Abstract
Dysregulation of endocytosis was viewed as an emerging feature of cancer development and progression. A large GTPase dynamin2 plays a significant role in receptor tyrosine kinases (RTKs) endocytosis. The study was designed to investigate its roles in hepatocellular carcinoma (HCC) metastasis and its underlying mechanism. Dynamin2 expression in cancer tissues from HCC patients was assessed by immunohistochemistry and its prognostic significance for the patients was conducted using univariate and multivariate analysis. Its role in tumor invasion and metastasis was evaluated in vitro by gene silence using siRNA-mediated approach and the small molecule inhibitor of Dynasore. EGFR expression in HCC cell lines and EGFR downstream signaling ERK1/2 was evaluated by Western-blot and immunofluorescence analyses after Dynamin2 inhibition. Our data indicated that low expression of dynamin2 was well correlated with invasion characteristics and shorter overall survival. HCC cell migration, colony formation and invasion were significantly increased after the inhibition of dynamin2 in HCC cells. Internalization of EGFR was markedly reduced when dynamin2 was knock down or inhibition. In addition, we observed that dynamin2 regulated EGF mediated EGFR downstream Ras/ERK1/2 signaling and p-ERK1/2 accumulation in nucleus. The results demonstrate a possible mechanism of dynamin involved EGFR endocytosis and modulation of metastasis in HCC. Dynamin2 inhibits the invasion and metastasis of HCC cells by the promotion of EGFR endocytosis and downregulation of ERK1/2 phosphorylation.
Keywords: Dynamin2, hepatocellular carcinoma, endocytosis, EGFR, ERK1/2
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer death, being the fifth most common cancer in the world [1]. Although radical surgery and liver transplantation remains the main modalities of curative treatment for HCC, but the further improvements in HCC has been hindered by high rate of postoperative recurrence or metastasis and the 5-year accumulative recurrence rate reaching to 50-70% [2]. Thus, understanding the mechanisms underlying the intrinsic tumor invasion and recurrence is imperative to improve efficacy of treatments for aggressive HCC. Emerging evidence, over the past decade, indicated that dysregulation of endocytosis may play a key role in human cancer progression. Recently, dynamin2, the key molecule in scission of clathrin- and caveolin-coated vesicles from cell membrane during endocytosis, was found markedly associated with membrane ruffles and lamellipodia, relying to increased cell migration [3]. On the contrary, Dynamin2 has been found also involved in preventing tumor invasion and metastasis, via interfere with extracellular matrix degradation, and may be a prognostic marker for early squamous cell carcinoma of the cervix [4]. However, the prognostic role of Dynamin2 in HCC progression and the underlying mechanism has not been fully addressed.
Dynamin2, deforming lipid bilayers and leading to vesicle scission, plays a significant role in receptor tyrosine kinases (RTKs) endocytosis. Dynamin2 is a 96-kDa large GTPase, originally known as a microtubule-binding protein in eukaryotic cells, and has been found to contain a GTP-binding domain [5]. Three isoforms including Dynamin1, Dynamin2 and Dynamin3 were found in mammals. Different from the Dynamin1 and Dynamin3, Dynamin2 is ubiquitously expressed [6], and has been shown to participate in several cellular physiological functions, including focal adhesion, cell migration and invasion, cancer cell proliferation [7], and endocytosis [8,9], which is closely related to the key role of dynamin2 in participating in receptor-mediated endocytosis, such as vesicle formation, caveolae internalization, synaptic vesicle recycling, and vesicle trafficking in and out of Golgi apparatus [10]. Through participation in several different cellular processes, overexpression of dynamin mutants defective in GTPase activity or obstruction of dynamin2 GTPase activity results in the insufficiency of internalization of many molecules, such as epidermal growth factor receptor (EGFR) [11].
EGFR, as the prototypical receptor tyrosine kinase, was found overexpression in around 40% to 70% of conventional HCC in most studies [12,13]. The effect of EGFR antagonists against HCC has been certified in cell lines and animal models [14]. One of the principle mechanisms by which EGFR signaling is regulated is ligand-mediated endocytosis. Formal proof coming from Michael Rosenfeld laboratory and other studies showed that endocytosis negatively regulates EGFR signaling [15,16], modulated by multiple molecules, such as clathrin, caveolin-1, etc. Thinking the role of dynamin2 on the receptor-mediated endocytosis, we wonder, in HCC, if the expression of dynamin2 promotes EGFR endocytosis and inhibit downstream signaling cascades.
On the basis of this information, we hypothesized that high dynamin2 expression in HCC could facilitate the endocytosis of EGFR, and downregulating dynamin2 expression may strengthen Erk1/2 phosphorylation, increase the recurrence rate, and result in a dismal survival rate.
Materials and methods
Reagents and cell lines
Dynasore (BD Corporation) was dissolved in sterile DMSO for in vitro experiments. DMSO was added to cultures at 0.1% (v/v) final concentration as a vehicle control. Primary antibodies, AKT and phosphorylated AKT (S473); ERK1/2 and phosphorylated ERK1/2 (T202/Y204); Anti-Rabbit EEA1; anti-mouse EGFR; anti-rabbit EEA1antibody were purchased from Cell Signaling Technology. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Santa Cruz. Anti-mouse dynamin2 mAb was purchased from BD Pharmingen. Alexa Fluor 488 conjugate second antibody (Invitrogen Corporation) was dissolved in PBS for in vitro experiments. Human or rat dynamin2 small interfering RNA (siRNA), control siRNA, were obtained from Shanghai GenePharma Co. (Shanghai, P.R.China).
Two human HCC cell lines, HCCLM3 (established in Liver Cancer Institute, Fudan University, Shanghai) and Huh7 (ATCC), were maintained in high-glucose DMEM supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin. Cell lines were cultured at 37°C in a humidified incubator in 5% CO2.
Patients and follow-up
HCC specimens used in tissue microarray (TMA) analysis were obtained with informed consent from patients who underwent radical resection between January 2008 and December 2009 at the Liver Cancer Institute and Zhongshan Hospital (Fudan University, Shanghai). The study was approved by the research ethics committee of Zhongshan Hospital. A total of 131 cases were used to examine the dynamin2 expression and prognosis in HCC. They were followed up until March 2013 with a median observation time of 59.0 months.
TMA and immunohistochemistry
A two-step method of immunohistochemistry (IHC) including a heat-induced antigen-retrieval procedure was performed as previously described [17]. Mouse anti-human dynamin2 mAb (BD) at 1:200 was used as primary detection antibody. Detection without primary antibody was considered as negative control. All samples IHC staining was independently assessed by two experienced pathologists. The staining intensity was graded from 0 to 2 (0, no staining; 1, weak; 2, strong). The staining extent was graded from 0 to 4 according to the percentage of immune reactive tumor cells (0%, 1%-5%, 6%-25%, 26%-75%, 76%-100%). A score ranging from 0 to 8 was calculated by multiplying the staining extent score with the staining intensity score, resulting in a low (0-4) expression level or a high (6-8) level for each sample.
Immunofluorescence
HCCLM3, Huh7 cells were seeded onto coverslips in six-well plates in DMEM supplemented with 10% FBS and allowed to adhere overnight. Cells were stimulated with or without Dynasore in serum-free media 30 min followed by treatment with EGF for 0, 10, 30 and 60 min. Cells were then washed twice in PBS, fixed in formaldehyde and permeabilized with PBS containing 0.1% Triton X-100. Following blocking in PBS-containing 5% Bovine Serum Albumin (BSA), cells were stained with EGFR antibody overnight 4°C and visualized with Alexa488-conjugated secondary antibody. Then cells were washed with ice-cold PBS and then incubated with 4’,6-diamidino-2-phenylindole (DAPI). The fluorescent images were visualized using a confocal microscope (Leica). For siRNA experiments, cells were transfected with control or dynamin2 siRNA as described above and seeded onto coverslips. After 48 h, cells were stimulated with EGF for 0-120 min, washed in PBS and stained with primary antibody for EGFR or p-ERK as mentioned above.
Western blot analysis
Western blot analysis was performed as previously described [18]. Briefly, total cell lysates were prepared, and proteins were separated by SDS-PAGE, followed by transfer to polyvinylidenedifluoride membrane. The membranes were washed, blocked, and incubated with the specific primary antihuman antibodies against Dynamin2 (1:500), p-AKTSer473 (1:1000), AKT (1:1000), p-ERK1/2Tyr202/Tyr204 (1:1000), ERK1/2 (1:1000), or GAPDH (1:1000), followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Proteins were detected by enhanced chemiluminescence assay (Pierce-Thermo Scientific).
Dynamin2 silencing by siRNA
Dynamin2 siRNA and negative control mismatch sequences were transfected into HCCLM3 and Huh7 cells using LipofectamineTM 2000 (Invitrogen) according to the manufacturer’s instructions. The following sense and anti-sense siRNA strands were used: dynamin2 (human) CCGGCCTGAGGTGTACATAGTCCTTCTCGAGAAGGACTATGTACACCTCAGGTTTTTG (sense), AATTCAAAAACCTGAGGTGTACATAGTCCTTCTCGAGAAGGACTATGTACACCTCAGG (antisense); After 72 h, cells were lysed, and protein was analyzed by Western blot.
In vitro migration and invasion assays
Cell migration and invasion were analyzed by a Transwell Permeable Supports system with 8-μm pores (Corning) following the instruction of the manufacture. Briefly, for motility assays, 5 × 104 cells were seeded into upper uncoated inserts; for invasion assays, 1.0 × 105 cells were seeded into upper inserts with a Matrigel-coated membrane (BD). Cells were seeded in 1% serum medium in upper chamber for 24 h or 48 h with lower chamber filled with 10% serum medium. After removal of the non-migrating or non-invading cells, the remaining cells were fixed, stained, and analyzed by inverted microscopy.
Colony formation assays
Cells were plated in 6-well plates (5 × 102 cells/plate) and cultured for 10 days. The colonies were stained with 1% crystal violet for 30 seconds after fixation with 10% formaldehyde for 5 minutes.
Statistical analysis
Statistical Analysis was done using SPSS 20.0 for Windows (SPSS, Inc.). Quantitative variables were analyzed using the student’s t-test or Pearson’s correlation test. Kaplan-Meier analysis was used to determine survival [19]. The log-rank test was used to compare patients’ survival between subgroups. The Cox regression model was used to perform multivariate analysis. P < 0.05 defined to be significant.
Results
Low dynamin2 protein expression in HCC is associated with worse clinical outcomes
Immunohistochemical analysis on tissue microarray was performed to measure dynamin2 protein levels in primary HCC samples from 131 postoperative HCC patients whose 5-year follow-up data were available. The clinical and pathologic characteristics of this patient cohort revealed that 57 (43.5%) patients were over 54 years of age and 108 (82.4%) were men with 112 (85.5%) infected with chronic hepatitis B. More than 70% of the patients were in the tumor-node metastasis (TNM) stage I or II, and the tumor size was larger than 5 cm in more than 60% of patients. Dynamin2 staining was mainly in the cytoplasm of HCC cells, and was rarely present in the nucleus (Figure 1A-C). Most of the stroma cells were negative staining. All the samples could be stratified into high dynamoin2 level and low dynamin2 level according to IHC staining intensity. To elucidate its biologic significance, we correlated the expressions of dynamin2 with clinicopathological characteristics of HCC. As shown in Table 1, dynamin2 expression in HCC was not related with any clinicopathologic characteristics studied except for associated with AFP (P = 0.032).
Figure 1.
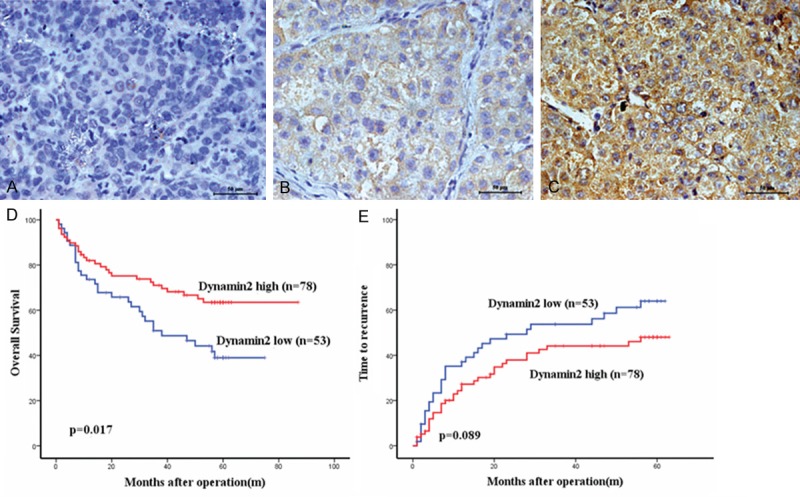
Immunohistochemistry expression of Dynamin2 in hepatocellular carcinoma (HCC) tissues and its prognostic implications for HCC patients are illustrated. (A-C) Dynamin2 staining was mainly in the cytoplasm of HCC cells, and was rarely present in the nucleus; original magnification, ×400. On the basis of these results, the expression of Dynamin2 was classified into a low expression group (negative and moderate; n = 53) and a high expression group (strong; n = 78). Curves illustrating (D) overall survival (OS) and (E) recurrence-free survival (RFS) were constructed using the Kaplan-Meier method and were evaluated using the log-rank test.
Table 1.
Correlation Between Dynamin2 Expression and Clinicopathological Characteristics
| Factors | Dynamin2 | P | |
|---|---|---|---|
|
| |||
| Negative No. | Positive No. | ||
| Gender | 0.207 | ||
| Male | 41 | 67 | |
| Female | 12 | 11 | |
| Age | 0.486 | ||
| ≤ 54 years | 25 | 32 | |
| > 54 years | 28 | 46 | |
| HBsAg | 0.174 | ||
| Negative | 5 | 14 | |
| Positive | 48 | 64 | |
| HCV-Ab | 0.686 | ||
| Negative | 52 | 75 | |
| Positive | 1 | 1 | |
| Cirr-nodul | 0.173 | ||
| None | 7 | 18 | |
| Micro | 45 | 60 | |
| AFP | 0.032 | ||
| ≤ 20 ng/ml | 14 | 35 | |
| > 20 ng/ml | 39 | 43 | |
| Thrumbi | 0.475 | ||
| No | 30 | 49 | |
| Yes | 23 | 29 | |
| Tumor size | 0.096 | ||
| < 5 cm | 13 | 30 | |
| > 5 cm | 40 | 48 | |
| Tumor number | 0.063 | ||
| Singer | 48 | 61 | |
| Multiple | 5 | 17 | |
| Encapsulation | 0.539 | ||
| Complete | 24 | 37 | |
| None | 29 | 41 | |
| Edmondson Stage | 0.822 | ||
| I/II | 39 | 56 | |
| III/IV | 14 | 22 | |
| TNM stage | 0.759 | ||
| I/II | 40 | 57 | |
| III | 13 | 21 | |
AFP, alpha-fetoprotein; TNM, tumor nodule metastasis.
At the time of the last follow-up, 64 patients had tumor recurrence, and 55 patients had died. In univariate analysis, the dynamin2 low expression group displayed significantly worse overall survival (OS) (median OS: 33.60 ± 22.615 months versus 41.01 ± 22.813 months; P = 0.017; Figure 1D) compared to dynamin2 high expression group. During the follow-up period, there were 30 deaths out of 53 patients (56.6%) of the dynamin2 low expression group compared with 25 deaths out of 78 patients (32.1%) of the dynamin2 high expression group. Univariate analysis also showed that dynamin2 high expression tended to decrease the recurrence free survival (RFS) rate, but did not reach significance (P = 0.089; Figure 1E). Vascular invasion, tumor size (≥ 5 cm), encapsulation and TNM stage were associated with both OS (P = 0.017, P = 0.000, P = 0.002, P = 0.010, P = 0.000 respectively) and RFS (P = 0.004, P = 0.006, P = 0.005, P = 0.004, respectively) (Table 2).
Table 2.
Univariate and Multivariate Analyses of Factors Associated With Recurrence and Survival*
| Factor | OS | RFS | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariate P | Hazard Ratio | Multivariate 95% CI | P | Univariate P | Hazard Ratio | Multivariate 95% CI | P | |
| Gender (male vs. female) | 0.120 | NA | 0.102 | NA | ||||
| Age (≤ 54 years vs. > 54 years) | 0.265 | NA | 0.542 | NA | ||||
| AFP (≤ 20 ng/ml vs. > 20 ng/ml) | 0.053 | NA | 0.070 | NA | ||||
| Thrumbi (No vs. Yes) | <.001 | 1.920 | 1.013-3.639 | 0.046 | 0.004 | 1.246 | 0.700-2.218 | 0.455 |
| Tumor size (≤ 5 cm vs. > 5 cm) | 0.002 | 1.628 | 0.795-3.335 | 0.182 | 0.006 | 1.697 | 0.909-3.167 | 0.097 |
| Tumor number (Singer vs. Multiple) | 0.323 | NA | 0.378 | NA | ||||
| Encapsulation (Complete vs. None) | 0.01 | 1.409 | 0.846-2.656 | 0.166 | 0.005 | 1.772 | 1.041-3.015 | 0.035 |
| Edmondson Stage (I/II vs. III/IV) | 0.276 | NA | 0.064 | NA | ||||
| TNM stage (I/II vs. III) | < .001 | 2.296 | 1.238-4.260 | 0.008 | 0.004 | 1.557 | 0.862-2.883 | 0.139 |
| Dynamin2 (Low vs. High) | 0.017 | 0.539 | 0.317-0.917 | 0.023 | 0.089 | 0.660 | 0.399-1.092 | NA |
Abbreviations: CI indcates confidence interval; TNM, tumor, node, metastasis;
Univariate analysis, Cox proportional hazards regression model.
Multivariate analysis, The Cox proportional hazards regression model was used for the multivariate analysis. Variables were adopted for their prognostic significance by univariate analysis and no obvious correlation between each other.
Multivariable analyses adjusting for the clinic pathological variables were adopted for their prognostic significance (P < 0 .05) by univariate analyses, which further indicated that the expression of dynamin2 was an independent prognostic factor for OS (P = 0.023, Table 2), while vascular invasion and tumor TNM stage were independent prognostic factors for OS (P = 0.046 and P = 0.008, respectively), and Encapsulation was an independent prognostic factor for RFS (P = 0.035).
Downregulation of dynamin2 promotes the colony formation of HCC cells
Clinical data analyses indicated that the expression of dynamin2 was an independent prognostic factor for OS, suggesting that dynamin2 low expression HCC cell has worse tumor biological features. Among HCC cell lines with relatively strong expression of dynamin2, we transfected HCCLM3 and Huh7 cells with dyamin2 siRNA. Compared with the HCCLM3 and Huh7 cells, Western blot revealed dynamin2 protein expression was downregulated in the HCCLM3-Dynamin2 RNAi and Huh7-Dyanmin2 RNAi cells (Figure 2A), which was used to perform the following studies, and were labeled as “Dynamin2 RNAi” in the figure.
Figure 2.
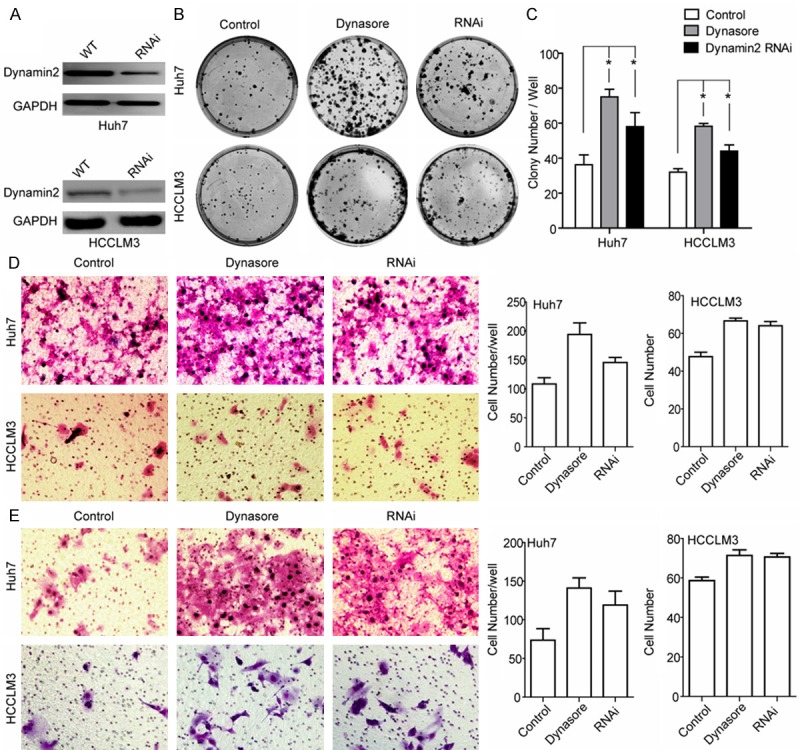
Interference with Dynamin2 by specific Dynamin2 siRNA or small molecular inhibitor Dynasore promotes HCCLM3 and Huh7 cell lines migration, colony formation and invasive potential in vitro. A. Dynamin2 expression in HCCLM3 and Huh7 cells were inhibited significantly after siRNA transfection for 72 hours. After selection with puromycin (3 ug/ml) for 48 hours, Dynamin2 protein expression levels were detected by Western blot analysis (*P < 0.05). B, C. Colony formation assay indicated that the growth rates increased in Dynamin2-silenced cells. Data shown are mean (± SD) from 3 independent experiments. D. Migration of HCCLM3 and Huh7 cells through a Matrigel-coated 8um transwell. After 48 hours, migrated cells on the bottom surface were fixed and counted after staining with Giemsa. Data shown are mean (± SD) from 3 independent experiments. 12 random fields were counted. Magnification, ×200. E. Cell migration was measured using 8 um Transwell migration assays. Values are mean (± SD) of 3 independent experiments. Magnification, ×200 *P < 0.05.
The role of dynamin2 on the HCC cells proliferation and stemness was tested by reducing dynamin2 levels via siRNA depletion or perturbing dynamin2 function with dynamin inhibitor-Dynasore [20,21], in HCCLM3 and Huh7 cell lines. As shown in Figure 2B, 2C, we found that silencing dynamin2 significantly increase HCC cells colony formation ability. Similarly, pretreatment of HCC cells with Dynasore (50 μΜ) for 30 minute, significantly promoted HCC colony formation to the same level as dynamin2 downregulation with siRNA.
Dynamin2 inhibited HCC cells migration and invasion
To examine whether cell motility was affected by dynamin2, we investigated the effect of dynamin2 on the migration and invasion of HCCLM3 and Huh7 cells with transwell permeable supports system. To test whether dynamin2 function is required for HCC cells migration, a transwell assay was used, and the results (Figure 2D) showed that both dynamin2 downregulation with siRNA (HCCLM3-Dynamin2 RNAi and Huh7-Dynamin2 RNAi cells) and the addition of the pharmacological dynamin inhibitory compounds Dynasore (pre-treated 50 μΜ, 30 min), increased migration significantly compared with control cells. Matrigel invasion studies revealed that the invasion ability of HCCLM3 and Huh7 was increased significantly when dynamin2 was downregulated or inhibited by Dynasore (Figure 2E). In conclusion, these results demonstrate that dynamin2 was associated with a modulated migration and invasion for HCCLM3 and Huh7 cells.
Dynamin2 downregulation delays EGFR endocytic trafficking and promotes EGFR signaling
Dynamin2 is a large GTPase that is essential for clathrin-dependent vesicle formation and trafficking. In view of the overexpression of EGFR in conventional HCC, and the small molecular selective inhibitor Dynasore inhibited the clathrin-dependent internalization of EGFR, we hypothesized that dynamin2 is the key negative modulator for EGFR activation and the following signaling, inhibiting HCC cell migration and invasion. To test this hypothesis, firstly, the early endosome marker EEA1 was detected in dynamin2 high and low expression conditions. The results of double immunofluorescence staining showed that the early endosome decreased significantly when dynamin2 was downregulated by siRNA or inhibited by Dynasore (Figure 3A). Secondly, the EGFR on HCC membrane was detected dynamically upon EGF stimulation by immunofluorescence. Compared with the control HCC cells, more EGFR were found on the membrane when HCC cells were pretreated by the dynasore (Figure 3B, 3C, first row) in the absence of EGF. However, after EGF-stimulated for 10 min, EGFR underwent a process of endocytosis and lower EGFR density was found in control HCC cells compared with Dynasore pretreated cells (Figure 3B, 3C, second row). Likewise, when the HCC cells were stimulated with EGF for 20 min, much more endogenous EGFR was found, in Dynasore pretreated HCC cells, diffused in the cytoplasma (Figure 3B, 3C, third row), which were close to the nucleus.
Figure 3.
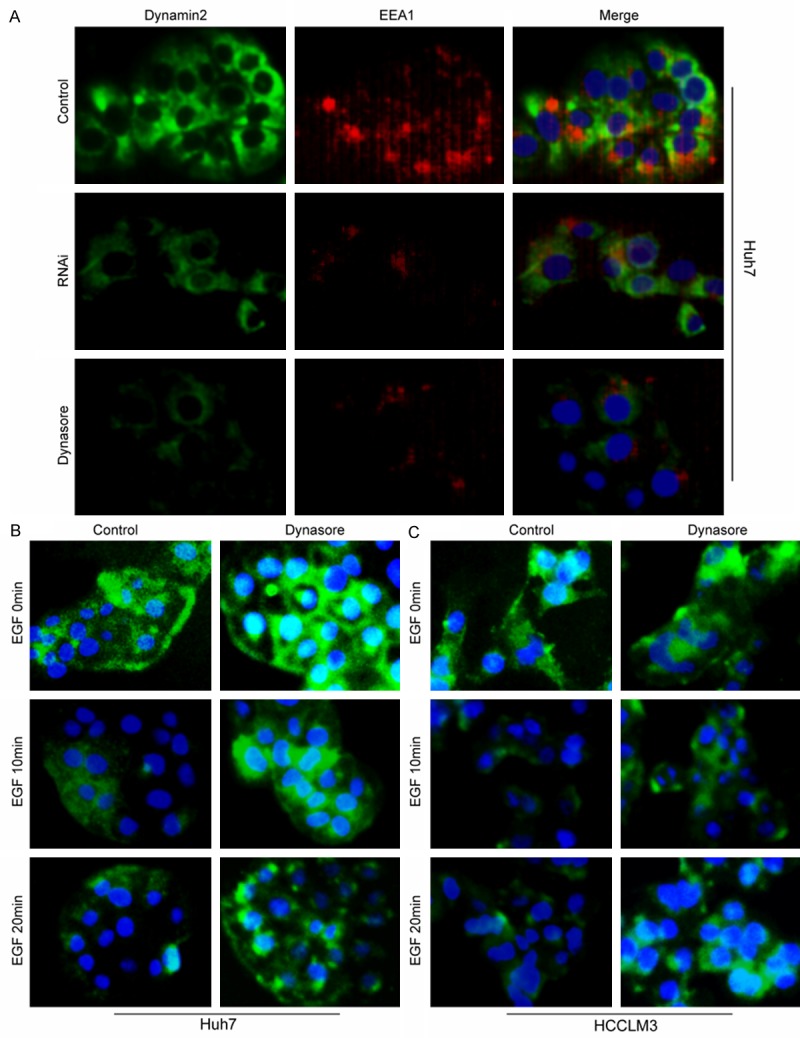
Dynamin2 and EEA1colocalized on endosomes. Huh7 cells were stained for Dynamin2 (green) and EEA1 (red) under full-serum conditions. The merged image indicated obvious overlap (yellow) between the two proteins. Bar, 50 μm (A). Dynamin2 inhibition by Dynasore blocks EGFR internalisation in HCCLM3 and Huh7 cell lines. (B, C) Huh7 and HCCLM3 cells were serum starved overnight, pre-treated with 50 μM Dynasore inhibitor for 30 min, then stimulated with 100 ng/ml of EGF for 10 and 20 min. Cells were then fixed and immunostained with mouse anti-EGFR antibody followed by Alexa-488 anti-mouse IgG. The internalisation of EGFR was analysed by microscopy; Scale bar Bar, 50 μm.
Inhibition of dynamin2 promotes ERK1/2 activation and accumulation in the nucleus
To test the effect of inhibition of dynamin2 on EGFR downstream signaling, Western blot and immunofluorescence was used to detect the p-ERK1/2 (Tyr202/Tyr204) protein levels and location. In HCCLM3 and Huh7 cells, p-ERK1/2 levels increased significantly as treated with Dynasore. Interestingly, more p-ERK1/2 was found in nuclear rather than in the cytoplasm (Supplementary Figure 1). The immunofluorescent findings were supported by the results of Western blot, the p-ERK1/2 level increased significantly in HCC cells when they were pretreated with Dynasore for 30min, and high p-ERK1/2 level maintained for a long time even the Dynasore was withdrawn (Figure 4A). Next, we detect the difference between control and Dynasore treated HCC cells stimulated with EGF. The results showed that p-ERK1/2 level, in Dynasore treated HCC cells, increased significantly and maintained longer time when compared with control HCC cells (Figure 4B).
Figure 4.
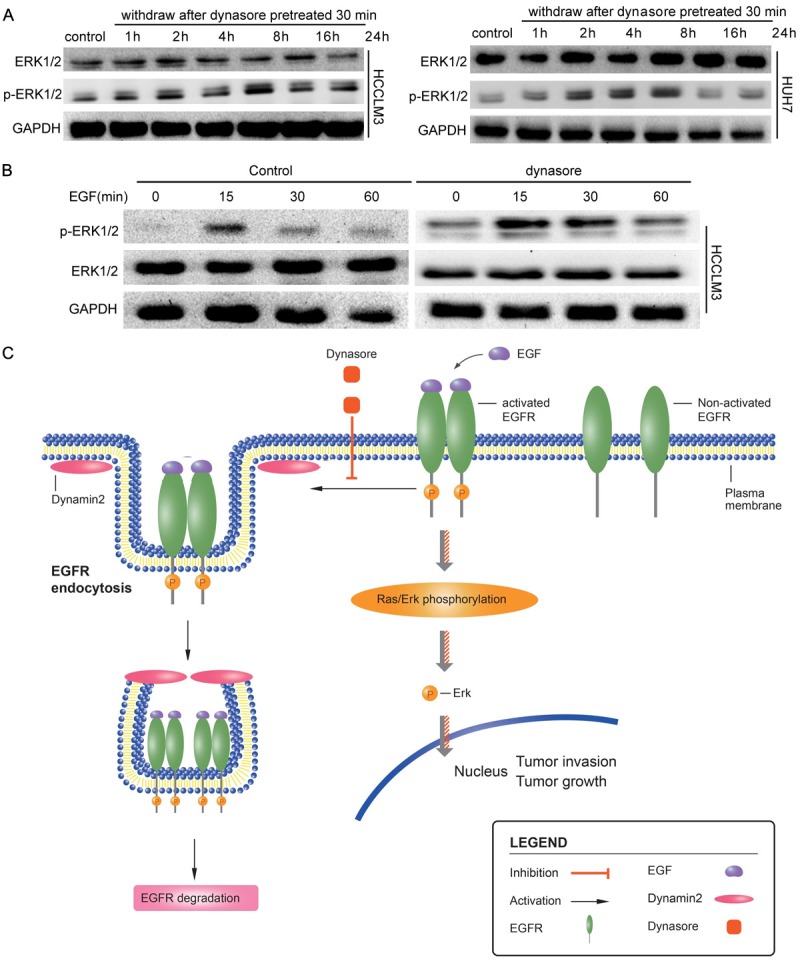
Dynamin2 inhibition by Dynasore or specific siRNA promotes ERK1/2 activation and a working model for the EGFRa-ERK1/2-Dynamin2 signaling in HCC. A. HCCLM3 and Huh7 cells were pre-treated with 50 μM Dynasore inhibitor for 30 min; Cells were then cultured with DMEM for 1 h, 2 h 4 h, 8 h, 16 h 24 h, then lysed andexamined for phosphorylation expression of phosphorylated ERK1/(T202/Y204) or total ERK1/2 by western blot analysis as described in ‘Materials and Methods’ section. B. HCCLM3 and Huh7 cells were pre-treated with 50 μM Dynasore inhibitor for 30 min. Cells were then stimulated with or without 100 ng/ml EGF for 15 min, 30 min, 60 min, lysed and examined for phosphorylation expression of phosphorylated ERK1/(T202/Y204) or total ERK1/2 by western blot analysis as described in ‘Materials and Methods’ section. C. EGFR endocytosis induced by Dynamin 2 association with its intracellular degradation. Inhibition of Dynamin2 reduce EGRF endocytosis and degradation and results in downstream effectors ERK persistent activation leading to ERK-dependent HCC growth and invasion.
Discussion
In this report, as illustrated in a working model (Figure 4C), we demonstrated a mechanism by which Dynamin2 regulates EGFR/ERK-stimulated HCC growth and invasion. By using an established in vitro HCC model that activation of the EGFR/ERK signaling promotes liver cancer cells growth and invasion in the HCCLM3 and Huh7 cell lines, we identified Dynamin2 as a modulator for EGFR-induced HCC cell migration and survival in vitro. Dynamin2 eliminated the persistent EGFR activation in HCC cell and modulated the HCC proliferation and metastasis. Inhibition of Dynamin2 by siRNA knockdown, a Dynamin2 inhibitor, promoted EGFRa-Dyn2-stimulated phosphorylation of ERK1/2, and HCC cell growth and migration, thereby establishing a link of p-ERK1/2 and Dynamin2 with EGFR activation, endocytosis and intracellular transport in HCC. Furthermore, we speculated that active EGFR are degraded or recycled with remarkable speed and precision in HCC. Dynamin2 mediated endocytosis of EGFR plays a key role in active EGFR signal vesicles reformation and degradation via lysosome/late endosome.
The acquired mutations confer new biological properties characteristic of malignant cells, which make the treatment of cancer harder than people think. Defective vesicular trafficking of growth factor receptors has emerged as a multifaceted hallmark of malignant cells. Endocytosis, the first step of intracellular vesicular trafficking, is involved in disassemble signaling and adhesion complexes. Dynamins, promoting constriction and fission of vesicle stalks, play cardinal roles in clathrin-mediated endocytosis, especially dynamin2, a ubiquitously expressed GTPase known to regulate endocytosis of EGFR and other cell surface receptors, but little is known about the role of dynamin2 in HCC progression. In the present study, we could observe the negative correlation between dynamin2 expression and OS, but we cannot show the recurrence in associated with low dynamin2 expression, even there is tendency that low dynamin2 expression has high recurrence rate. This may result from small sample size that is major limitation of this study. In vitro experiments showed that dynamin2 played important roles in HCC cell colony formation, migration and invasion. In addition, we observed that internalization of EGFR was severely reduced when cells were depleted of dynamin2 or by pre-treating of chemical dynamin inhibitor Dynasore.
Our clinical data and in vitro experiment support that downregulation of dynamin2 is favorable for HCC progression and predict poor overall survival, which is different from the role of dynamin2 in glioblastoma and pancreatic tumor [22-24].
To explore the underlying mechanism, we focus our study on the regulation of dynamin2 to cell surface receptors. In a variety of human cancers, EGFR was found overexpressed. For HCC, it was reported that overexpression of EGFR was found in around 40% to 70% of conventional HCC. So we use two methods, siRNA knockdown, or chemical dynamin inhibitor Dynasore, to inhibit dynamin2 to explore the role played by dynamin2 in the control of EGFR display on the cell surface, in regulation of EGFR activation, degradation, and in cell signaling via EGFR. We found that early endosome marker EEA1 is decreased significantly, which means the inhibition of endocytosis and formation of early endosomes. Furthermore, we detect the expression of cell surface EGFR at different timepoints after EGF stimulation. The results showed that the expression of EGFR on HCC cell surface was much higher in Dynasore pretreated cells than control cells. EGF induced endocytosis of EGFR is strongly impaired in Dynasore pretreated HCC cells, and the degradation of EGFR is decreased too, supported by the evidence that more intracellular EGFR was found in membrane and in perinuclear area in Dynasore pretreated HCC cells. This find is in according with the report of Sousa LP [25].
These experiments clearly demonstrate that inhibition of dynamin2 in favorable for the location of EGFR at the cell surface. Thinking the ERK activation is primarily mediated by the activated EGFR located at the plasma surface, and the key role of the signal for various downstream signaling cascades that control important cellular processes such as proliferation, migration and invasion [26], we postulated that dynamin2 might regulate the ERK activation through influencing the EGFR endocytosis and degradation. This hypothesis was supported by the exploration of the phosphorylation and the location of ERK under condition in which dynamin2 was inhibited. The results of immunofluorescence detection showed that the level of p-ERK was weak and the location is mainly in cytoplasma. Along with time extending, the level of p-ERK increased gradually. What’s more, p-ERK nuclear translocation was found obviously. With EGF stimulation, the p-ERK level in Dynasore pretreated HCC cells is much higher than that in control cells at the same timepoint.
The results of our study are also supported by the finding of Yoo-Young Lee in their research. They found dynamin 2 might involved in preventing invasion and metastasis in carcinoma of cervix. What is different is they attribute the phenomenon of dynamin 2 inhibiting invasion to the downregulation of MMP-2. In our study, we further demonstrate that dynamin2 promoted EGFR endocytosis and following inhibition of ERK activation, which is the key signal pathway to activate MMP-2 expression. Thinking the promoting role of dynamin2 on invasion in pancreatic tumor and glioblastoma, we wonder the role of dynamin 2 in tumor pregression is determined by the different gene expression profile, which deserves to study further to find and testify the protein interaction with dynamin2 and the functions of the complex in EGFR and other cell surface receptor degradation, sorting or recycling.
In conclusion, our study demonstrate that dynamin2, an independent predictive factor for favorable clinical outcomes, may be involved in HCC migration and invasion possibly associated with the inhibition of dynamin2 on EGFR endocytosis and inactivate of ERK signal pathway. Discovery of the means to upregulate dynamin2 expression may provide novel therapies for improve OS for HCC patients after hepatectomy.
Acknowledgements
This study was granted in part by The National Clinical Key Special Subject of China and The Nation Natural Science Foundation of China (81172275 and 21272565).
Disclosure of conflict of interest
The authors declare that they have no conflicts of interest.
Supporting Information
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruchten AE, McNiven MA. Dynamin as a mover and pincher during cell migration and invasion. J Cell Sci. 2006;119:1683–1690. doi: 10.1242/jcs.02963. [DOI] [PubMed] [Google Scholar]
- 4.Lee YY, Do IG, Park YA, Choi JJ, Song SY, Kim CJ, Kim MK, Song TJ, Park HS, Choi CH, Kim TJ, Kim BG, Lee JW, Bae DS. Low dynamin 2 expression is associated with tumor invasion and metastasis in invasive squamous cell carcinoma of cervix. Cancer Biol Ther. 2010;10:329–335. doi: 10.4161/cbt.10.4.12275. [DOI] [PubMed] [Google Scholar]
- 5.Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- 6.Sontag JM, Fykse EM, Ushkaryov Y, Liu JP, Robinson PJ, Südhof TC. Differential expression and regulation of multiple dynamins. J Biol Chem. 1994;269:4547–4554. [PubMed] [Google Scholar]
- 7.Joshi S, Perera S, Gilbert J, Smith CM, Mariana A, Gordon CP, Sakoff JA, McCluskey A, Robinson PJ, Braithwaite AW, Chircop M. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol Cancer Ther. 2010;9:1995–2006. doi: 10.1158/1535-7163.MCT-10-0161. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- 10.Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, McNiven MA. McNiven. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc Natl Acad Sci U S A. 2010;107:5863–5868. doi: 10.1073/pnas.0915123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Hirata M, Shinonome S, Wada T, Iguchi M, Dohi K, Inoue M, Ishioka Y, Hojo K, Yamada T, Sugimoto T, Masuno K, Nezasa K, Sato N, Matsuo K, Yonezawa S, Frenkel EP, Shichijo M. Preclinical Antitumor Activity of S-222611, an oral reversible tyrosine kinase inhibitor of EGFR and HER2. Cancer Sci. 2014;8:1040–1048. doi: 10.1111/cas.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Takeda T, Sakon M, Tsujimoto M, Higashiyama S, Noda K, Miyoshi E, Monden M, Matsuura N. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84:1377–1383. doi: 10.1054/bjoc.2000.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 15.Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Rosenfeld, Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- 16.Grandal MV, Zandi R, Pedersen MW, Willumsen BM, van Deurs B, Poulsen HS. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis. 2007;28:1408–1417. doi: 10.1093/carcin/bgm058. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J. Clin. Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang XW, Tang ZY. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 20.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eppinga RD, Krueger EW, Weller SG, Zhang L, Cao H, McNiven MA. Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene. 2012;31:1228–1241. doi: 10.1038/onc.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razidlo GL, Wang Y, Chen J, Krueger EW, Billadeau DD, McNiven MA. Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Dev Cell. 2013;24:573–585. doi: 10.1016/j.devcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng H, Liu KW, Guo P, Zhang P, Cheng T, McNiven MA, Johnson GR, Hu B, Cheng SY. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene. 2012;31:2691–2702. doi: 10.1038/onc.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Sousa LP, Lax I, Shen H, Ferguson SM, De Camilli P, Schlessinger J. Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc Natl Acad Sci U S A. 2012;109:4419–4424. doi: 10.1073/pnas.1200164109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Salomon, Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


