Abstract
To investigate the association between preoperative HBsAg (hepatitis B surface antigen) level and risk of HCC (hepatocellular carcinoma) recurrence following curative resection, we enrolled 826 HBV-related HCC patients who underwent curative resection and received long-term follow-up at the Eastern Hepatobiliary Surgery Hospital (Shanghai, China). Multivariate analyses showed that serum HBsAg ≥ 2000 S/CO, seropositive hepatitis B e antigen (HBeAg), γ-glutamyl transpeptidase > 61 U/L, prothrombin time > 13 s, multinodularity, lager tumor size, and major portal vein invasion were independently associated with a increased risk of HCC recurrence. Compared with HCC patients with HBsAg level < 2000 S/CO, HCC patients with HBsAg level ≥ 2000 S/CO had a higher prevalence of seropositive HBeAg, antiviral therapy, and cirrhosis; were younger; and had a higher levels of alanine transaminase (ALT), aspartate aminotransferase (AST), and HBV viral load. Multivariable stratified analyses showed HCC patients with HBsAg level < 2000 S/CO tended to have a lower incidence of HCC recurrence in following subgroups of patients, including for noncirrhotic (HR, 0.561; 95% CI, 0.345-0.914), HBV DNA < 2000 IU/mL (HR, 0.604; 95% CI, 0.401-0.912), ALT ≤ 41 U/L (HR, 0.643; 95% CI, 0.440-0.942), AST ≤ 37 U/L (HR, 0.672; 95% CI, 0.459-0.983), and seronegative HBeAg (HR, 0.682; 95% CI, 0.486-0.958). When we evaluated HBeAg-negative patients with HBV DNA < 2000 IU/mL, HBsAg level still determined risk of HCC recurrence (p = 0.014), but not HBV DNA (p = 0.550) and ALT (p = 0.186). These results suggest high levels of HBsAg increase risk of HCC recurrence following curative resection. HBsAg level might serve as a new marker to complement HBV DNA level in predicting HCC recurrence, especially in HBeAg-negative patients with low viral load.
Keywords: Hepatitis B surface antigen, hepatocellular carcinoma, recurrence
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. Hepatitis B virus (HBV) infection, especially infection with a high HBV viral load, is a major risk factor for the development of HCC [1]. Surgery is considered the standard curative treatment option for HCC. However, despite advances in surgical modalities, survival after tumor resection remains poor mainly due to persistent high incidences of HCC recurrence [2,3]. Many factors affect HCC recurrence risk after curative resection, including alanine transaminase (ALT) level; virological factors such as hepatitis B e antigen (HBeAg) status, HBV viral load, HBV genotype C, and antiviral treatment; cirrhosis; and tumor-associated variables such as serum α-fetoprotein (AFP) level, tumor number, tumor size, capsule formation, vascular invasion, Edmonson grading, and TNM stage [4-10]. Among these factors, HBV viral load is the most clinically correctable. Higher viral load has been reported to be an independent risk factor for HCC recurrence after surgery [11]. Nucleoside analogues are effective in suppressing HBV replication and ameliorating HBV-related liver disease. In addition, they have been shown to be associated with a lower risk of HCC development and postoperative HCC recurrence [8-10].
Recently, quantification of HBsAg levels has attracted much attention as an important marker for evaluating viral activity. Throughout the natural history of HBV infection, HBsAg levels vary markedly across different phases of chronic HBV infection and across different HBV genotypes [12-15]. Previous studies have shown a positive correction between HBsAg level and HBV viral load [16]. A lower HBsAg level was also shown to be associated with a higher chance of HBsAg loss and a lower risk of hepatitis activity in patients who were infected HBV genotype B or C [17]. One study has proposed that an HBsAg level < 1000 IU/mL and an HBV DNA level < 2000 IU/mL can be used together as a marker for inactive HBV genotype D carriers [15]. Furthermore, another recent study has shown that high levels of HBsAg increase the risk of HCC in patients with low HBV load [16]. However, it is still unclear whether higher levels of HBsAg increase the risk for HBV-related HCC recurrence following curative resection, especially in patients with low viremia.
To address this issue, we enrolled 826 HBV-related HCC patients who underwent curative resection and received long-term follow-up into the present study and sought to elucidate the association between HBsAg level at time of tumor resection and risk of HCC recurrence following resection in this cohort.
Materials and methods
Patient cohort
We identified all hospitalized patients who were admitted with a primary diagnosis of HCC and received curative liver resection (Admission Code: M81700/3) from Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University (Shanghai, China) between October 2009 and August 2010. The diagnosis of HCC was confirmed by pathology.
Only HCC patients with HBV infection were included in our study cohort. We applied the following exclusion criteria: diagnosis of hepatitis C or other viral hepatitis; presence of malignant tumor; lack of data on tumor recurrence; tumor rupture before or during operation; and patients who received liver resection, transarterial chemoembolization, percutaneous ethanol injection, radiofrequency ablation, or liver transplantation before the index hospitalization. Patients with HCC recurrence in the first 3 months after the index hospitalization for liver resection were also excluded. Death before the recurrence of HCC was defined as competing mortality, and such patients were also excluded.
We first identified 1665 potentially eligible HCC patients who received curative tumor resection. Using our inclusion and exclusion criteria (Figure 1), we excluded 839 patients because of seronegative HBsAg (n = 205), infection with HCV (hepatitis C virus) (n = 8), tumor rupture before or during operation (n = 47), receiving liver tumor therapy before enrollment (n = 146), having intrahepatic recurrence and extrahepatic metastasis within the first 3 months after the index hospitalization for liver resection (n = 218) [8], death prior to recurrence (n = 73), or no data on tumor recurrence (n = 142). Therefore, 826 HBV-related HCC patients were enrolled into the study. The project was approved by the Eastern Hepatobiliary Surgery Hospital Ethical Committee, China. All patients gave written informed consent to participate. The data do not contain any information that could identify the patients.
Figure 1.

Flow chart of study cohort selection. HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; RFA, radiofrequency ablation; TAE, therapeutic arterial embolization; TACE, transcatheter arterial chemoembolization.
Biochemical and serological markers
Antibody against HBsAg (anti-HBs), HBeAg, antibody against HBeAg (anti-HBe), and antibody against HBcAg (anti-HBc) were tested using a radioimmunoassay kit (Roche, Mannheim, Germany). Antibody against HCV (anti-HCV) was measured by means of a second-generation enzyme immunoassay (Chemclin Biotech Co., Ltd., China). Serum biochemical tests were performed by a systemic multiautoanalyzer (Technicon SMAC, Technicon Instruments Corp., Tarrytown, NY). Serum alpha-fetoprotein (AFP) level was also measured by a radioimmunoassay (Serono Diagnostic SA, Coinsins, Switzerland).
Quantification of HBsAg and HBV DNA
Serum HBsAg was tested using a radioimmunoassay kit (Roche, Mannheim, Germany). The range of detection was from 0 S/CO to 4000 S/CO. HBV DNA was extracted from 100 μL of serum using a standard commercial diagnostic kit for the quantification of hepatitis B viral DNA (Shanghai Kehua Laboratory System Co., Ltd., China) according to the manufacturer’s instructions with a final elution volume of 2 μL. The extracted DNA was then quantified with the same kit on the ABI7500 Fast Real-time PCR System (Applied Biosystems, Mortlake, USA) according to the manufacturer’s instructions. To ensure the specificity of the test and to prevent diagnostic errors, the limit of detection was defined as 1000 IU/mL.
Follow-up
All patients were regularly followed for AFP measurement, ultrasonography (USG), and/or computed tomography (CT) or magnetic resonance imaging (MRI) scan every one to two months for the first six months after operation and every three months afterwards. Tumor recurrence was suspected when there was a progressive elevation of serum AFP and/or ultrasonographic evidence of a new hepatic lesion that was confirmed by dynamic CT scan, MRI or position emission tomography (PET). HCC recurrence was defined as re-hospitalization with a primary diagnosis of HCC after the index admission date and a treatment modality for HCC recurrence, such as surgery, transarterial chemoembolization, percutaneous ethanol injection, radiofrequency ablation, or liver transplantation, during the study period. Disease-free survival was measured from the date of surgery to the date of recurrence. Follow-up of patients was continued until HCC recurrence, death, or August 5, 2013. Causes of death were also investigated.
Statistical methods
Descriptive analyses of the variables were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Univariate analyses were performed using the Chi-squared test for categorical variables and the independent-samples t-test for discrete variables. Cumulative incidences were calculated using the Kaplan-Meier method. Clinicopathological prognostic factors were evaluated using the univariate Kaplan–Meier method and compared with the log-rank test to identify the prognostic predictors for recurrence. Multivariate regression analysis was performed using Cox proportional hazards models to identify the independent prognostic factors for recurrence. Variables to be entered into the multivariate analysis were selected based on the results of the univariate analyses (p < 0.1). In order to compare the predictive values of different factors for HCC recurrence, receiver operating characteristic (ROC) curve analysis was used to compute the area under the ROC curves for different factors. The performances of the factors in predicting HCC recurrence were compared using the Chi-squared test for categorical variables and the t-test for discrete variables. A value of p < 0.05 was considered statistically significant.
Results
Clinicopathological characterization of HCC patients
The demographic, biochemical, virological, surgical, and pathological data of the 826 patients are shown in Table 1. There were 717 men (86.8%) and 109 women (13.2%). The median age was 51.1 (range, 13-79) years. The median hospital stay was 15.2 (range, 7-72) days. Of the 826 patients, 232 (28.1%) were seropositive for HBeAg, 534 (64.6%) had cirrhosis, and 341 (41.3%) had alanine transarninase (ALT) levels > 41 U/L. Serum HBsAg levels in most patients (72.4%) were ≥ 4000 S/CO. HBV DNA levels in nearly half of the patients (44.6%) were < 2000 IU/mL. The tumor stage distribution according to the 7th edition of the AJCC/UICC staging system was as follows: stage I, 468 (56.7%); stage II, 221 (26.8%); stage III, 135 (16.3%); and stage IVA, 2 (0.2%). A total of 691 patients had accurate information on history of antiviral therapy. Of the 691 patients, 483 patients (69.9%) received antiviral therapy.
Table 1.
Characterization of 826 Patients with HCC
| Variables | Patients, n (%) |
|---|---|
| Patient demographics | |
| Gender | |
| Male | 717 (86.8) |
| Female | 109 (13.2) |
| Age (years) | |
| < 50 | 361 (43.7) |
| 50-59 | 293 (35.5) |
| ≥ 60 | 172 (20.8) |
| Cirrhosis | 534 (64.6) |
| Diabetes | 46 (5.6) |
| Metformin usea | 8 (1.0) |
| Viral factors | |
| Anti-HBV therapy (n = 691)b | 483 (69.9) |
| Serum HBsAg level (S/CO) | |
| < 1000 | 99 (12.0) |
| 1000-1999 | 38 (4.6) |
| 2000-2999 | 42 (5.1) |
| 3000-3999 | 49 (5.9) |
| ≥ 4000 | 598 (72.4) |
| Seropositive HBeAg | 232 (28.1) |
| Serum HBV DNA level (IU/mL) (n = 773)b | |
| < 1000 | 309 (40.0) |
| 1000-1999 | 36 (4.7) |
| 2000-19999 | 102 (13.2) |
| 20000-199999 | 132 (17.1) |
| ≥ 200,000 | 194 (25.1) |
| Biochemical factors | |
| ALT (> 41 U/L) | 341 (41.3) |
| AST (> 37 U/L) | 333 (40.3) |
| TBIL (> 18.8 μmol/L) | 146 (17.7) |
| r-GT (> 61 U/L) | 391 (47.3) |
| ALP (> 129 U/L) | 97 (11.7) |
| ALB (< 34 g/L) | 14 (1.7) |
| Prealbumin (< 170 g/L) | 211 (25.5) |
| PT (> 13 s) | 122 (14.8) |
| WBC (< 4.0*109/L) | 155 (18.8) |
| PLT (< 100.0*109/L) | 153 (18.5) |
| Tumor characteristics | |
| AFP (> 20 μg/L) | 491 (59.4) |
| Tumor number | |
| single | 677 (82.0) |
| multiple | 149 (18.0) |
| Tumor size (cm) | |
| ≤ 3 | 259 (31.4) |
| 3~5 | 272 (32.9) |
| 5~10 | 216 (26.2) |
| > 10 | 79 (9.6) |
| Portal vein invasion | 62 (7.5) |
| Cutting margin ≥ 1 cm (n = 590)b | 112 (19.0) |
| Histo-pathological findings | |
| Capsule formation | 691 (83.7) |
| Edmonson grading | |
| I | 9 (1.1) |
| II | 199 (24.1) |
| III | 598 (72.4) |
| IV | 20 (2.4) |
| Microvascular invasion | 261 (31.6) |
| pTNM stage | |
| I | 468 (56.7) |
| II | 221 (26.8) |
| III | 135 (16.3) |
| IVA | 2 (0.2) |
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; WBC, white blood cell; PLT, platelet; AFP, alpha-fetoprotein; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; PT, prothrombin time; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Drug users indicate patients using a drug at least 1 day pen month on average.
Number of available data.
Cumulative incidence of HCC recurrence following curative resection
During a follow-up of 35.8 ± 9.9 months, 395 patients (47.8%) developed recurrent HCC. Cumulative incidence of HCC recurrence at 1 year and 3 years after curative resection was 25.5% and 46.6%, respectively. Most recurrences (80.0%) clustered within two years of surgery (early recurrence). The tumor stage according to the 7th edition of the AJCC/UICC staging system was as follows: stage I, 468 (56.7%); stage II, 221 (26.8%); stage III, 135 (16.3%); and stage IVA, 2 (0.2%). The survival curves according to AJCC/UICC staging showed significant differences between stages I, II, III, and IV (p < 0.001). Univariate analysis revealed that HBeAg seropositivity, and higher levels of ALT, serum HBsAg, and HBV viral load were associated with a higher cumulative incidence of HCC recurrence (Figure 2A-D). In addition, other risk factors, including aspartate aminotransferase (AST) > 37 U/L,peptidase (GT) > 61 U/L, alkaline phosphatase (ALP) > 129 U/L, prothrombin time (PT) > 13 s, AFP > 20 μg/L, multinodularity, larger tumor size, cutting margin ≥ 1 cm, no capsule formation, portal vein invasion, microvascular invasion, and higher rank of Edmonson grading were found to be associated with HCC recurrence (Table 2). Those patients who received antiviral therapy tended to have a lower incidence of HCC recurrence than those who did not receive antiviral therapy, although the difference was not statistically significant (p = 0.158). In Supplementary Table 1, we stratified patients by the time of tumor recurrence. We found that antiviral therapy was significantly associated with a lower risk of HCC late recurrence (p = 0.049), but not early recurrence (p = 0.475) (Figure 2E, 2F).
Figure 2.

Cumulative incidences of hepatocellular carcinoma (HCC) recurrence following liver resection. (A-D) hepatitis B e antigen (HBeAg) seropositivity (A), and higher levels of serum hepatitis B surface antigen (HBsAg) (B), hepatitis B virus (HBV) viral load (C), and alanine transaminase (ALT) (D) were associated with a higher cumulative incidence of HCC recurrence. (E, F) Antiviral therapy was significantly associated with low risk of HCC late recurrence (E), but not HCC early recurrence (F).
Table 2.
Univariate and multivariate analysis of factors associated with HCC recurrence
| Factor | N | Recurrence rate (%) | Univariate analysis (p value) | Multivariate Analysis (p value) | Hazard ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1-year | 3-year | ||||||
| Gender | 0.255 | NA | NA | NA | |||
| Male | 717 | 26.1 | 47.3 | ||||
| Female | 109 | 22.0 | 42.2 | ||||
| Age | 0.531 | NA | NA | NA | |||
| < 50 | 361 | 26.6 | 48.8 | ||||
| 50-59 | 293 | 27.0 | 45.4 | ||||
| ≥ 60 | 172 | 20.9 | 44.2 | ||||
| Cirrhosis | 0.178 | NA | NA | NA | |||
| Yes | 534 | 25.8 | 48.5 | ||||
| No | 292 | 25.0 | 43.2 | ||||
| Diabetes | 0.441 | NA | NA | NA | |||
| Yes | 46 | 23.9 | 52.2 | ||||
| No | 780 | 25.6 | 46.3 | ||||
| Metformin usea | 0.574 | NA | NA | NA | |||
| Yes | 8 | 12.5 | 37.5 | ||||
| No | 818 | 25.7 | 46.7 | ||||
| Anti-HBV therapy (n = 282)b | 0.158 | NA | NA | NA | |||
| Yes | 216 | 26.4 | 44.4 | ||||
| No | 66 | 34.8 | 51.5 | ||||
| Serum HBsAg level (S/CO) | 0.006 | 0.026 | 1.538 | 1.054-2.243 | |||
| < 2000 | 137 | 21.2 | 36.5 | ||||
| ≥ 2000 | 689 | 26.4 | 48.6 | ||||
| Seropositive HBeAg | 0.032 | 0.022 | 1.424 | 1.052-1.928 | |||
| Negative | 594 | 26.3 | 44.3 | ||||
| Positive | 232 | 23.7 | 52.6 | ||||
| Serum HBV DNA level (IU/ml) (n = 773)c | 0.013 | 0.910 | 1.017 | 0.761-1.359 | |||
| < 2000 | 345 | 22.0 | 42.6 | ||||
| ≥ 2000 | 428 | 28.3 | 50.0 | ||||
| ALT (U/L) | 0.014 | 0.804 | 0.960 | 0.693-1.329 | |||
| ≤ 41 | 485 | 23.7 | 42.5 | ||||
| > 41 | 341 | 28.2 | 52.5 | ||||
| AST (U/L) | < 0.001 | 0.857 | 1.033 | 0.727-1.467 | |||
| ≤ 37 | 493 | 20.7 | 41.6 | ||||
| > 37 | 333 | 32.7 | 54.1 | ||||
| TBIL (μmol/L) | 0.285 | NA | NA | NA | |||
| ≤ 18.8 | 680 | 24.9 | 45.7 | ||||
| > 18.8 | 146 | 28.8 | 50.7 | ||||
| γ-GT (U/L) | < 0.001 | 0.003 | 1.561 | 1.164-2.093 | |||
| ≤ 61 | 435 | 18.2 | 36.1 | ||||
| > 61 | 391 | 33.8 | 58.3 | ||||
| ALP (U/L) | 0.036 | 0.053 | 0.648 | 0.417-1.006 | |||
| ≤ 129 | 729 | 24.0 | 45.5 | ||||
| > 129 | 97 | 37.1 | 54.6 | ||||
| AFP (μg/L) | 0.020 | 0.546 | 1.092 | 0.821-1.453 | |||
| ≤ 20 | 335 | 17.9 | 43.0 | ||||
| > 20 | 491 | 30.8 | 49.1 | ||||
| ALB (g/L) | 0.195 | NA | NA | NA | |||
| < 34 | 14 | 14.3 | 28.6 | ||||
| ≥ 34 | 812 | 25.7 | 46.9 | ||||
| Prealbumin (mg/L) | 0.118 | NA | NA | NA | |||
| < 170 | 211 | 33.2 | 50.7 | ||||
| ≥ 170 | 615 | 22.9 | 45.2 | ||||
| PT (s) | 0.043 | 0.025 | 1.451 | 1.047-2.011 | |||
| ≤ 13 | 704 | 25.1 | 45.0 | ||||
| > 13 | 122 | 27.9 | 55.7 | ||||
| WBC (*109/L) | 0.676 | NA | NA | NA | |||
| < 4 | 155 | 22.6 | 45.8 | ||||
| ≥ 4 | 671 | 26.2 | 46.8 | ||||
| PLT (*109/L) | 0.280 | NA | NA | NA | |||
| < 100 | 153 | 26.1 | 51.6 | ||||
| ≥ 100 | 673 | 25.4 | 45.5 | ||||
| Tumor number | < 0.001 | 0.003 | 1.621 | 1.183-2.222 | |||
| single | 677 | 21.0 | 42.2 | ||||
| multiple | 149 | 46.3 | 66.4 | ||||
| Tumor size (cm) | < 0.001 | 0.012 | 1.219 | 1.044-1.424 | |||
| ≤ 3 | 259 | 13.1 | 35.1 | ||||
| 3~5 | 272 | 23.5 | 44.5 | ||||
| 5~10 | 216 | 33.3 | 56.9 | ||||
| > 10 | 79 | 51.9 | 63.3 | ||||
| Capsule formation | < 0.001 | 0.149 | 0.787 | 0.569-1.089 | |||
| Yes | 691 | 22.7 | 44.6 | ||||
| No | 135 | 40.0 | 57.0 | ||||
| Edmonson grading | 0.032 | 0.521 | 0.911 | 0.684-1.212 | |||
| I | 9 | 11.1 | 22.2 | ||||
| II | 199 | 13.1 | 39.7 | ||||
| III | 598 | 29.6 | 49.2 | ||||
| IV | 20 | 35.0 | 50.0 | ||||
| Portal vein invasion | < 0.001 | 0.007 | 1.832 | 1.184-2.836 | |||
| Yes | 62 | 59.7 | 79.0 | ||||
| No | 764 | 22.8 | 44.0 | ||||
| Microvascular invasion | < 0.001 | 0.083 | 1.285 | 0.968-1.705 | |||
| Yes | 261 | 41.0 | 58.6 | ||||
| No | 565 | 18.4 | 41.1 | ||||
| Cutting margin (cm) (n = 590)c | 0.001 | 0.119 | 0.735 | 0.499-1.082 | |||
| < 1 | 478 | 25.9 | 46.7 | ||||
| ≥ 1 | 112 | 12.5 | 30.4 | ||||
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; WBC, white blood cell; PLT, platelet; AFP, alpha-fetoprotein; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; PT, prothrombin time; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Drug users indicate patients using a drug at least 1 day pen month on average.
Only determined in patients with HBV DNA≥ 2000IU/mL and recieving antivirual therapy within 1 month pre-operation or within 3 months post-operation.
Number of available data.
Multivariate analyses revealed that HBsAg level (95.0% CI: 1.054-2.243, p = 0.026), HBeAg status (95.0% CI: 1.052-1.928, p = 0.022), γ-GT (95.0% CI: 1.164-2.093, p = 0.003), PT (95.0% CI: 1.047-2.011, p = 0.025), tumor number (95.0% CI: 1.183-2.222, p = 0.003), tumor size (95.0% CI: 1.044-1.424, p = 0.012), and portal vein invasion (95.0% CI: 1.184-2.836, p = 0.007) were independent prognostic factors affecting cumulative incidence of HCC recurrence following curative resection (Table 2).
Correlation of serum HBsAg levels at time of resection with clinicopathological features
To further understand the impact of serum HBsAg levels at time of resection on HCC recurrence, the clinicopathological characteristics between HCC patients with HBsAg levels < 2000 S/CO and HCC patients with HBsAg levels ≥ 2000 S/CO were compared. Compared with HCC patients with lower HBsAg levels, those with HBsAg levels ≥ 2000 S/CO had a higher prevalence of seropositive HBeAg, history of antiviral therapy, and cirrhosis; were younger; and had higher levels of ALT, AST, and serum HBV viral load. There appeared to be a higher percentage of patients with prealbumin levels < 170 mg/L in the high-HBsAg group than in the low-HBsAg group, although the statistical significance of the difference was just above the threshold (p = 0.054) (Table 3). Other factors including sex, hospital stay, diabetes, metformin use, PLT, WBC count, ALB, TBIL, γ-GT, ALP, and all the tumor-associated characteristics had no statistically significant difference between the two groups (p > 0.05) (Supplementary Table 2).
Table 3.
Comparison of clinicopathological features of HCC patients according to the serum HBsAg level
| HBsAg level (S/CO) | p value | ||
|---|---|---|---|
|
|
|||
| < 2000 (n = 137) | ≥ 2000 (n = 689) | ||
| Age (mean ± SD, years) | 54.6 ± 10.4 | 50.4 ± 10.6 | < 0.001 |
| Cirrhosis (%) | 72 (52.6) | 462 (67.1) | 0.001 |
| Anti-HBV therapy (%) (n = 691)a | < 0.001 | ||
| No | 61 (52.1) | 147 (25.6) | |
| Yes | 56 (47.9) | 427 (74.4) | |
| Seropositive HBeAg (%) | 25 (18.2) | 207 (30.0) | 0.005 |
| Serum HBV DNA level (IU/mL) (%) (n = 773)a | < 0.001 | ||
| ≥ 2000 | 36 (28.8) | 392 (60.5) | |
| < 2000 | 89 (71.2) | 256 (39.5) | |
| ALT (U/L) | 0.010 | ||
| > 41 | 43 (31.4) | 298 (43.3) | |
| ≤ 41 | 94 (68.6) | 391 (56.7) | |
| AST (U/L) | 0.020 | ||
| > 37 | 43 (31.4) | 290 (42.1) | |
| ≤ 37 | 94 (68.6) | 399 (57.9) | |
| Prealbumin (mg/L) | 0.054 | ||
| < 170 | 26 (19.0) | 185 (26.9) | |
| ≥ 170 | 111 (81.0) | 504 (73.1) | |
HCC: hepatocellular carcinoma; HBV: hepatitis B virus; HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B e antigen; ALT: alanine transaminase; AST: aspartate aminotransferase.
Number of available data.
Multivariable stratified analysis
To eliminate confounding factors and further investigate the impact of perioperative HBsAg level on postoperative HCC recurrence, we stratified the patients according to HBeAg status, cirrhosis status, and levels of ALT, AST, and HBV viral load. We found that an HBsAg level < 2000 S/CO was associated with a reduced risk of HCC recurrence in the following categories: noncirrhotic (HR, 1.782; 95% CI, 1.094-2.901), HBV DNA < 2000 IU/mL (HR, 1.655; 95% CI, 1.097-2.497), ALT ≤ 41 U/L (HR, 1.554; 95% CI, 1.062-2.275), AST ≤ 37 U/L (HR, 1.489; 95% CI, 1.017-2.180), and seronegative HBeAg (HR, 1.466; 95% CI, 1.044-2.059) (Figure 3).
Figure 3.
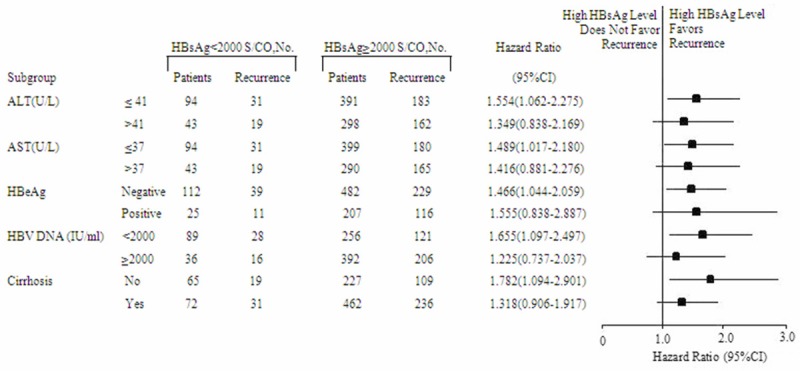
Multivariable stratified analyses on the association between HBsAg (hepatitis B surface antigen) level and HCC (hepatocellular carcinoma) recurrence. HCC patients with HBsAg levels < 2000 S/CO had lower cumulative incidences of recurrence than those with higher HBsAg levels when alanine transaminase (ALT) level was ≤ 41 U/L, aspartate aminotransferase (AST) level was ≤ 37 U/L, hepatitis B e antigen (HBeAg) status was seronegative, hepatitis B virus (HBV) DNA level was < 2000 IU/mL, or cirrhosis was absent.
Factors affecting HCC recurrence risk in HBeAg-negative patients with low viral load
In HBeAg-negative patients with low viral load (HBV DNA level < 2000 IU/mL), univariate analysis showed that HCC recurrence was associated with diabetes, HBsAg level ≥ 2000 S/CO, AST level > 37 U/L, γ-GT level > 61 U/L, prealbumin level < 170 mg/L, multinodularity, larger tumor size, cutting margin ≥ 1 cm, lack of capsule formation, portal vein invasion, microvascular invasion, and higher rank of pTNM stage but not with ALT or HBV DNA level (Figure 4A-C). Of this population, noncirrhotic patients appeared to have a lower cumulative incidence of HCC recurrence than cirrhotic patients, although the difference was not statistically significant (p = 0.098) (Table 4). In HBeAg-negative and noncirrhotic patients with low viral load, we found that higher HBsAg level was associated with late recurrence of HCC (p = 0.032) (Figure 4D) but not with early recurrence. HBV DNA level was not associated with either early (p = 0.206) or late recurrence (p = 0.557).
Figure 4.
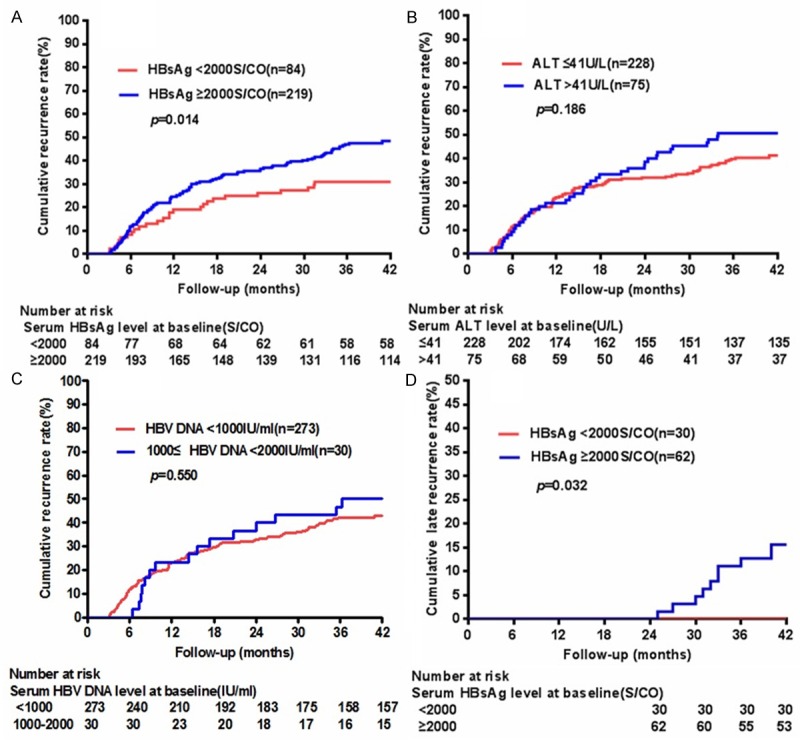
HCC recurrence in HBeAg-negative patients with low viral load. HCC recurrence was associated with HBsAg level ≥ 2000S/CO (A), but not with ALT (B) or HBV DNA level (C). (D) In HBeAg-negative and noncirrhotic patients with low viral load, higher HBsAg level was associated with late recurrence of HCC.
Table 4.
Univariate and multivariate analysis of factors associated with HCC recurrence in HBeAg negative patients with low viral load
| Factor | n | Recurrence rate (%) | Univariate analysis (p value) | Multivariate Analyses (p value) | Hazard ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1-year | 3-year | ||||||
| Gender | 0.331 | NA | NA | NA | |||
| Male | 253 | 24.5 | 43.5 | ||||
| Female | 50 | 16.0 | 40.0 | ||||
| Age | 0.670 | NA | NA | NA | |||
| < 50 | 118 | 22.9 | 39.8 | ||||
| 50-59 | 105 | 24.8 | 45.7 | ||||
| ≥ 60 | 80 | 21.3 | 43.8 | ||||
| Cirrhosis | 0.098 | 0.075 | 1.550 | 0.957-2.509 | |||
| Yes | 170 | 24.7 | 47.6 | ||||
| No | 133 | 21.1 | 36.8 | ||||
| Diabetes | 0.001 | 0.188 | 1.649 | 0.783-3.472 | |||
| Yes | 13 | 15.4 | 84.6 | ||||
| No | 290 | 23.4 | 41.0 | ||||
| Serum HBsAg level (S/CO) | 0.014 | 0.107 | 1.549 | 0.910-2.638 | |||
| < 2000 | 84 | 19.0 | 31.0 | ||||
| ≥ 2000 | 219 | 24.7 | 47.5 | ||||
| Serum HBV DNA level (IU/ml) | 0.550 | NA | NA | NA | |||
| < 1000 | 273 | 23.1 | 42.5 | ||||
| 1000-1999 | 30 | 23.3 | 46.7 | ||||
| ALT (U/L) | 0.186 | NA | NA | NA | |||
| ≤ 41 | 228 | 23.7 | 40.4 | ||||
| > 41 | 75 | 21.3 | 50.7 | ||||
| AST (U/L) | 0.045 | 0.483 | 0.818 | 0.465-1.436 | |||
| ≤ 37 | 226 | 20.4 | 39.8 | ||||
| > 37 | 77 | 31.2 | 51.9 | ||||
| TBIL (μmol/L) | 0.918 | NA | NA | NA | |||
| ≤ 18.8 | 252 | 22.2 | 43.3 | ||||
| > 18.8 | 51 | 27.5 | 41.2 | ||||
| γ-GT (U/L) | < 0.001 | 0.001 | 2.346 | 1.430-3.849 | |||
| ≤ 61 | 181 | 17.1 | 30.4 | ||||
| > 61 | 122 | 32.0 | 61.5 | ||||
| ALP (U/L) | 0.103 | NA | NA | NA | |||
| ≤ 129 | 269 | 21.9 | 41.6 | ||||
| > 129 | 34 | 32.4 | 52.9 | ||||
| AFP (μg/L) | 0.140 | NA | NA | NA | |||
| ≤ 20 | 135 | 14.8 | 40.0 | ||||
| > 20 | 168 | 29.8 | 45.2 | ||||
| ALB (g/L) | 0.285 | NA | NA | NA | |||
| < 34 | 2 | 0.0 | 0.0 | ||||
| ≥ 34 | 301 | 23.3 | 43.2 | ||||
| Prealbumin (mg/L) | 0.006 | 0.383 | 1.265 | 0.746-2.147 | |||
| < 170 | 59 | 35.6 | 57.6 | ||||
| ≥ 170 | 244 | 20.1 | 39.3 | ||||
| PT (s) | 0.392 | NA | NA | NA | |||
| ≤ 13 | 270 | 21.9 | 42.2 | ||||
| > 13 | 33 | 33.3 | 48.5 | ||||
| WBC (*109/L) | 0.825 | NA | NA | NA | |||
| < 4 | 51 | 25.5 | 45.1 | ||||
| ≥ 4 | 252 | 22.6 | 42.5 | ||||
| PLT (*109/L) | 0.805 | NA | NA | NA | |||
| < 100 | 44 | 25.0 | 45.5 | ||||
| ≥ 100 | 259 | 22.8 | 42.5 | ||||
| Tumor number | < 0.001 | 0.941 | 1.020 | 0.606-1.716 | |||
| single | 250 | 19.2 | 38.4 | ||||
| multiple | 53 | 41.5 | 64.2 | ||||
| Tumor size (cm) | < 0.001 | 0.230 | 1.178 | 0.901-1.541 | |||
| ≤ 3 | 95 | 8.4 | 30.5 | ||||
| 3~5 | 99 | 20.2 | 39.4 | ||||
| 5~10 | 82 | 35.4 | 56.1 | ||||
| > 10 | 27 | 48.1 | 59.3 | ||||
| Capsule formation | 0.002 | 0.007 | 0.488 | 0.291-0.819 | |||
| Yes | 244 | 20.9 | 39.8 | ||||
| No | 59 | 32.2 | 55.9 | ||||
| Edmonson grading | 0.071 | 0.649 | 0.897 | 0.563-1.431 | |||
| I | 6 | 16.7 | 33.3 | ||||
| II | 70 | 10.0 | 32.9 | ||||
| III | 216 | 25.9 | 45.4 | ||||
| IV | 11 | 54.5 | 63.6 | ||||
| Portal vein invasion | < 0.001 | 0.812 | 1.080 | 0.571-2.043 | |||
| Yes | 29 | 55.2 | 79.3 | ||||
| No | 274 | 19.7 | 39.1 | ||||
| Microvascular invasion | < 0.001 | 0.001 | 2.215 | 1.367-3.589 | |||
| Yes | 104 | 42.3 | 58.7 | ||||
| No | 199 | 13.1 | 34.7 | ||||
| Cutting margin (cm) (n = 224)a | 0.004 | 0.067 | 0.470 | 0.210-1.055 | |||
| < 1 | 187 | 25.7 | 45.5 | ||||
| ≥ 1 | 37 | 10.8 | 18.9 | ||||
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; WBC, white blood cell; PLT, platelet; AFP, alpha-fetoprotein; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; PT, prothrombin time; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Number of available data.
Discussion
Although the association between HBsAg level and risk for HBV-related HCC development has been previously documented, the impact of HBsAg level on HBV-related HCC recurrence following curative resection remains unclear. In the present study, we not only showed that a higher level of HBsAg was associated with a higher cumulative incidence of postoperative HCC recurrence but also found that HBsAg level among HBeAg-negative patients with low viral load, but not HBV DNA or ALT level, was still predictive of recurrence risk. These data suggested that HBsAg level might complement HBV DNA level in predicting HCC recurrence, especially in HBeAg-negative patients with low viral load.
Surgical resection has been an effective cure for some HCC patients. However, postoperative prognosis is poor because of the high recurrence rate. The 1- and 3-year cumulative incidences of HCC recurrence following surgical resection have been reported to be 9.4%-30.1% and 36.9%-62.3%, respectively [5,6,18-21]. These values are similar to those of our study. Many factors are reported to be associated with postoperative HCC recurrence, including hepatic functional parameters such as albumin level, PT, and Child-Pugh class; cirrhosis; and tumor characteristics such as AFP level, tumor number, tumor size, capsule formation, vascular invasion, Edmonson grading, and pTNM stage [4-7]. In the current study, we demonstrated that a higher cumulative incidence of postoperative HCC recurrence was associated with the following disease characteristics: elevated levels of ALT, AST, γ-GT, ALP, PT, and AFP, multinodularity, larger tumor size, cutting margin < 1 cm, lack of capsule formation, portal vein invasion, microvascular invasion, and higher rank of Edmonson grading or pTNM stage.
Recently, the roles of virological factors such as HBV viral load, HBeAg status, and antiviral therapy in HCC recurrence have also been investigated [8-11]. Kubo et al first reported that high viral load is an independent risk factor for HCC recurrence after liver resection in HBV-related HCC patients [22]. Hung et al found that HBV viral load of > 2000 IU/mL is associated with HCC recurrence after liver resection with an odds ratio as high as 22.3 [11]. Our data also suggested that higher HBV viral load is significantly associated with an increased risk of postoperative HBV-related HCC recurrence. In addition, Sun et al reported that seropositive HBeAg is associated with higher risk of early recurrence and is an independent risk factor for postoperative recurrence of HBV-related HCC [23], and a recent meta-analysis study further confirmed the result [24]. Our data also demonstrated that HBeAg positivity is a significant independent risk factor for HCC recurrence. Nucleoside analogues have been shown to be associated with a lower risk of HCC and other cirrhosis-related complications in those with chronic hepatitis and cirrhosis [25-28]. Several recent studies further demonstrated that nucleoside analogues are associated with a lower risk of recurrence among patients with HBV-related HCC after liver resection [8-10]. Our data suggested that nucleoside analogues may significantly reduce the risk of HCC late recurrence and further confirmed that antiviral therapy may play an important role in reducing HCC recurrence after curative resection.
HBsAg is produced by HBV and serves as an important serological marker for the diagnosis and monitoring of patients with chronic hepatitis B. Recently, HBsAg quantification has become increasingly recognized as an important method for evaluating viral activity and possible host immune control over HBV infection as well as predicting HBsAg loss and virological response to antiviral therapy [12,14,17,29-32]. A lower HBsAg level was shown to be associated with a higher chance of HBsAg loss and lower risk of hepatitis activity in patients with HBV genotype B or C infection [15,17]. There is also a known positive correlation between HBsAg and HBV DNA levels [16]. This correlation has been shown to be higher at the HBeAg-positive phase, lower at the HBeAg-negative phase, and lowest at the lowly replicative phase [13,14]. In our cohort, HCC patients with higher levels of HBsAg also had a higher prevalence of seropositive HBeAg as well as a higher level of HBV DNA and hepatitis activity. Another recent study suggested that a high level of HBsAg may increase the risk of HCC in patients with low HBV load [16]. Because a higher HBsAg level usually signifies a worse prognosis, it is of clinical interest to know whether a higher HBsAg level would also indicate a higher risk of HCC recurrence. Very recently, Sohn et al first reported that HBsAg levels were associated with late recurrence after curative resection in HBV-related HCC [33]. Huang et al further found a preoperative HBsAg level of 1000 IU/mL or greater is an independent risk factor for HCC recurrence in patients with low HBV DNA levels. In this study, we also found that a higher HBsAg level was an independent risk factor associated with HCC recurrence after curative resection [34]. Multivariable stratified analyses showed that HCC patients with higher HBsAg levels tended to have a higher incidence of HCC recurrence in several subgroups of patients with the following characteristics: noncirrhotic; low viral load; normal ALT or AST; and seronegative HBeAg. These observations further confirmed the association between higher levels of HBsAg and increased risk of HCC recurrence in HBV-related HCC patients after tumor resection. Furthermore, when we evaluated patients with negative HBeAg and low viral load, HBsAg level was still predictive of recurrence risk, while HBV DNA or ALT levels were not. These data suggested that HBsAg level might complement HBV DNA level in predicting HCC recurrence, especially in HBeAg-negative patients with low viral load.
There are several limitations to the present study. First, the unit of HBsAg level is S/CO in our study instead of IU/mL. Thus, we could not use the cutoff value of ≥ 1000 IU/mL, the usefulness of which has been confirmed in previous studies [15,16,35,36]. We recalculated the cutoff level of HBsAg with the unit of S/CO based on the ROC curves and found that Youden’s index (YI) reached a maximum near HBsAg = 2000 S/CO. Second, we did not exclude patients who received antiviral therapy when we determined whether higher levels of HBsAg were associated with increased risk of recurrence in HBeAg-negative patients with low viral load. In this study, more patients in the high-HBsAg cohort had received antiviral therapy than in the low-HBsAg cohort. Because antiviral therapy is associated with lower risk of HCC recurrence, the higher baseline HBsAg level in the patients who received antiviral therapy may have led to a more conservative estimation of the association in the present study.
In conclusion, we found that higher levels of HBsAg were associated with a higher risk of HCC recurrence among patients with HBV-related HCC after curative resection. Among HBeAg-negative patients with low viral load, HBsAg level was still predictive of recurrence risk, but HBV DNA and ALT levels were not. Therefore, HBsAg level may be used as an additional marker to complement HBV DNA level in predicting HCC recurrence, especially in HBeAg-negative patients with low viral load.
Acknowledgements
This project is supported by National key scientific instrument and equipment development grant 2012YQ220113 (H.H.) and Natural Science Foundation of China (NSFC) grants 81372672 (H.H.) after highlight test.
Disclosure of conflict of interest
None to disclose.
Supporting Information
References
- 1.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011;26:1354–1360. doi: 10.1111/j.1440-1746.2011.06812.x. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 6.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 9.Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, Yamamoto Y, Nakanishi M, Kohara T, Sho T, Yamamoto K, Horimoto H, Kobayashi T, Yokoo H, Matsushita M, Todo S, Asaka M. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44:991–999. doi: 10.1007/s00535-009-0093-z. [DOI] [PubMed] [Google Scholar]
- 10.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, Wong J, Lee KF, Lai PB, Chan HL. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 11.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 12.Brunetto MR. A new role for an old marker, HBsAg. J Hepatol. 2010;52:475–477. doi: 10.1016/j.jhep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Jaroszewicz J, Calle SB, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, Romagnoli V, Cherubini B, Moscato G, Maina AM, Cavallone D, Bonino F. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–490. doi: 10.1053/j.gastro.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149. e13–e14. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Tseng TC, Liu CJ, Su TH, Wang CC, Chen CL, Chen PJ, Chen DS, Kao JH. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141:517–525. 521–525. doi: 10.1053/j.gastro.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 20.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Yamamoto T, Ikebe T, Wakasa K, Nishiguchi S, Kinoshita H. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88:1016–1024. doi: 10.1002/(sici)1097-0142(20000301)88:5<1016::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, Ren N, Zhuang PY, Zhu XD, Fan J, Tang ZY. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47:684–690. doi: 10.1016/j.jhep.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Qu LS, Zhu J, Chen H, Jin F, Ni RZ, Lu CH. Effects of hepatitis B e-antigen on recurrence of hepatitis B-related hepatocellular carcinoma after curative resection: A meta-analysis. Hepatol Res. 2013;43:347–354. doi: 10.1111/j.1872-034X.2012.01079.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, Chan HY, Wong VW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients With liver cirrhosis. Hepatology. 2013;58:1537–47. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 26.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kobayashi M, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 27.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57:399–408. doi: 10.1002/hep.25937. [DOI] [PubMed] [Google Scholar]
- 28.Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, Fu SY, Wu MC. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 29.Manesis EK, Hadziyannis ES, Angelopoulou OP, Hadziyannis SJ. Prediction of treatment-related HBsAg loss in HBeAG-negative chronic hepatitis B: a clue from serum HBsAg levels. Antivir Ther. 2007;12:73–82. [PubMed] [Google Scholar]
- 30.Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S, Wolf E, McCloud P, Batrla R, Marcellin P. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–1150. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- 31.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, Valla D, Vidaud M, Nicolas-Chanoine MH, Marcellin P. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 32.Tangkijvanich P, Komolmit P, Mahachai V, Sa-Nguanmoo P, Theamboonlers A, Poovorawan Y. Comparison between quantitative hepatitis B surface antigen, hepatitis B e-antigen and hepatitis B virus DNA levels for predicting virological response to pegylated interferon-alpha-2b therapy in hepatitis B e-antigen-positive chronic hepatitis B. Hepatol Res. 2010;40:269–277. doi: 10.1111/j.1872-034X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 33.Sohn W, Paik YH, Kim JM, Kwon CH, Joh JW, Cho JY, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21:2429–2435. doi: 10.1245/s10434-014-3621-x. [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Lau WY, Zhou WP, Shen F, Pan ZY, Yuan SX, Wu MC. Prediction of Hepatocellular Carcinoma Recurrence in Patients With Low Hepatitis B Virus DNA Levels and High Preoperative Hepatitis B Surface Antigen Levels. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, Chen CJ. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J. Clin. Oncol. 2010;28:2437–2444. doi: 10.1200/JCO.2009.27.4456. [DOI] [PubMed] [Google Scholar]
- 36.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


