complex
Autophagy is activated by the formation of the PI3KC3/Beclin-1 complex and inhibited by the formation of the Bcl 2/Beclin-1 complex [12,24]. Beclin-1 plays a key role in the initiation of autophagy by increasing the levels of the PI3KC3/Beclin-1 complex [25,26]; therefore, the expression levels of Beclin-1 and PI3KC3 were measured by immunoblotting after bortezomib treatment, and we found that Beclin-1 and PI3KC3 were not induced by bortezomib in any of the examined concentrations and time points in Nalm-6 cells (Figure 3A). The study above showed that PI3KC3 is involved in bortezomib-induced autophagy. To further elucidate the detailed mechanism, we explored the effect on the formation of the Bcl-2/Beclin-1 complex and Beclin-1/PI3KC3 complex by treatment with bortezomib; Nalm-6 cells were treated with bortezomib and then the cell lysates were prepared for the co-immunoprecipitation with an anti-Beclin-1 antibody. As we expected, there was disruption of the Beclin-1/Bcl-2 complex and formation of the Beclin-1/PI3KC3 complex in the bortezomib-treated cells (Figure 3B). These results demonstrate that bortezomib induces autophagy via decreasing the interaction of the Beclin-1/Bcl-2 complex, while increasing the interaction of the Beclin-1/PI3KC3 complex.
Figure 3.

Bortezomib increases the formation of the Beclin-1/PI3KC3 complex. A. Nalm-6 cells were exposed to increasing concentrations of bortezomib for 24 h or with 10 nM bortezomib for 0-24 h followed by Beclin-1 and PI3KC3 level analysis. B. Nalm-6 cells were treated with or without 10 nM bortezomib for 24 h, which was followed by immunoprecipitation with an anti-Beclin-1 antibody and then evaluation of Beclin-1, Bcl-2, and PI3KC3 expression.
Bortezomib inhibits growth and induces apoptosis in B-ALL cells
To assess the anti-ALL effect of bortezomib, Nalm-6 or CD34+ primary cells was exposed to bortezomib at different concentrations for 24 h. Cell growth and apoptosis were measured with the MTT assay and FACS analysis after Annexin V-FITC/propidium iodide (PI) staining, respectively. After treatment, inhibition of proliferation and apoptosis were markedly increased in a dose-dependent manner (Figure 4A and 4B). Furthermore, we examined the expression of proteins relative to apoptosis by immunoblotting. The expression of cleaved Caspase-3, Bax, and cytoplasm cytochrome C were up-regulated and Bcl-2 was down-regulated in Nalm-6 cells after treatment (Figure 4C). Consistently, the cleaved Caspase 3 level was up-regulated in CD34+ primary cells from 2 B-ALL patients (Figure 4D). These results suggest that bortezomib inhibits growth and induces apoptosis as well as has a therapeutic impact on B-ALL.
Figure 4.
Bortezomib shows anti-leukemia activity in ALL cells. A. Bortezomib inhibits the growth of Nalm-6 cells. Nalm-6 cells and CD34+ primary cells were exposed to increasing concentrations of bortezomib for 24 h. Cell viability was evaluated with the MTT assay. Data are shown as the mean ± SD (**, p < 0.01, ***, p < 0.001 versus control). B. Bortezomib induces apoptosis in Nalm-6 cells. Nalm-6 cells were treated with increasing concentrations of bortezomib for 24 h, which was followed by analysis of apoptosis by staining with PI and Annexin-V FITC. Annexin-V positive cells were measured by flow cytometry. Columns represent the average percent of Annexin-V positive cells from 3 independent experiments, which are shown as the mean ± SD (***, p < 0.001 versus control). Representative images are shown in the left panel. C and D. Nalm-6 cells and CD34+ primary cells were treated with increasing concentrations of bortezomib for 24 h, which was followed by Western blot analysis for the expressions of Caspase 3, cleaved Caspase 3, Bax, Bcl-2 and cytoplasm cytochrome C.
Bortezomib arrests the cell cycle at the G2/M phase
We explored the effect of bortezomib on the cell cycle and found that bortezomib suppressed cell cycle progression at the G2/M phase. Our data showed that the percentage of cells in the G2/M phase was increased from 17.72% to 55% with increasing concentrations of bortezomib (Figure 5A and 5B), indicating that cell cycle arrest contributed to growth inhibition by bortezomib in the Nalm-6 cell line.
Figure 5.
Bortezomib arrested the cell cycle progression at the G2/M phase in Nalm-6 cells. Nalm-6 cells were exposed to increasing concentrations of bortezomib for 24 h, which was followed by staining with PI; then, the DNA content was assayed for cell cycle analysis by flow cytometry. Columns represent the average percent of cells in the G2/M phase from 3 independent experiments, which were shown as the mean ± SD (**, p < 0.01, ***, p < 0.001 versus control). Representative images are shown in the upper panel.
Inhibition of autophagy enhances the anticancer activity of bortezomib
To examine the effect of autophagy on the anticancer activity of bortezomib, we analyzed the cell viability, apoptosis and cell cycle after treatment with bortezomib alone or in combination with autophagy inhibitors, including 3-MA and JNKi. Both Nalm-6 and CD34+ cells had more significantly inhibited growth after treatment with bortezomib in combination with 3-MA or JNKi compared with bortezomib alone (Figure 6A). Furthermore, 3-MA and JNKi increased the bortezomib-induced apoptosis by flow cytometric analysis and up-regulated the expression of apoptosis proteins, including cleaved-Caspase 3 and cytoplasm cytochrome C (Figure 6B). Additionally, we investigated the cell cycle process of Nalm-6 cells that were exposed to either bortezomib alone or in combination with 3-MA or JNKi, and we found that only JNKi enhanced the effect of G2/M arrest by bortezomib (Figure 6C). These findings suggest that autophagy prevents Nalm-6 cells from apoptosis and promotes cells survival after bortezomib treatment.
Figure 6.

Inhibition of autophagy enhances the anti-leukemia activity of bortezomib. Nalm-6 cells or CD34+ primary cells from patients were treated with 10 nM bortezomib for 24 h in the presence or absence of 3-MA (5 mM) or JNKi (10 µM). A. Cell viability was evaluated with the MTT assay. Data are shown as the mean ± SD (***, p < 0.001 versus control). B. Apoptosis was analyzed by measuring annexin-V positive cells by flow cytometry. Columns represent the average percent of Annexin-V positive cells from 3 independent experiments, which were shown as the mean ± SD (***, p < 0.001 versus control). Western blot analysis for the expressions of cleaved Caspase 3 and cytoplasm cytochrome C is shown in the right panel. C. Cell cycle analysis by staining the DNA content with PI. Columns represent the average percent of cells in the G2/M phase from 3 independent experiments, which were shown as the mean ± SD (*, p < 0.05, **, p < 0.01, ***, p < 0.001 versus control). Representative images are shown in the left panel. D. Nalm-6 cells were treated with 10 nM bortezomib for 24 h in the presence or absence of chloroquine (50-200 µM). Cell viability was evaluated with the MTT assay. Data are shown as the mean ± SD (*, p < 0.05, ***, p < 0.001 versus control). Western blot analysis for the expressions of LC3, cleaved Caspase 3 and cytoplasm cytochrome C is shown in the right panel.
Recent studies have shown that chloroquine, a lysosomal inhibitor, can potentiate the cytotoxicity of chemotherapy drugs by inhibiting autophagy in established cancers, including prostate cancer [27], colorectal cancer [28], chronic myeloid leukemia [29], etc. To investigate the effect of chloroquine in combination with bortezomib treatment in B-ALL, we examined autophagic flux, cell viability, and apoptosis proteins and observed that the LC3-II level was notably increased with the combination of bortezomib and chloroquine. Co-treatment with bortezomib and chloroquine markedly inhibited cell growth (Figure 6D); moreover, the expression of cleaved-Caspase 3 and cytoplasm cytochrome C, induced by bortezomib, were enhanced by treatment with chloroquine (Figure 6D). Taken together, our data indicated that the effect of bortezomib on B-ALL is partly attenuated by autophagy, and autophagy inhibitors can enhance the anti-B-ALL activity of bortezomib.
Discussion
The outcomes of refractory or relapsed ALL are still disappointing. Bortezomib is a new treatment choice for these refractory or relapsed patients. However, the molecular mechanism of the anti-ALL effect of bortezomib is not clear. In this study, we demonstrated that bortezomib inhibited cell growth, induced apoptosis, arrested the cell cycle and induced autophagy in Nalm-6 cells and CD34+ primary cells. Additionally, the inhibition of autophagy could enhance the anti-ALL function of bortezomib; meanwhile, we explored bortezomib-induced autophagy in association with the interaction of three types of proteins, including PI3KC3, Beclin 1 and Bcl-2.
The ubiquitin proteasome system (UPS) plays a key role in maintaining cellular protein homeostasis through the selective degradation of damaged, misfolded and short-lived regulatory proteins that control essential cellular processes [30]. Dysfunction of this system has been related to transformation and oncogenesis; therefore, the UPS becomes an attractive target for the anticancer therapies. Bortezomib, as a reversible proteasome inhibitor, blocked the degradation of intracellular proteins, affecting multiple cellular processes, including cell cycle progression, cell apoptosis, endoplasmic reticulum stress, angiogenesis, and DNA repair, which contribute to the anti-tumor effect of bortezomib [31]. Currently, the therapeutic strategies, including bortezomib alone or in combination with other chemotherapy drugs for treating acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) [32-35], have led to further investigation of its utility in these malignancies. In this study, we observed that bortezomib inhibited cell growth in both the Nalm-6 cell line and CD34+ primary cells. To explore the reasons for inhibition of cell proliferation, we examined apoptosis, the cell cycle and the autophagy process in B-ALL cells and observed that bortezomib increased Bax and decreased Bcl-2, promoting the release of cytochrome C and activated mitochondrial apoptotic pathway, resulting in cell death. Additionally, the cell cycle process was arrested at the G2/M phase by bortezomib, which also contributed to cell growth inhibition. Moreover, we found that bortezomib induced autophagy in the aforementioned cells, and we further explore the molecular mechanism of bortezomib-induced autophagy and whether autophagy plays a pro-survival or pro-death role in B-ALL cells.
Recent findings have shown that in cells undergoing starvation-induced autophagy, JNK1 phosphorylates serine 70 on Bcl-2, leading to disruption of the Bcl-2/Beclin-1 complex [16,17] and releasing Beclin-1 to form a complex with the class III phosphatidylinositol 3-kinase (PI3KC3), which engages in the early stage of autophagic vesicle formation [15]. In our study, bortezomib downregulated the Bcl-2 expression, liberating Beclin-1 from Bcl-2 and increasing Beclin-1/PI3KC3 complex formation. Because PI3KC3 positively regulates autophagy activation while Bcl-2 negatively regulates autophagy activation, our results demonstrated that bortezomib decreased the interaction between Beclin-1 and Bcl-2, antagonized the inhibition of autophagy by Bcl-2 and facilitated the formation of the Beclin-1/PI3KC3 complex, activating autophagy.
The role of autophagy in cell survival and cell death is like a “double-edged sword” [36]. On the one hand, autophagy maintains the nutrient and energy homeostasis in the case of exposure to various stresses conditions. On the other hand, autophagy might permit cancer cells to become chemotherapy resistant or too much autophagy might lead to undesirable cell death [37]. Therefore, we asked whether bortezomib-induced autophagy exerts a pro-survival or pro-death role in B-ALL cells; to do so, we used autophagy inhibitors to block the autophagy process, which was followed by assessing the effect on cell growth, apoptosis and cell cycle process. Our findings suggested that inhibition of autophagy enhanced the growth inhibition and cell cycle arrest by bortezomib, promoting apoptosis by up-regulating the expressions of cleaved Caspase 3 and cytoplasm cytochrome C, indicating that bortezomib-induced autophagy promotes B-ALL cell survival and plays a protective role, and an inhibitor of autophagy could enhance the cytotoxicity of bortezomib treatment.
In conclusion, this study is the first report that bortezomib induces autophagy in B-ALL cells by increasing formation of the PI3KC3/Beclin 1 complex and, in combination with autophagy inhibitors, can enhance the anticancer activity of bortezomib. This study increases our understanding of bortezomib in the treatment of hematological malignancies.
Disclosure of conflict of interest
None.
References
- 1.Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Hüttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A, Schaich M, Schwartz S, Serve H, Starck M, Stelljes M, Stuhlmann R, Viardot A, Wendelin K, Freund M, Hoelzer D German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 2.Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C, Doyen C, Garderet L, Randriamalala E, Araujo C, Lepeu G, Marit G, Caillot D, Escoffre M, Lioure B, Benboubker L, Pégourié B, Kolb B, Stoppa AM, Fuzibet JG, Decaux O, Dib M, Berthou C, Chaleteix C, Sebban C, Traullé C, Fontan J, Wetterwald M, Lenain P, Mathiot C, Harousseau JL. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–8. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 3.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Invest. 2004;22:304–11. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 5.Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005;5:18. doi: 10.1186/1475-2867-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyama D, Kikuchi J, Hiraoka N, Wada T, Kurosawa H, Chiba S, Furukawa Y. Proteasome inhibitors exert cytotoxicity and increase chemosensitivity via transcriptional repression of Notch1 in T-cell acute lymphoblastic leukemia. Leukemia. 2014;28:1216–1226. doi: 10.1038/leu.2013.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastian L, Hof J, Pfau M, Fichtner I, Eckert C, Henze G, Prada J, von Stackelberg A, Seeger K, Shalapour S. Synergistic Activity of Bortezomib and HDACi in Preclinical Models of B-cell Precursor Acute Lymphoblastic Leukemia via Modulation of p53, PI3K/AKT, and NF-kB. Clin Cancer Res. 2013;19:1445–1457. doi: 10.1158/1078-0432.CCR-12-1511. [DOI] [PubMed] [Google Scholar]
- 8.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 9.Laane E, Tamm KP, Buentke E, Ito K, Kharaziha P, Oscarsson J, Corcoran M, Björklund AC, Hultenby K, Lundin J, Heyman M, Söderhäll S, Mazur J, Porwit A, Pandolfi PP, Zhivotovsky B, Panaretakis T, Grandér D. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16:1018–1029. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- 10.Lou Z, Ren T, Peng X, Sun Y, Jiao G, Lu Q, Zhang S, Lu X, Guo W. Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro. J Int Med Res. 2013;41:1505. doi: 10.1177/0300060513490618. [DOI] [PubMed] [Google Scholar]
- 11.Li CY, Johnson DE. Bortezomib induces autophagy in head and neck squamous cell carcinoma cells via JNK activation. Cancer Lett. 2012;314:102–107. doi: 10.1016/j.canlet.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selimovic D, Porzig BB, El-Khattouti A, Badura HE, Ahmad M, Ghanjati F, Santourlidis S, Haikel Y, Hassan M. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal. 2013;25:308–318. doi: 10.1016/j.cellsig.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 15.Klionsky DJ, Abdalla FC, Abeliovich H. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitinin selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–62. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backer JM. The regulation and function of class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 19.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeffler HP, Taguchi H. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–180. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–518. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 24.He C, Levine B. The Beclin-1 interactome. Curr Opin Cell Biol. 2010;22:140–9. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin-1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 26.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin-1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol. 2014;11:508–516. doi: 10.1038/nrurol.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonewolf CA, Mehta M, Schiff D, Wu H, Haffty BG, Karantza V, Jabbour SK. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World J Gastrointest Oncol. 2014;6:74–82. doi: 10.4251/wjgo.v6.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Cao L, Yu Y, Yang L, Yang M, Liu K, Huang J, Kang R, Livesey KM, Tang D. Inhibiting autophagy potentiates the anticancer activity of IFN1@/IFNα in chronic myeloid leukemia cells. Autophagy. 2013;9:317–327. doi: 10.4161/auto.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford LJ, Irvine AE. Targeting the ubiquitin proteasome system in haematological malignancies. Blood Rev. 2013;27:297–304. doi: 10.1016/j.blre.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Crawford LJ, Irvine AE, Gupta IA. Multiple Myeloma - An Overview. 2012. Proteasome inhibitors in the treatment of multiple myeloma; pp. 3–32. [Google Scholar]
- 32.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, DeAngelo DJ, Kolitz JE, Powell BL, Voorhees P, Wang ES, Blum W, Stone RM, Marcucci G, Bloomfield CD, Moser B, Larson RA. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J. Clin. Oncol. 2013;31:923–9. doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum W, Schwind S, Tarighat SS, Geyer S, Eisfeld AK, Whitman S, Walker A, Klisovic R, Byrd JC, Santhanam R, Wang H, Curfman JP, Devine SM, Jacob S, Garr C, Kefauver C, Perrotti D, Chan KK, Bloomfield CD, Caligiuri MA, Grever MR, Garzon R, Marcucci G. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood. 2012;119:6025–31. doi: 10.1182/blood-2012-03-413898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker AR, Klisovic RB, Garzon R, Schaaf LJ, Humphries K, Devine SM, Byrd JC, Grever MR, Marcucci G, Blum W. Phase I study of azacitidine and bortezomib in adults with relapsed or refractory acute myeloid leukemia. Leukemia Lymphoma. 2014;55:1304–1308. doi: 10.3109/10428194.2013.833333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messinger YH, Gaynon PS, Sposto R, van der Giessen J, Eckroth E, Malvar J, Bostrom BC Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Consortium. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood. 2012;120:285–90. doi: 10.1182/blood-2012-04-418640. [DOI] [PubMed] [Google Scholar]
- 36.Shintani T, Klionsky DJ. Autophagy in Health and Disease: A Double-Edged Sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang ZF, Klionsky DJ. Eaten alive: a history of Macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]