Abstract
Circular RNAs (circRNAs) are a large class of RNAs that, unlike linear RNAs, form covalently closed continuous loops and have recently shown huge capabilities as gene regulators in mammals. These circRNAs mainly arise from exons or introns, and are differentially generated by back splicing or lariat introns. Interestingly, they are found to be enormously abundant, evolutionally conserved and relatively stable in cytoplasm. These features confer numerous potential functions to circRNAs, such as acting as microRNA (miRNA) sponges, binding to RNA-associated proteins to form RNA-protein complexes and then regulating gene transcription. Importantly, circRNAs associate with cancer-related miRNAs and the circRNA-miRNA axes are involved in cancer-related pathways. Some synthetic circRNAs have shown the remarkable anti-cancer effects. Though circRNAs are ancient molecules, the huge therapeutic potentials of circRNAs are recently being discovered from the laboratory to the clinic. Here, we review the current understanding of the roles of circRNAs in cancers and the potential implications of circRNAs in cancer targeted therapy.
Keywords: Circular RNA, microRNA sponge, anti-cancer, targeted therapy
Introduction
The concept of “circular RNA” was first proposed in 1976, Sanger and colleagues found that viroids are single-stranded covalently closed circular RNA (circRNA) molecules pathogenic to certain higher plants [1]. Unlike linear RNA, the 3’ and 5’ ends in circRNA normally present in an RNA molecule have been jointed together. This feature confers numerous properties to circRNA, many of which have only recently been validated. CircRNAs are a large class of RNAs that have shown huge capability as gene regulators in humans [2]. Beyond being a potentially major approach of gene regulation [3], circRNAs may represent new roles in cancer diagnosis and targeted therapy.
Origins of circRNA
CircRNA is a type of RNA that forms a covalently closed continuous loop. The early studies showed that some circRNAs arised from protein coding genes [4], and they were produced by seemingly rare errors in canonical and non-canonical RNA splicing [5,6]. Evidence is emerging that circRNA molecules are predominantly generated by a process called back-splicing, where in downstream exons are spliced to upstream exons in reverse order [7]. In other words, a splice donor site being joined to a splice acceptor site further upstream in the primary transcript, yielding a circular transcript (Figure 1A-i). In addition, the exon circularization is dependent on flanking intronic complementary sequences and is evolutionarily dynamic [8]. The long-held belief that the circularized transcript is an accidental byproduct resulting from very rare error during splicing has recently been challenged. Zhang and colleagues identified another new class of intron-derived circular RNAs (circRNAs) in derived from introns of protein coding genes and demonstrated their capacity to enhance transcription of their host genes (Figure 1A-ii) [9]. Interestingly, the latest study reported that RNA circularization and pre-mRNA splicing compete against each other in the manner of tissue specific and conserved feature in animals [10]. A handful of circRNAs generated from eukaryotic genomes, although have been identified, their roles are unclear as they are generated by seemingly rare errors in RNA splicing.
Figure 1.

CircRNAs are generated in nucleus and are very stable in cytoplasm. A. The origin of circRNAs. i. The unusual splicing machinery “backsplice” and generate exonic circRNAs whose 5’ and 3’ ends are covalently linked. ii. The intron-derived circRNAs are generated from lariat introns that escape debranching. Sequences near the 5-splice site (yellow box) and branch point (red box) are minimally sufficient for an intron to escape debranching and become a stable circRNA. B. CircRNAs locate in cytoplasm. CircRNAs either undergo nuclear export or are released to the cytoplasm via nuclear pore complex (NPC), where they enjoy extraordinary stability, likely as a result of resistance to debranching enzymes (DBE) and RNA exonucleases (REN).
Properties of circRNA: abundant, conserved and stable
More than 20 years ago, circRNAs were discovered from a handful of transcribed genes [5,6,11,12]. At that time, these RNA molecules had generally been considered to be of low abundance and likely representing errors in splicing. Recently, the old circRNAs have been found to be abundant, conserved and stable in cytoplasm.
CircRNAs are abundant
By genomic methods, Salzman et al found that the scrambled exons are in a large number of both normal and cancer cells. At least 10% of total transcript isoforms are expressed at levels comparable to the canonical linear isoforms. The vast majority of scrambled exons (~98%) represent circular transcripts [4]. Moreover, by greater sequencing depth, Jeck et al. revealed that about 1 in 8 expressed genes produce detectable levels of circRNAs including the “low” category and the abundance of circular molecules exceeds that of associated linear mRNA by > 10-fold [3]. Interestingly, Memczak et al also found that numerous circRNAs (1,950 circRNAs in humans, 1,903 circRNAs in mouse and 724 circRNAs in C. elegans) specifically express in a cell type or developmental stage [2]. Taken together, in contrast to the linear mRNAs, the circRNAs appear more abundant than expected.
CircRNAs are conserved
As an additional type of noncoding RNAs (ncRNAs), the endogenous circRNA retains a similar genomic structure. Despite the exonic circRNAs (arise from exons), the intronic circRNAs (arise from introns) can be further distinguished by the presence of a 2’-5’ junction [9]. Exonic circRNAs do not feature a 2’-5’ linkage but consist of 3’-5’ links throughout the molecule. These characters indicate a high degree of conservation of specific back splicing events and these two distinct species are evolutionarily conserved. For all circRNA types studied to date, although only a small fraction of the theoretically possible circRNA isoforms from a given gene are actually observed, most of them are produced across the tree of eukaryotic life [13]. CircRNA probably shows an ancient, conserved feature of eukaryotic gene expression programs.
CircRNAs are stable
CircRNAs either undergo nuclear export or are released to the cytoplasm during cell mitosis, where they enjoy extraordinary stability, likely as a result of resistance to debranching enzymes (DBE) and RNA exonucleases (REN) (Figure 1B) [4,11]. Exonic circRNA is very stable in cells [6], with most species exhibiting a half-life over 48 h [3], compared to an average half-life of 10 h for mRNAs [14]. However, exonic circRNA is not stable in serum, with a half-life of < 15 s, presumably due to circulating RNA endonucleases [15]. Possibly due to this stability, some exonic circRNAs have been shown to be at higher levels than the linear RNA gene product [16]. In addition, about 9,000 lariat intronic RNAs are stable and occur in oocyte cytoplasm [17]. Other studies have reported circRNA cytoplasmic localization [18-20]. Thus, although these features of circRNAs all point to the possibility of important functional roles, their nature and mechanisms are still to be discovered.
Possible functions of circRNA: old tree with new flower
CircRNAs represent a novel class of conserved endogenous RNAs that regulate gene expression in mammals. The evolutionary conservation of circularization usually conceals certain important function for a particular circRNA.
CircRNAs act as miRNA sponge
Animal microRNAs (miRNAs) are a large class of noncoding small (~21nt) RNAs that repress translation of mRNAs involved in a diverse set of biological processes [21]. They directly bind to target mRNAs by complementary base pairs and can trigger cleavage of mRNAs depending on the degree of complementarity. Both artificial and natural sponge RNAs contain complementary binding sites to a miRNA of interest. Due to a sponge’s binding sites are specific to the miRNA seed region, these RNAs can “sponge up” miRNAs of a particular family, thereby serving as competitive inhibitors that suppress the ability of the miRNA to bind its mRNA targets [22,23]. Mammals have thousands of circRNAs with predicted miRNA binding sites, many of these predicted miRNA binding sites in circRNAs are functional and under similar selective pressure as miRNA binding sites in mRNAs [24]. Thus, this phenomenon yields a clue that they may function in sponging.
For instance, CDR1as/ciRS-7 is encoded in the genome antisense to the human CDR1 gene locus (namely CDR1as) [2], and targets miR-7 (namely ciRS-7: circular RNA sponge for miR-7) [25]. It was found that there are over 60 miR-7 binding sites, far more than any known linear sponge [2,25]. Though CDR1as/ciRS-7 can be endonucleolytically cleaved by miR-671 in an AGO2-dependent manner, it cannot be cleaved by miR-7 and AGO2. This is because miRNA cleavage activity depends on complementarity beyond the 12th nucleotide position, while none of miR-7’s binding sites meet this requirement. To silence miR-7 expression in zebrafish which do not have the CDR1 locus in their genome, Memczak and colleagues took advantage of a tool called morpholino, which can base-pair and sequester target molecules [26]. Interestingly, morpholino treatment had the same severe effect on midbrain development as ectopically expressing ciRS-7 in zebrafish brains using injected plasmids [2]. This indicates a significant interaction between ciRS-7 and miR-7 in vivo.
Another notable circular miRNA sponge is sex-determining region Y (SRY). SRY is highly expressed in murine testes and generates a circRNA that contains 16 miR-138 binding sites, functions as a miR-138 sponge [5,25]. In the genome, SRY is flanked by long inverted repeats (IRs) over 15.5 kb in length. When one or both of the IRs are deleted, circularization does not occur [18]. This finding introduces the idea of inverted repeats enabling circularization. So far, circRNA sponges have been characterized with the high expression levels and a large number of miRNA binding sites (Figure 2A). They are likely to be more effective sponges than those that are linear [7]. Currently, the sponging activity is the main function of some circRNAs.
Figure 2.
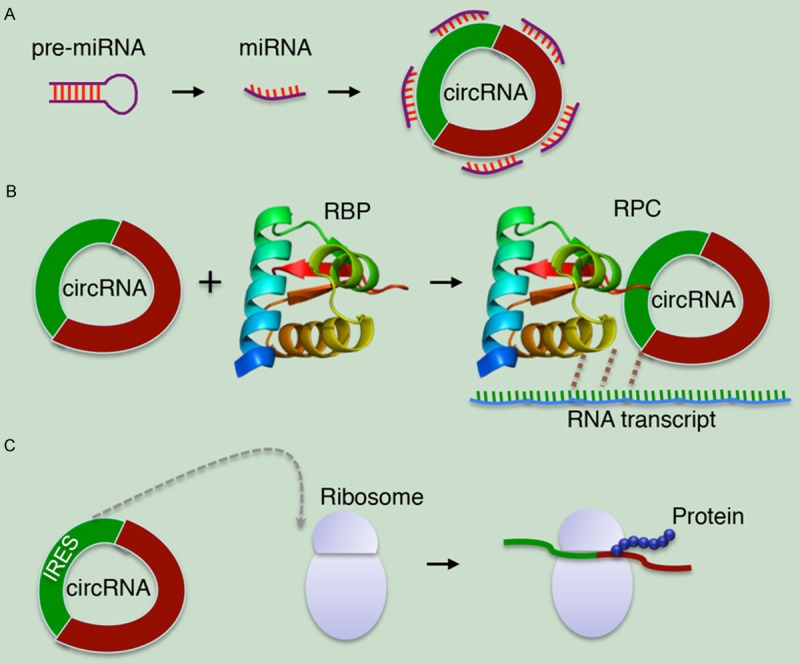
Models of circRNA-mediated gene expression regulation. A. CircRNA functions as a miRNA inhibitor. B. CircRNA binds to RNA-binding protein (RBP) to form RNA-protein complex (RPC) and then interacts with the linear transcript of gene. C. The synthetic circRNA that contains an internal ribosome entry site (IRES) can be translated into a protein product in vitro.
CircRNAs regulate linear RNA transcription and protein production
Though recent attention has been focused on the sponge functions of circRNAs, researchers are considering several other functional possibilities as well. Besides regulating miRNAs, circRNAs may bind and sequester RNA-binding proteins (RBPs) or even base-pair with RNAs besides miRNAs, resulting in the formation of large RNA-protein complexes (RPCs) [7]. These RPCs can regulate the pool of RBPs or small RNAs capable of interacting with the canonical linear RNA counterpart (Figure 2B) [4,27]. Other circRNAs may encode proteins with functions distinct from those of their canonical counterparts. Although the tested circRNA is a purely artificial construct, the synthetic circRNA that contains an internal ribosome entry site (IRES) can be efficiently translated (Figure 2C) [28]. As even linear mRNAs are thought to circularize during translation through protein-protein interactions between factors binding the 5’ and 3’ ends of the mRNA, RNA circularization, whether by direct covalent bonds or non-covalent means such as protein bridging or Watson-Crick base pairing, may be much more common than is currently appreciated [29]. The discovery of such a large class of previously unknown circRNAs also raises the question of what other RNAs might have been missed. Considering that RNA structural elements, such as triple helices, efficiently prevent degradation from RNA termini [30], it is becoming clear that circRNAs likely have key regulatory functions in RNA transcription and protein production.
CircRNAs and cancer: common molecules with huge potentials
Since the Nature paper firstly ascribed an actual function to one of these circRNA molecules [2], the potential of circRNA has elevated the scientific community’s consciousness [31]. Though circRNAs are old molecules, the huge therapeutic potentials of circRNAs are recently being discovered from the laboratory to the clinic (Figure 3).
Figure 3.
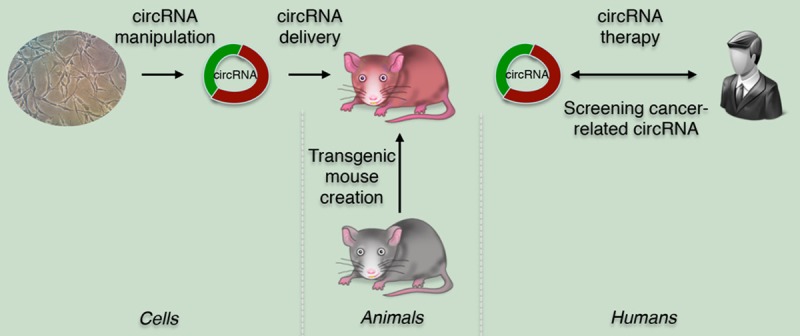
CircRNAs involvement in cancer: from bench to clinic. At the cell level, the specific circRNA and its role in function are discovered and identified. In the in vivo experiment, the transgenic mouse is created and then the specific circRNA is delivered to this animal model to analyze the changes in phenotype. Prior to the clinic trial, the functional target circRNA is screened from cancer patient. The targeted therapy can be used in humans by delivering the therapeutic circRNA to various cancer patients.
CircRNAs associate with cancer-related miRNAs
The fact that circRNAs are targeted by endogenous miRNAs was discovered by Hansen et al [25]. It has recently come to light that these circRNAs play critical roles in fine-tuning the level of miRNA-mediated regulation of gene expression by sequestering the miRNAs. After a measure for the likelihood of a circRNA to be associated with a disease in terms of the statistical significance of the circRNA’s interaction with miRNAs associated with the concerned disease, Ghosal et al. further performed the Gene ontology (GO) enrichment analysis on the set of protein coding genes in the miRNA-circRNA interactome of diseases to check the enrichment of genes associated with particular biological processes. They found such enrichment for biological processes for mRNAs in 90 diseases. Among these mRNAs, there are 22 genes in response to light stimulus and 43 cell cycle-related genes associated with breast cancer. 194 and 68 genes involved in different biological processed associate with cervical cancer and gastric cancer respectively. In addition, they also found that 12 genes in response to DNA damage stimulus associate with oral carcinoma [32]. This is the first report that reveals the global view of the potential association of circRNAs with cancer based on a comprehensive data analysis. However, the direct circRNA:miRNA association needs more biological evidences.
CircRNA-miRNA axes regulate cancer-related pathways
The newly identified ciRS-7, as a circular miR-7 inhibitor, harbors more than 60 conventional miR-7 binding sites. To uncover the important role of ciRS-7 in cancers, the regulatory network in which miR-7 participates must be definitely considered. Up to date, the increasing evidences involve miR-7 in a lot of pathways since miR-7 directly down-regulates major oncogenic factors in cancer-related signaling pathways including EGFR [33], IRS-1 [33], IRS-2 [33], Raf1 [34], Pak1 [35], Ack1 [36], PA28 gama [37], YY1 [38], IGF1R [39], PIK3CD and mTOR [40], suggesting a distinct tumor suppressive role of miR-7. Additionally, miR-7 indirectly up-regulates E-cadherin by targeting IGF1R [39,41] and FAK [42,43], resulting in lessened epithelial to mesenchymal transition (EMT), decreased anchorage-independent growth and suppression of metastasis.
In spite of the tumor suppressive role of miR-7, the adverse effect has also been investigated. As an example, lung carcinomas and poor prognosis was found to be associated with miR-7 overexpression [44]. In this work, the authors also showed that tumor volume in nude mice increased significantly with a concomitant decrease in survival rate when injected with CL1-5 cells expressing miR-7 artificially. Furthermore, inhibition of miR-7 caused decreased proliferation and increased apoptosis in human caner cells [45], indicating that high miR-7 expression is not necessarily beneficial in terms of inhibiting carcinogenesis. Together, these apparently contradictory functions of miR-7 may well be explained by an ambiguous role of miR-7 in regulation of the complex networks of oncogenes and tumor suppressors resulting in a cancer type (and perhaps ciRS-7) dependent outcome.
Human ciRS-7 is highly and widely associated with AGO proteins in a miR-7-dependent manner. Although the circRNA is completely resistant to miRNA-mediated target destabilization, it strongly suppresses miR-7 activity, resulting in increased levels of miR-7 targets [25]. Mechanically, considering the high miR-7 and ciRS-7 expression in brain, a considerable amount of RNA-induced silencing complexes (RISC) would be tethered by miR-7 to the circular RNA. Thus, the abundant miR-7/ciRS-7 association may significantly affect the cellular pool of available RISC components. Consequently, miRNA activity and regulation by miRNAs in general would be less pronounced in miR-7/ciRS-7-expressing tissues [2,46]. Together, miR-7 is closely coupled to ciRS-7 and fine-tuning of the miR-7/miR-671/ciRS-7 axis will likely play profound roles in cancer-associated biological processes.
CircRNAs exhibit potential anti-cancer effects
If circular sponge activity can indeed help in countering harmful miRNA activity, we need to figure out the best way to introduce sponge expression in vivo, probably via a transgene. It is also important to consider how transgene can be expressed only in specific location of the cell or tissue, or expressed only when induced [22]. CircRNA can be produced both in vitro and in vivo using two methodologies. The first makes use of ligase to ligate both ends of the linear form of RNA transcripts [47,48], while the second uses a spontaneous group I intron self-splicing system, designated as the permuted intron-exon (PIE) method [49]. The latter technique is the only methodology available for in vivo circRNA production because it has no requirement for proteinaceous components, such as ligases [50]. Therefore, the PIE method is a promising economical methodology for producing circRNA drugs.
The circularized miRNA sponges displayed superior anti-cancer activities compared to the linear sponges in malignant melanoma cell lines. As an alternative to the use of the vectors expressing linear sponges, the use of the expression vector for RNA circles opens new way to deliver miRNA sponges with persistent effects [51]. Circular sponges may be optimized with tough decoy RNAs to further strengthen the suppression of miRNA activity in cell lines. Addition of more miRNA binding sites may infinitely increase the potency of the circular sponges [52,53]. The availability of circularizing the miRNA sponge in cells is a candidate for a new methodology for RNA-based cancer therapy.
Since certain circRNA has many binding sites for a specific miRNA, it is more effective than typical miRNA inhibitors. With the new discovery of miRNA management by naturally occurring circRNAs, RNA circles may prove to be well-suited carriers of decoy-type miRNA inhibitors as the second-generation miRNA inhibitor [52]. Such synthetic circRNA inhibitors might be future targets for therapies and soon appear as new therapeutic strategies in cancers.
Future perspectives
For the recently identified circRNA, both CDR1as and ciRS-7 refer the same circular RNA molecule. “CDR1as” assumes that circRNAs will bear some relationship to a named gene-in this case, an antisense sequence to the cerebellar degeneration-related gene. While “ciRS-7” denotes binding to miR-7, and therefore assumes that other circRNAs in this category will also neatly align with a single miRNA. With the discovery of numerous circRNAs, a better naming system for circRNAs is extremely needed. Based on the naming rules for miRNAs, we strongly suggest that this first one of circRNAs is called circR-1. As miRNA sponges, circRNAs have contributed to maintaining each of the roughly 21-nt miRNAs unchanged over major parts of evolution. The selection pressure on each miRNA nucleotide is undoubtedly high. Despite the enormous number of possible miRNA sequences, the small amount of change in miRNAs implies that the remaining evolutionary space for innovation is limited. Possibly the hairpin-shaped miRNA precursors could work as potential regulators and fit into a lot of genomic noncoding sequences, including circRNAs, could act as a mover of evolution [54].
The current methods to detect and characterize circRNAs are still limited and challengeable [55,56]. The emerging roles of the highly complex network of ceRNAs, which communicate via miRNA and the overwhelming number of identified circRNA structures prompts for exciting new avenues of research to uncover the full biologic functions of these ncRNAs in cellular regulation and human disease. The clinical trial treating human diseases with synthetic miRNA inhibitors is already in phase 2a [57]. Like these small RNA-based drugs (e.g. miRNAs), such circRNA molecules face multiple delivery challenges such as lack of targeted delivery in cancer targeted therapy [58]. As a novel strategy for miRNA suppression by using circRNAs, the biological complexity and the clinical applicability of circRNAs needs to be further unraveled.
Acknowledgements
The authors thank their respective laboratory members and collaborators for critical review of this article. The authors apologize that space constraints prevent them from citing all relevant publications. This work was supported in part by research grants from the Natural Science Foundation of Zhejiang Province (LY15C060006/LY15H160127), the Academic Climbing Project of Zhejiang Provincial Universities Discipline Leaders (pd2013103), the Public Technology Applied Research Program of Zhejiang Province (2012C37102/ 2013C37029), the Sci-Tech Research Project of Ningbo (2014C50058), the Scientific Innovation Team Project of Ningbo City (2011B82014) and the K. C. Wong Magna Fund at Ningbo University.
Disclosure of conflict of interest
None.
References
- 1.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 3.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 6.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 7.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA Biogenesis Competes with Pre-mRNA Splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 12.Zaphiropoulos PG. Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol Cell Biol. 1997;17:2985–2993. doi: 10.1128/mcb.17.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, Dinneny JR, Brown PO, Salzman J. Circular RNA is expressed across the eukaryotic tree of life. PLoS One. 2014;9:e90859. doi: 10.1371/journal.pone.0090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 15.Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem Pharmacol. 2006;71:702–710. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talhouarne GJ, Gall JG. Lariat intronic RNAs in the cytoplasm of Xenopus tropicalis oocytes. RNA. 2014;20:1476–1487. doi: 10.1261/rna.045781.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubin RA, Kazmi MA, Ostrer H. Inverted repeats are necessary for circularization of the mouse testis Sry transcript. Gene. 1995;167:245–248. doi: 10.1016/0378-1119(95)00639-7. [DOI] [PubMed] [Google Scholar]
- 19.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XF, Lytton J. A circularized sodium-calcium exchanger exon 2 transcript. J Biol Chem. 1999;274:8153–8160. doi: 10.1074/jbc.274.12.8153. [DOI] [PubMed] [Google Scholar]
- 21.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 22.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20:R858–861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas LF, Saetrom P. Circular RNAs are depleted of polymorphisms at microRNA binding sites. Bioinformatics. 2014;30:2243–2246. doi: 10.1093/bioinformatics/btu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 26.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 27.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 29.Martineau Y, Derry MC, Wang X, Yanagiya A, Berlanga JJ, Shyu AB, Imataka H, Gehring K, Sonenberg N. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008;28:6658–6667. doi: 10.1128/MCB.00738-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilusz JE, JnBaptiste CK, Lu LY, Kuhn CD, Joshua-Tor L, Sharp PA. A triple helix stabilizes the 3’ ends of long noncoding RNAs that lack poly(A) tails. Genes Dev. 2012;26:2392–2407. doi: 10.1101/gad.204438.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkel JM. Assume nothing: the tale of circular RNA. Biotechniques. 2013;55:55–57. doi: 10.2144/000114061. [DOI] [PubMed] [Google Scholar]
- 32.Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 34.Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284:5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- 35.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saydam O, Senol O, Wurdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO, Yi M, Stephens RM, Krichevsky AM, Saydam N, Brenner GJ, Breakefield XO. miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res. 2011;71:852–861. doi: 10.1158/0008-5472.CAN-10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Qian J, Gu J, Chang L, Ge D, Chu Y. PA28gamma emerges as a novel functional target of tumour suppressor microRNA-7 in non-small-cell lung cancer. Br J Cancer. 2014;110:353–362. doi: 10.1038/bjc.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Li X, Wu CW, Dong Y, Cai M, Mok MT, Wang H, Chen J, Ng SS, Chen M, Sung JJ, Yu J. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene. 2013;32:5078–5088. doi: 10.1038/onc.2012.526. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, Wu K, Nie Y, Fan D. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–1372. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 40.Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J. 2010;432:199–205. doi: 10.1042/BJ20100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong X, Li G, Yuan Y, He Y, Wu X, Zhang W, Wu Z, Chen T, Wu W, Lobie PE, Zhu T. MicroRNA-7 inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via targeting FAK expression. PLoS One. 2012;7:e41523. doi: 10.1371/journal.pone.0041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun LH, Wang XF, Zhang JX, Cao L, Wang XR, You YP, Liu N. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin Med J (Engl) 2011;124:2616–2621. [PubMed] [Google Scholar]
- 44.Chou YT, Lin HH, Lien YC, Wang YH, Hong CF, Kao YR, Lin SC, Chang YC, Lin SY, Chen SJ, Chen HC, Yeh SD, Wu CW. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res. 2010;70:8822–8831. doi: 10.1158/0008-5472.CAN-10-0638. [DOI] [PubMed] [Google Scholar]
- 45.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 47.Beaudry D, Perreault JP. An efficient strategy for the synthesis of circular RNA molecules. Nucleic Acids Res. 1995;23:3064–3066. doi: 10.1093/nar/23.15.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CY, Sarnow P. Internal ribosome entry sites tests with circular mRNAs. Methods Mol Biol. 1998;77:355–363. doi: 10.1385/0-89603-397-X:355. [DOI] [PubMed] [Google Scholar]
- 49.Puttaraju M, Been MD. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992;20:5357–5364. doi: 10.1093/nar/20.20.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umekage S, Kikuchi Y. In vitro and in vivo production and purification of circular RNA aptamer. J Biotechnol. 2009;139:265–272. doi: 10.1016/j.jbiotec.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Tay FC, Lim JK, Zhu H, Hin LC, Wang S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Adv Drug Deliv Rev. 2015;81:117–127. doi: 10.1016/j.addr.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Bak RO, Hollensen AK, Mikkelsen JG. Managing microRNAs with vector-encoded decoy-type inhibitors. Mol Ther. 2013;21:1478–1485. doi: 10.1038/mt.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haraguchi T, Ozaki Y, Iba H. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009;37:e43. doi: 10.1093/nar/gkp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kosik KS. Molecular biology: Circles reshape the RNA world. Nature. 2013;495:322–324. doi: 10.1038/nature11956. [DOI] [PubMed] [Google Scholar]
- 55.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 58.Gong Z, Yang J, Li J, Yang L, Le Y, Wang S, Lin HK. Novel insights into the role of microRNA in lung cancer resistance to treatment and targeted therapy. Curr Cancer Drug Targets. 2014;14:241–258. doi: 10.2174/1568009614666140305104845. [DOI] [PubMed] [Google Scholar]


