Abstract
Hypoxia-inducible factor (HIF)-1α is the key cellular survival protein under hypoxia, and is associated with tumor progression and angiogenesis. We have recently shown the inhibitory effects of minocycline on ovarian tumor growth correlated with attenuation of vascular endothelial growth factor (VEGF) and herein report a companion laboratory study to test if these effects were the result of HIF-1α inhibition. In vitro, human ovarian carcinoma cell lines (A2780, OVCAR-3 and SKOV-3) were utilized to examine the effect of minocycline on HIF-1 and its upstream pathway components to elucidate the underlying mechanism of action of minocycline. Mice harboring OVCAR-3 xenografts were treated with minocycline to assess the in vivo efficacy of minocycline in the context of HIF-1. Minocycline negatively regulated HIF-1α protein levels in a concentration-dependent manner and induced its degradation by a mechanism that is independent of prolyl-hydroxylation. The inhibition of HIF-1α was found to be associated with up-regulation of endogenous p53, a tumor suppressor with confirmed role in HIF-1α degradation. Further studies demonstrated that the effect of minocycline was not restricted to proteasomal degradation and that it also caused down-regulation of HIF-1α translation by suppressing the AKT/mTOR/p70S6K/4E-BP1 signaling pathway. Minocycline treatment of mice bearing established ovarian tumors, led to suppression of HIF-1α accompanied by up-regulation of p53 protein levels and inactivation of AKT/mTOR/p70S6K/4E-BP1 pathway. These data reveal the therapeutic potential of minocycline in ovarian cancer as an agent that targets the pro-oncogenic factor HIF-1α through multiple mechanisms.
Keywords: Minocycline, HIF-1, p53, AKT, mTOR, VEGF, ovarian cancer
Introduction
Hypoxia-induced stress response has been implicated in propagation of human malignancies including ovarian cancer. Outgrowth of solid tumors beyond their vasculature results in hypoxia which promotes tumor transition towards a more aggressive phenotype with increased proliferation, invasiveness and metastasis [1]. HIF-1, the central regulator of hypoxia response, is a heterodimer protein comprising an oxygen-regulated subunit (HIF-1α) and a constant constitutively expressed subunit HIF-1β. Hydroxylation of HIF-1α proline residues under normoxic conditions results in poly-ubiquitination and subsequent proteasomal degradation of HIF-1α protein. Under hypoxia, the absence of oxygen blocks the hydroxylases from modifying HIF-1α thus allowing HIF-1α to accumulate [2]. HIF-1α can also be regulated independent of oxygen availability. p53 loss, the most frequent oncogenic mutation in human ovarian tumors [3], is known to be associated with increased HIF-1α levels [4]. It has been shown that p53 promotes ubiquitination and proteasomal degradation of HIF-1α as well as decreasing its transcriptional activity [5]. HIF-1α is also controlled by multiple oncogenic signaling pathways including AKT/mammalian target of rapamycin (mTOR) cascade which is known to be frequently up-regulated in ovarian cancer [6]. Activation of AKT/mTOR in response to cytokines, growth factors and other oncogenes leads to phosphorylation and activation of ribosomal protein S6 kinase (p70S6K) and eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) which in turn lead to enhancement of HIF-1α mRNA translation thus allowing HIF-1α accumulation [7,8]. Convincing data supports over-expression of HIF-1α in ovarian tumors correlated with high malignant pathological grades [9] and poor prognosis [10,11]. Specifically, HIF-1α has been defined critical in promotion of tumor cell invasiveness [12] and angiogenesis in ovarian cancer [13]. It directly activates the expression of the pro-angiogenic factor VEGF thus promoting neoangiogenesis to restore the supply of oxygen and nutrients enabling continuous tumor growth [14]. Based on these detrimental effects, HIF-1α represents an important target for antitumor intervention.
Minocycline (7-dimethylamino-6-desoxytertracycline) is a safe antibiotic from the second-generation tetracycline family. Preclinical evidence strongly supports the anti-tumor and anti-metastatic activities of minocycline and a number of other tetracyclines in various tumors [15]. Along this line we have recently communicated the preliminary results demonstrating the inhibitory effects of minocycline on tumor growth [16] and malignant ascites formation in ovarian cancer [17]. We have also established that in preclinical models of ovarian cancer, minocycline attenuates invasiveness of tumor cells [18] along with inhibition of VEGF expression [17]. Since minocycline influences the expression of VEGF, which is regulated by HIF-1α and also suppresses tumorigenic cellular processes which are, at least in part, HIF-1α mediated, we postulated that minocycline exerts these effects through modulation of HIF-1α.
To test this notion, we used ovarian carcinoma cell lines, A2780 (p53 wild type), OVCAR-3 (p53 mutated at its DNA binding domain) and SKOV-3 (p53 null) [19] as model systems to investigate the effects and mechanisms of minocycline in the context of HIF-1. Our results revealed that minocycline decreases HIF-1α expression and stability associated with enhancement of p53 and suppression of AKT/mTOR/p70S6K/4E-BP1 cascade. To confirm our in vitro observations, next we conducted in vivo-based investigations in an experimental model of ovarian cancer in mice. The results clearly show minocycline-induced inhibition of tumoral HIF-1α and AKT/mTOR/p70S6K/4E-BP1 pathway, accompanied by up-regulation of p53 protein. The evidence presented here point to the huge potential of minocycline in dismantling the oncogenic transcription factor HIF-1α in ovarian cancer.
Materials and methods
Chemicals and antibodies
Unless otherwise stated, all drugs and chemicals used in this study were obtained from Sigma-Aldrich (Australian subsidiary). The following primary antibodies were used throughout this study: rabbit polyclonal antibodies specific for HIF-1α, HIF-1β, p53, AKT, phospho (p)-AKT (Ser473), p-mTOR (Ser2448), mTOR, p-p70S6K (Thr389) , p70S6K, p-4E-BP1 (Thr37/46), 4E-BP1, p21 and BAX (Cell Signaling Technology, Sydney, Australia) and mouse Monoclonal anti-β-actin (R&D Systems). Secondary antibodies were goat anti rabbit or anti mouse immunoglobulin G conjugated with horseradish peroxidase (HRP, Santa Cruz Biotechnology).
Cell culture
The human ovarian cancer cell lines; A2780, OVCAR-3 and SKOV-3 cells, were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in RPMI 1640 medium with 2 mM l-glutamine, 2 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1 mM sodium pyruvate (Invitrogen, Sydney, Australia) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin (50 U/ml) at 37°C in a humidified atmosphere containing 5% CO2 as suggested by the manufacturer. Cell lines were routinely assessed by cell morphology and their average doubling time.
In vitro hypoxia tests
At 60% to 80% confluency, cells were treated with the indicated concentrations of minocycline (ranging from 0 to 100 μmol/L) dissolved in 5% FBS media before being placed in a sealed modular hypoxic chamber (Billups-Rothenburg, Del Mar, CA) flushed with 1% O2, 5% CO2 and 94% N2. The chamber was then placed in an incubator at 37°C for 4 h. For induction of chemical hypoxia, the same procedure was used, except that instead of placement in hypoxic chamber, cells were treated with the chemical hypoxic agent desferrioxamine (DFO, 50 μM) for 4 h. Cells not exposed to hypoxia or hypoxia-mimetic agents were run in parallel as controls. Following treatment, cells were subjected to immunocytochemistry or western blotting to determine HIF-1α and HIF-1β protein expressions.
Immunocytochemistry staining
Immunofluorescent staining was conducted as previously described [17]. Immunostaining of HIF-1α and HIF-1β was carried out on cells seeded on sterilized cover slips and exposed to minocycline (0/100 µM) followed by incubation in normoxia or hypoxia for 4 h. Expression of p53 was examined in cells subjected to the indicated treatments with minocycline. Immunostained cells were analyzed for protein expression using Olympus IX71 laser scanning microscopy with × 60 oil immersion lens. Images were acquired and processed with FV1200 software.
Western blot analysis
50 μg of cellular lysate (collected as previously described [17]) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Following electro transfer, polyvinylidene difluoride membrane (Millipore Corporation) was probed with specific antibodies. Immune complexes were detected using HRP conjugated with either anti-mouse or anti-rabbit followed by chemiluminescence detection (Perkin Elmer Cetus). To show equal protein loading, blots were stripped and re-probed with specific antibodies recognizing β-actin.
Establishment of i.p. xenografts and assessment of minocycline activities
Female nude athymic Balb C nu/nu mice (6 weeks old) were purchased from Biological Resources (Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia). The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions in facilities approved by the University of New South Wales Animal Care and Ethics Committee (ACEC). All procedures carried out on mice were in strict accordance with the protocol approved by ACEC (approval number: 9/23B), and all efforts were made to minimize suffering. I.p. tumors were established according to the method previously described [20]. Briefly, 10 × 106 log-phase growing OVCAR-3 cells suspended in 0.5 mL PBS were injected intraperitoneally to each mouse. On day 28 after cell inoculation, mice were randomly assigned to one of the treatment or control groups (8 mice per group). Minocycline was dissolved in sterile normal saline (0.6 mg/mL). Mice were injected intraperitoneally with a single dose of minocycline (30 mg/kg). Control group received sterile normal saline instead. At the end of treatment periods (4 or 24 h), peritoneal cavity was washed according to the procedure explained earlier [17]. Then animals were euthanized using Lethabarb R (100 mg/kg) i.p. injection (VIRBAC) and tumors were immediately dissected and preserved at -80°C for later analysis. Whole-cell tumor lysates were used to determine HIF-1α, p53, AKT, p-AKT, p-mTOR, mTOR, p-p70S6K, p70S6K, p-4-EBP1 and 4E-BP1 expression.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism Software version 6.0. Data are presented as mean ± SD. The student t test was used to compare 2 independent group means. One-way ANOVA was used to determine the statistical differences between more than 2 groups; a significant interaction was interpreted by a subsequent simple effects analysis with Bonferroni correction. Statistical significance was established at the P < 0.05 level.
Results
Effects of minocycline on accumulation of HIF-1α during hypoxia
To investigate the effect of minocycline on HIF-1α expression in ovarian cancer cells exposed to hypoxia, immunofluorescent staining was performed after 4 h treatment of A2780 and OVCAR-3 cell lines with minocycline (100 μM) under either normal (21%) or low (1%) oxygen concentrations. The results demonstrated that minocycline significantly decreased the HIF-1α surge induced by hypoxia in both ovarian cancer cell lines. However, as expected HIF-1β expression was not affected by hypoxia or minocycline (Figure 1A).
Figure 1.
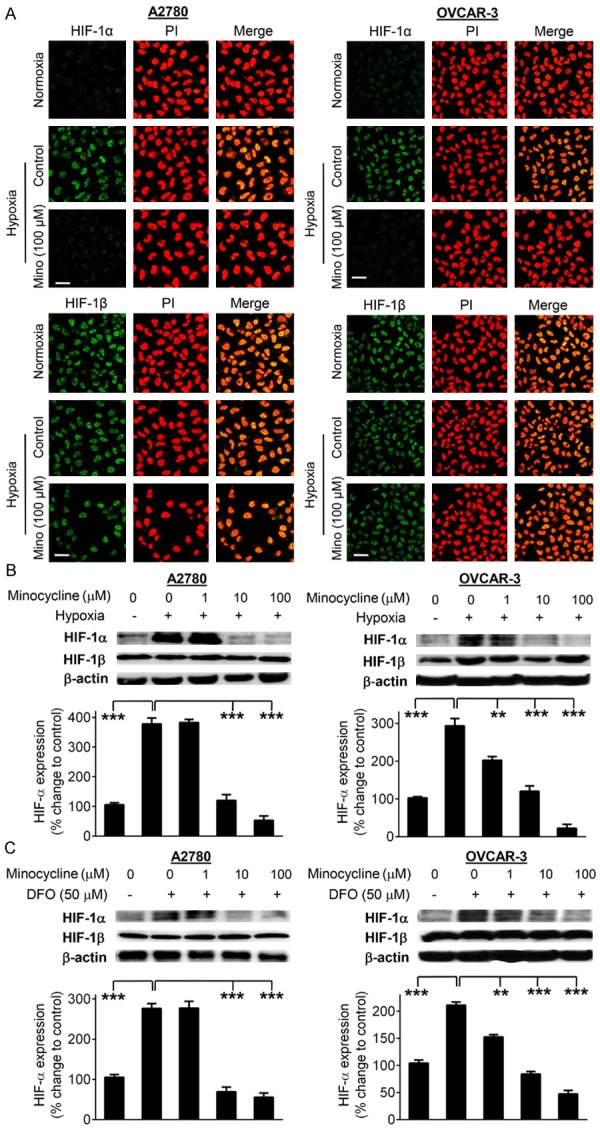
Minocycline inhibits HIF-1α expression induced by hypoxia or hypoxia mimetic agent DFO. A. Representative confocal images of HIF-1α or HIF-1β (green) in A2780 and OVCAR-3 cells subjected to normoxic or hypoxic conditions. The cells under hypoxia were either vehicle-treated or exposed to minocycline (100 μM) for 4 h. Cells were also stained with propidium iodide (red). Images were obtained at 60 × magnification. The scale bars represent 50 µm. B. Cellular levels of HIF-1α and HIF-1β proteins in A2780 and OVCAR-3 cells treated with indicated concentrations of minocycline for 4 h in normoxia/hypoxia conditions as examined by Western blotting. C. Western blot analysis of HIF-1α and HIF-1β proteins in A2780 and OVCAR-3 cells exposed to varying concentrations of minocycline in the presence or absence of DFO (50 μM). Densitometric analysis of immunoblotting data was carried out using the Quantity One image program (Bio-Rad). The values are normalized to β-actin as loading control and are expressed as the percentage of control group. Columns, means of three independent experiments; bars, SD. **P < 0.01 and ***P < 0.001 vs. control cells.
Results obtained from Western blot analysis also show significant inhibitory effect of minocycline at 10 and 100 μM concentrations on hypoxia-induced HIF-1α expression in A2780 and OVCAR-3 cells (p < 0.001). HIF-1β expression in the whole cell extract remained unchanged after exposure to hypoxia and/or minocycline (Figure 1B).
Next utilizing the hypoxia mimetic agent DFO, chemically induced hypoxia was used to obtain further evidence on the effect of minocycline on cellular HIF-1α expression. Exposure of cells to DFO, led to a significant increase in HIF-1α protein expression (p < 0.001). Pre-treatment of cells with minocycline led to concentration-dependent reduction in HIF-1α level. Consistent with previous results, HIF-β levels were not changed in cells subjected to DFO and/or minocycline treatment (Figure 1C).
Minocycline decreases HIF-1α stability
Regulation of HIF-1α seems to occur principally at the protein level by HIF-1α stabilization [21]. To determine the possible mechanism by which minocycline regulates HIF-1α expression, we first examined the effect of minocycline on HIF-1α stability using cycloheximide (CHX) to inhibit new protein synthesis in the cells. A2780 and OVCAR-3 cells were treated with CHX or CHX plus minocycline as indicated in Figure 2A. The half-life of HIF-1α protein was 5.2 min and 4.6 min in A2780 and OVCAR-3 cells respectively when the cells were treated with CHX alone. Pretreatment with minocycline significantly decreased HIF-1α protein half-life in cells exposed to CHX (2 min in A2780 cells and 2.7 min in OVCAR-3) (Figure 2B). These data suggest that minocycline inhibits HIF-1α expression at least in part through decreasing HIF-1α protein stability.
Figure 2.
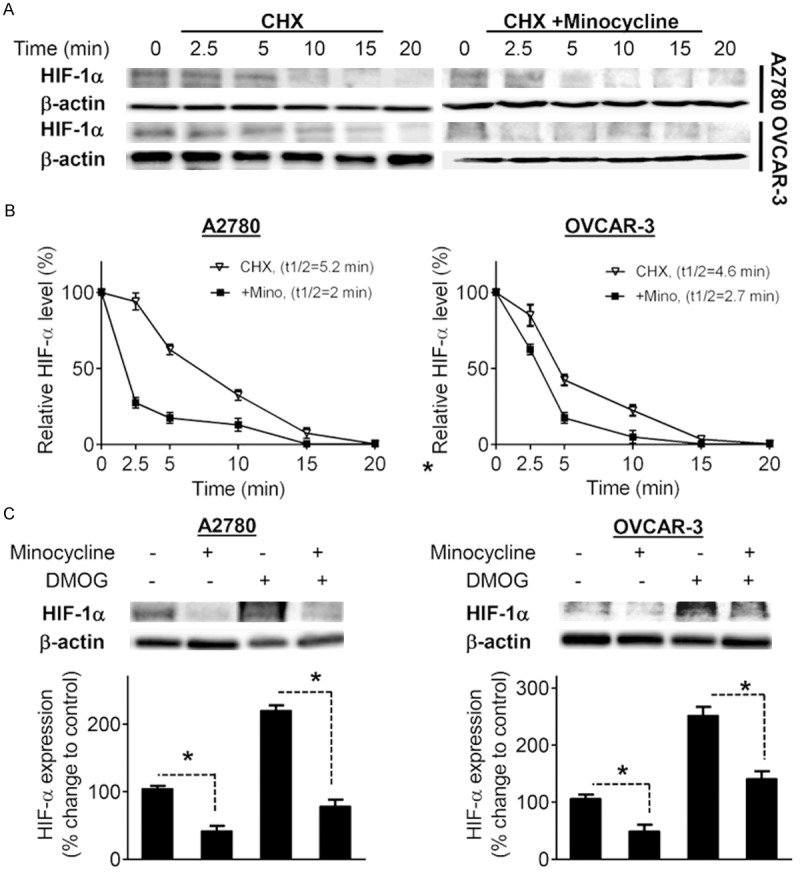
Minocycline decreases HIF-1α protein stability independent of prolyl-hydroxylase enzyme. A. A2780 and OVCAR-3 cells pretreated with vehicle or minocycline (100 μM) for 30 min, were exposed to CHX (100 μM). Whole cell extracts were collected at different time points and subjected to Western blot analysis using HIF-1α antibody. B. The densitometric values quantified using the Quantity One image program (Bio-Rad) were corrected based on β-actin and were expressed as the percentage of the values corresponding to the HIF-1α protein levels at time zero. C. Western blot analysis of HIF-1α protein in A2780 and OVCAR-3 cells pretreated with vehicle or minocycline (100 μM) for 1 h and then exposed to DMOG (5 mM) or vehicle for an additional 4 h. β-actin, loading control. Densitometric values of the bands obtained by using the Quantity One image program (Bio-Rad) were corrected and expressed relative to that of control cells which was set as 100. Columns, means of three independent experiments; bars, SD. *P < 0.05 vs. vehicle-treated cells.
The effect of minocycline on HIF-1α is hydroxylase-independent
Oxygen dependent proteolysis is the primary means of regulating HIF-1α stability [2]. In normoxia, hydroxylation of prolyl residues 402 and 564 at the oxygen-dependent degradation domain of HIF-1α triggers its association with von Hippel Lindau protein, leading to HIF-1α degradation via ubiquitin-proteasome pathway [22,23]. To examine whether the inhibitory effect of minocycline on HIF-1α is dependent on classical oxygen sensing pathway, we pretreated A2780 and OVCAR-3 cells with the prolyl hydroxylase inhibitor dimethyloxaloylglycine (DMOG) prior to exposure to minocycline. As expected, treatment with DMOG led to overexpression of HIF-1α in both A2780 and OVCAR-3 cells (Figure 2C), however DMOG did not block the inhibitory effect of minocycline on HIF-1α expression. These results suggest that the inhibition of HIF-1α by minocycline is regardless of oxygen sensing through hydroxylation of HIF-1α.
Minocycline up-regulates p53 expression
Accumulated evidence supports the critical role of the tumor suppressor p53 in determining HIF-1α stability [5,24,25]. p53 functions to promote HIF-1α proteasomal degradation [5,24,25] as well as inhibition of HIF transcriptional activity [5,26]. Interestingly, mutation of p53 in its DNA binding domain retains its ability to block HIF-1α expression [27] and transcriptional activities [28]. Therefore, we next investigated whether minocycline-induced inhibition of HIF-1α is associated with up-regulation of p53 in both A2780 (p53-wild) and OVCAR-3 (p53-mutated) cells. Immunocytochemistry staining (Figure 3A) and Western blot analysis (Figure 3B) revealed that minocycline markedly increased the endogenous expression of p53 in both cell lines in a concentration-dependent fashion.
Figure 3.
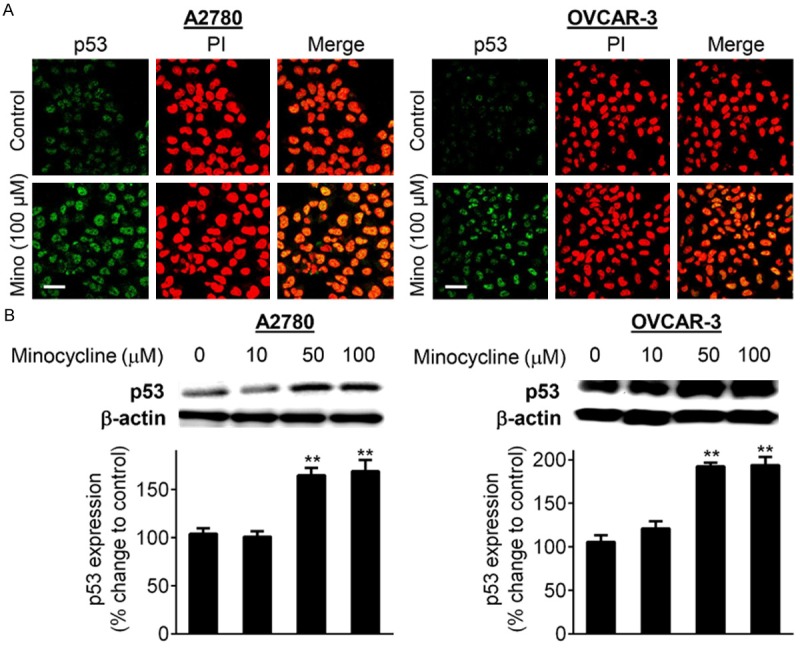
Minocycline up-regulated the expression of p53. A. Confocal imaging analysis of p53 (green) in A2780 and OVCAR-3 cells under control conditions and exposed to minocycline (100 μM) for 24 h. Cells were also stained with propidium iodide (red). Images were obtained at 60 × magnification. The scale bars represent 50 μm. B. Cellular levels of p53 in A2780 and OVCAR-3 cells treated with increasing concentrations of minocycline for 24 h as examined by Western blotting. Densitometric analysis of immunoblotting data was carried out using the Quantity One image program (Bio-Rad). The values are normalized to β-actin as loading control and are expressed as the percentage of vehicle-treated group. Columns, means of three independent experiments; bars, SD. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control cells.
Minocycline affects HIF-1α expression in p53-null cells
Because p53 accumulates with minocycline, we considered that this might be the mechanism behind attenuation of HIF by minocycline. To test this notion we utilized SKOV-3, an ovarian cancer cell line that is p53 null [19]. Immunofluorescent staining revealed that minocycline could effectively block HIF-α expression in these cells under hypoxia (Figure 4A). This was also confirmed by Western blot analysis showing the concentration-dependent decrease in HIF-α under low oxygen condition (Figure 4B). In line with this, minocycline suppressed the HIF-α surge induced by the hypoxia mimetic agent DFO in a concentration-dependent fashion in these cells (Figure 4C). These data suggest that the accumulation of p53 cannot completely explain the effects of minocycline on HIF-1.
Figure 4.
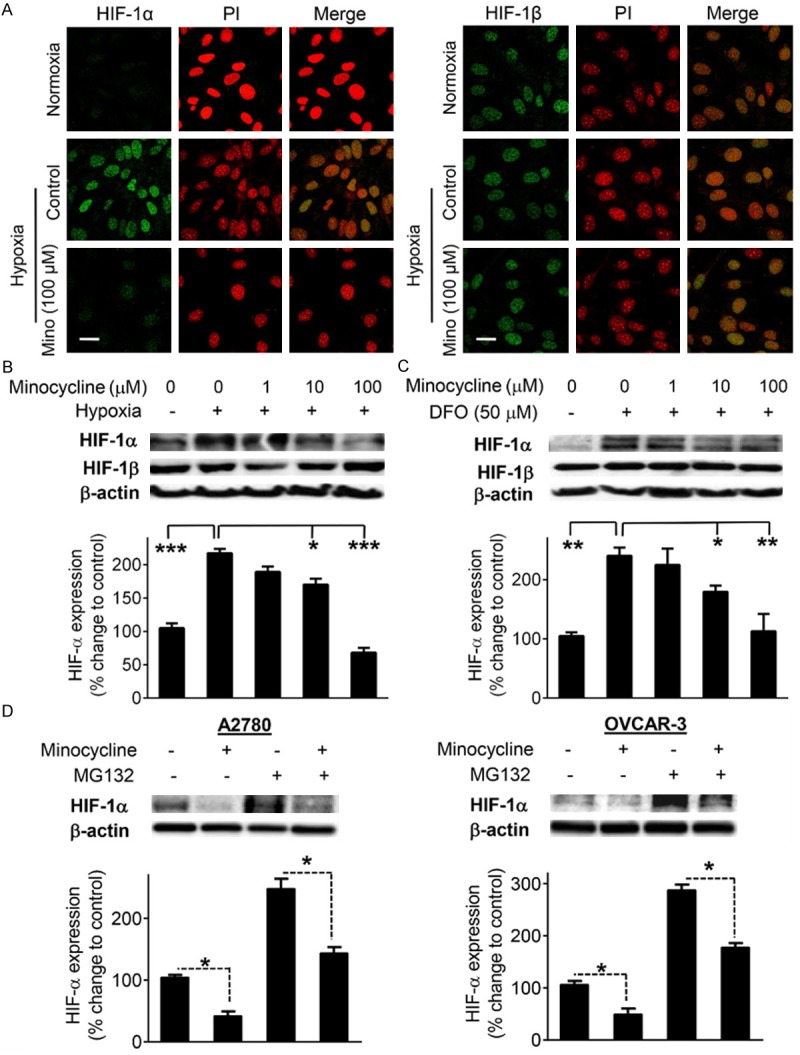
Minocycline suppresses HIF-1α protein independent of p53 status or proteasomal degradation. A. Confocal immunocytochemistry of HIF-1α and HIF-1β (green) in SKOV-3 cells subjected to specified treatments for 4 h. The nuclei are counterstained with propidium iodide (red). Images were obtained at 60 × magnification. The scale bars represent 50 μm. B, C. The expression levels of HIF-1α and HIF-1β in SKOV-3 cells exposed to the indicated treatments for 4 h as estimated by Western blot analysis. D. Western blot analysis of HIF-1α protein in A2780 and OVCAR-3 cells pretreated with vehicle or minocycline (100 μM) for 1 h and then exposed to MG132 (10 μM) or vehicle for an additional 4 h. β-actin, loading control. Densitometric values of the bands obtained by using the Quantity One image program (Bio-Rad) were corrected and expressed relative to that of control cells which was set as 100. Columns, means of three independent experiments; bars, SD. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control cells.
The effect of minocycline on HIF-1α is proteasome-independent
Next, to examine whether the inhibitory effect of minocycline on HIF-1α is only through acceleration of its proteasomal degradation, we pretreated OVCAR-3 and SKOV-3 cells with the proteasome inhibitor carbobenzoxy-Leu-Leu-leucinal (MG132) prior to exposure to minocycline. Treatment with MG132 led to an expected increase in HIF-1α expression in both A2780 and OVCAR-3 cells, but had no effect on inhibition of HIF-1α expression that was mediated by minocycline (Figure 4D). These results suggest that the inhibition of HIF-1α is not only due to increased HIF-1α proteasomal degradation. This raises the possibility that the effect of minocycline on HIF-1α might be at protein synthesis level as well.
Minocycline inhibits AKT/mTOR/p70S6K1/4E-BP1 pathway
Besides HIF-1α stabilization, increased translation [29] and possibly transcription [30] have also emerged as regulatory mechanisms of HIF-1. The AKT pathway and its downstream target mTOR have been shown to increase levels of HIF-1α protein [7] by phosphorylating the components required for HIF-1α protein translation namely p70S6K and 4E-BP1 [7,8]. These kinases control translation of HIF-1 mRNA to protein [31,32]. To test whether inhibition of HIF-1α expression by minocycline is associated with modulation of AKT/mTOR pathway, we examined the effect of minocycline on this pathway in OVCAR-3 and SKOV-3 cells both harboring constitutively active AKT/mTOR pathway [33,34]. As shown in Figure 5, minocycline down-regulated phospho-AKT (Ser473) in both cell types in a dose-dependent manner whereas protein levels of total AKT were not altered. Furthermore, minocycline inhibited phosphorylation of the downstream targets of AKT, mTOR (Ser2448), p70S6K (Thr389) and 4E-BP1 (Thr37/46) in both cell lines and in a way that parallels the decrease in HIF-1α protein levels. Since AKT/mTOR/p70S6K/4E-BP1 pathway is required for HIF-1α protein synthesis, their effective inhibition by minocycline might explains its negative effects on HIF-1α in ovarian cancer cells.
Figure 5.
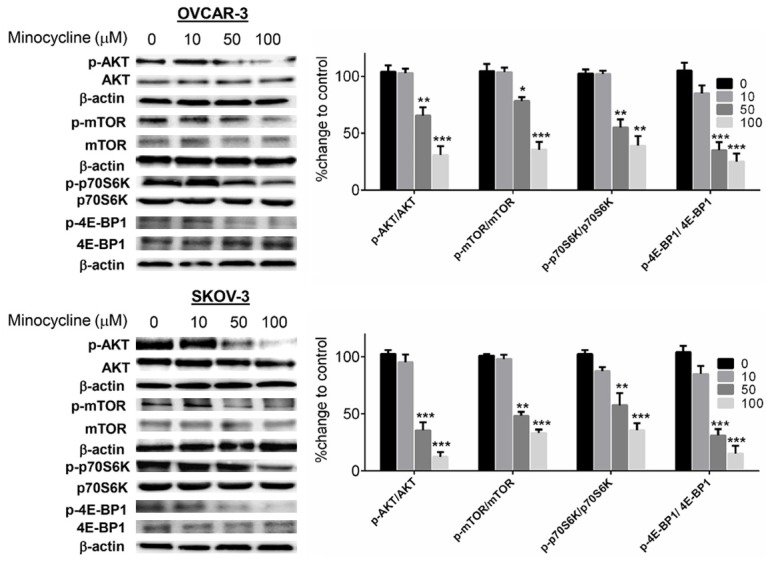
Effect of minocycline on AKT/mTOR/p70S6K1/4E-BP1 pathway (A) Whole cell extracts of OVCAR-3 and SKOV-3 cells after treatment with minocycline (0-100 μM) for 24 h were analyzed by Western blotting using the indicated antibodies. The densitometric values quantified using the Quantity One image program (Bio-Rad) were corrected based on β-actin and were expressed as the percentage of the values corresponding to control cells. Numbers opposite column indicators, concentration of minocycline (μM); Columns, means of three independent experiments; bars, SD. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. vehicle-treated cells.
Tumoral inhibition of HIF-1α correlates with both up-regulation of p53 and suppression of the AKT/mTOR/P70S6K/4E-BP1 signaling
To assess whether the inhibitory effect of minocycline on HIF-1α in ovarian cancer cells could occur in vivo, we next evaluated the effect of minocycline treatment on tumoral HIF-1α expression in an experimental model of ovarian cancer in mice. As shown in Figure 6, a single dose of minocycline resulted in significant decrease in tumor HIF-1α levels after both 4 (65 ± 5%, p < 0.001) and 24 h (80 ± 6%, p < 0.001). Furthermore, compared to control group, the expression of p53 protein was increased by 120 ± 20% (p < 0.01) in the minocycline-treated mice after 4 h. Obviously, the effect was more pronounced in the group receiving minocycline dose for 24 h where p53 expression increased by 160 ± 15% (p < 0.01). In line with our in vitro results phosphorylation of AKT and mTOR was significantly blocked in tumors excised from single-dose minocycline treated animals after both 4 and 24 h (p < 0.001). To further confirm the inhibitory effect of minocycline on AKT/mTOR pathway in vivo, we next examined the effect of minocycline on the phosphorylation of p70S6K and 4E-BP1 the downstream targets of mTOR. Western blot analysis revealed significant down-regulation of the endogenous levels of phosphorylated p70S6K (p < 0.01) and 4E-BP1 (p < 0.001) in the tumor samples excised from mice treated with minocycline. These in vivo observations fully support our in vitro results described above, suggesting effective inhibition of HIF-α associated with up-regulation of p53 and suppression of AKT/mTOR/p70S6K/4E-BP1 cascade by minocycline leading to its reported biological effects in cancer.
Figure 6.
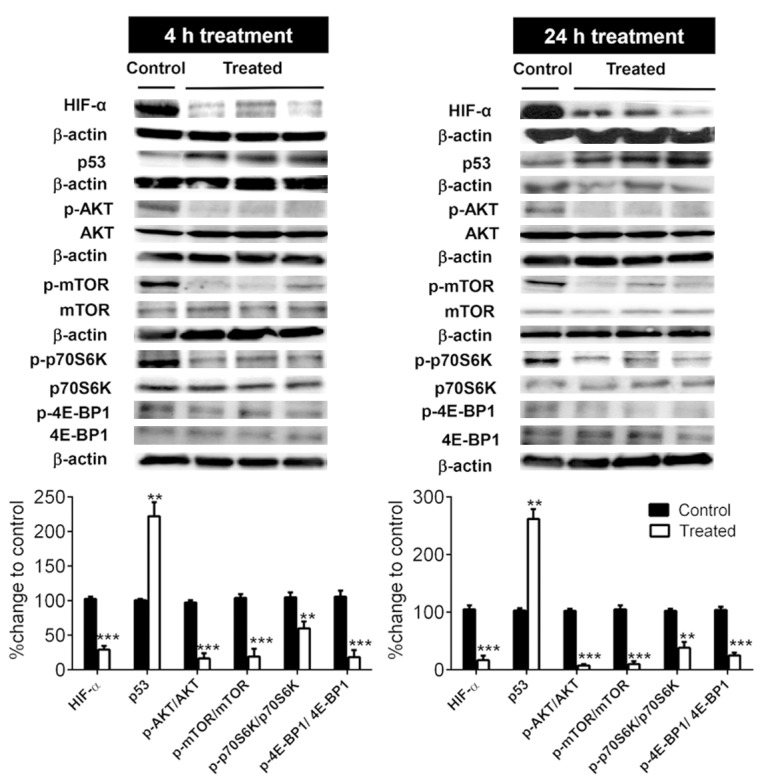
Minocycline suppresses HIF-1α associated with p53 up-regulation and inhibition of AKT/mTOR/p70S6K/4E-BP1 pathway in female BALB/c athymic nude mice bearing i.p. OVCAR-3 tumors. Whole cell protein lysates of tumor tissues excised from mice subjected to a single dose of minocycline (30 mg/kg, i.p.) for 4 h or 24 h were subjected to Western blotting using the indicated antibodies. The densitometric values quantified using the Quantity One image program (Bio-Rad) were corrected based on β-actin and were expressed as the percentage of the values corresponding to control group. Columns, means of densitometry values obtained from analyzing tumor samples of animals in each group (n = 8), Bars, SD. **p < 0.01 and ***p < 0.001 vs. control.
Discussion
HIF-1 is a key cellular survival protein with critical roles in tumor progression, angiogenesis and metastasis [1]. We and others have recently indicated the anti-tumor activities of minocycline [16,35] correlated with its inhibitory effects on angiogenesis [36] and VEGF expression [17]. However, the mechanisms behind these effects is not well-defined. Based on the pivotal role of HIF-1 in tumor angiogenesis and VEGF expression, it is possible that modulation of this transcription factor is one of the key mechanisms of action of minocycline. The major aim of the current study was to determine the effects of minocycline on HIF-1 in ovarian cancer. In vitro, using ovarian cancer cells, we found that minocycline inhibited the HIF-1α surge induced by hypoxia or hypoxic mimetic agent DFO. This was followed by acute (4 and 24 hours) treatment of ovarian tumor-bearing mice with minocycline. Analysis of tumors collected from these mice revealed down-regulation of HIF-1α in minocycline-treated animals.
HIF-1α protein expression is predominantly regulated via its stability regulation [21]. Thus in order to define the molecular mechanism by which minocycline inhibited HIF-1α expression, we first examined whether minocycline decreased HIF-1α protein stability. It was observed that the half-life of HIF-1α protein was shortened significantly in the presence of minocycline, demonstrating that minocycline induced HIF-1α protein degradation. HIF-1α destruction is initiated by a family of enzymes designated as oxygen sensors i.e. prolyl hydroxylases. In the presence of oxygen these enzymes hydroxylate the proline residues (Pro 402 and Pro 564) of HIF-1α protein thus allowing its association with von Hippel Lindau protein to facilitate ubiquitination and proteasomal degradation of HIF-1α [22,23]. To further clarify the mechanism of effect of minocycline, we next checked whether the inhibitory effect of minocycline on HIF-1α is dependent on the activity of prolyl hydroxylases. Interestingly, it was found that the effect of minocycline is preserved in the presence of hydroxylase inhibitor (DMOG) indicating that the effect of minocycline is regardless of the classical oxygen-dependent degradation pathway. More recent reports suggest that besides this traditional degradation pathway, HIF-1α stability can be controlled by a number other factors including the tumor suppressor p53. Convincing data supports the pivotal role of p53 in proteasomal degradation of HIF-1α [37] as well as repressing its transcriptional activity [5,26]. In our study, it was found that minocycline up-regulates the endogenous levels of p53 both in vitro in A2780 (p53 wild type) and OVCAR-3 (p53 mutated) cells; as well as in vivo in tumors excised from minocycline-treated mice. As minocycline enhances p53 expression in cells harboring endogenous p53, this might explain its effect on HIF-1α expression. We tested this notion using SKOV-3 cells which are p53 null. It was observed that minocycline effectively blocked the HIF-1α surge induced by hypoxia or hypoxic-mimetic agent DFO in these cells suggesting that the accumulation of p53 cannot fully explain the effects of minocycline on HIF-1α. This was further confirmed by our results showing that the effect of minocycline is proteasomal-independent and is preserved in the presence of the proteasome inhibitor MG132. Based on this data, the attenuation of HIF-1α by minocycline is not solely through enhancement of its degradation.
HIF-1α expression also depends on its rate of de novo synthesis. Thus, we next examined whether minocycline affected HIF-1α protein synthesis. PI3K/AKT signaling is known to be overexpressed in ovarian cancer [6] and is directly implicated in the control of HIF-1α and VEGF expression [38]. Indeed, PI3K/AKT pathway regulates HIF-1α protein translation through mTOR signaling [39,40]. Activation of the serine/threonine kinase AKT which is the central downstream effector of PI3K leads to phosphorylation and activation of mTOR. Activated mTOR in turn, controls HIF-1α protein translation by phosphorylation of two downstream effectors, namely p70S6K and 4E-BP1 [7,8]. In this study we found that minocycline at 50-100 µM significantly suppressed the phosphorylation of p70S6K and 4E-BP1 in OVCAR-3 and SKOV-3 cells, both expressing constitutively active AKT/mTOR pathway [33,34], and this paralleled with its inhibitory effect on AKT and mTOR activation. The same effect was observed in tumor samples collected from mice treated with a single dose of minocycline for 4 and 24 h. Given the key role of AKT/mTOR/p70S6K/4E-BP1 pathway in the regulation of HIF-1α translation, our results strongly suggested that minocycline-induced suppression of this pathway might be involved in the inhibition of HIF-1α translation rate. The activity of the translation regulators p70S6K and 4E-BP1 is controlled by multiple phosphorylation events induced by multiple kinases. The mitogen activated protein kinase (MAPK) pathway is also known to increase HIF-1α and VEGF protein synthesis through its effects on hyper phosphorylation and activation of p70S6K and 4E-BP1 [41]. Interestingly, we have previously shown that minocycline attenuated MAPK pathway as well [17,18]. Hence, these data indicate that minocycline interfered with protein translation regulation, which might contribute -at least in part- to its inhibitory effect on HIF-1α protein expression. On the other hand, activated p70S6K negatively regulates p53 expression via its downstream effector Mdm2 which is also a positive regulator of HIF-1α [8,42]. Thus, suppression of p-p70S6K by minocycline might account for up-regulation of p53 to provide another mechanism for the inhibitory effect of minocycline on HIF-1α.
Overall, the in vitro and in vivo data presented here demonstrate that minocycline exerts suppressive effects at multiple levels on HIF-1α protein expression in ovarian cancer cells. This novel finding provides new insight into the potential mechanism of the anti-cancer properties of minocycline. Molecular targeting of HIF-1α by minocycline might be a useful and novel strategy for treatment of ovarian cancer.
Disclosure of conflict of interest
None to disclose.
References
- 1.Jin Y, Wang H, Liang X, Ma J, Wang Y. Pathological and prognostic significance of hypoxia-inducible factor 1alpha expression in epithelial ovarian cancer: a meta-analysis. Tumour Biol. 2014;35:8149–59. doi: 10.1007/s13277-014-2059-x. [DOI] [PubMed] [Google Scholar]
- 2.Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol (Oxf) 2013;208:148–165. doi: 10.1111/apha.12096. [DOI] [PubMed] [Google Scholar]
- 3.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Schmid T, Zhou J, Kohl R, Brune B. p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1) Biochem J. 2004;380:289–295. doi: 10.1042/BJ20031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Zhang L, Greshock J, Colligon TA, Wang Y, Ward R, Katsaros D, Lassus H, Butzow R, Godwin AK, Testa JR, Nathanson KL, Gimotty PA, Coukos G, Weber BL, Degenhardt Y. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer. 2011;50:606–618. doi: 10.1002/gcc.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. doi: 10.2174/1568009611313030003. [DOI] [PubMed] [Google Scholar]
- 8.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Basecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeber LM, Horree N, Vooijs MA, Heintz AP, van der Wall E, Verheijen RH, van Diest PJ. The role of hypoxia inducible factor-1alpha in gynecological cancer. Crit Rev Oncol Hematol. 2011;78:173–184. doi: 10.1016/j.critrevonc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Shimogai R, Kigawa J, Itamochi H, Iba T, Kanamori Y, Oishi T, Shimada M, Sato S, Kawaguchi W, Sato S, Terakawa N. Expression of hypoxia-inducible factor 1alpha gene affects the outcome in patients with ovarian cancer. Int J Gynecol Cancer. 2008;18:499–505. doi: 10.1111/j.1525-1438.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Garner EI, Welch WR, Berkowitz RS, Mok SC. Over-expression of hypoxia-inducible factor 1 alpha in ovarian clear cell carcinoma. Gynecol Oncol. 2007;106:311–317. doi: 10.1016/j.ygyno.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu P, Ning Y, Yao L, Chen M, Xu C. The proliferation, apoptosis, invasion of endothelial-like epithelial ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res. 2010;29:124. doi: 10.1186/1756-9966-29-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant CS, Munkarah AR, Kumar S, Batchu RB, Shah JP, Berman J, Morris RT, Jiang ZL, Saed GM. Reduction of hypoxia-induced angiogenesis in ovarian cancer cells by inhibition of HIF-1 alpha gene expression. Arch Gynecol Obstet. 2010;282:677–683. doi: 10.1007/s00404-010-1381-9. [DOI] [PubMed] [Google Scholar]
- 14.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 15.Ataie-Kachoie P, Badar S, Morris DL, Pourgholami MH. Minocycline targets the NF-kappaB Nexus through suppression of TGF-beta1-TAK1-IkappaB signaling in ovarian cancer. Mol Cancer Res. 2013;11:1279–1291. doi: 10.1158/1541-7786.MCR-13-0239. [DOI] [PubMed] [Google Scholar]
- 16.Pourgholami MH, Mekkawy AH, Badar S, Morris DL. Minocycline inhibits growth of epithelial ovarian cancer. Gynecol Oncol. 2012;125:433–440. doi: 10.1016/j.ygyno.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Pourgholami MH, Ataie-Kachoie P, Badar S, Morris DL. Minocycline inhibits malignant ascites of ovarian cancer through targeting multiple signaling pathways. Gynecol Oncol. 2013;129:113–119. doi: 10.1016/j.ygyno.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Ataie-Kachoie P, Morris DL, Pourgholami MH. Minocycline suppresses interleukine-6, its receptor system and signaling pathways and impairs migration, invasion and adhesion capacity of ovarian cancer cells: in vitro and in vivo studies. PLoS One. 2013;8:e60817. doi: 10.1371/journal.pone.0060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appierto V, Villani MG, Cavadini E, Gariboldi M, De Cecco L, Pierotti MA, Lambert JR, Reid J, Tiberio P, Colombo N, Formelli F. Analysis of gene expression identifies PLAB as a mediator of the apoptotic activity of fenretinide in human ovarian cancer cells. Oncogene. 2007;26:3952–3962. doi: 10.1038/sj.onc.1210171. [DOI] [PubMed] [Google Scholar]
- 20.Pourgholami MH, Yan Cai Z, Lu Y, Wang L, Morris DL. Albendazole: a potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin Cancer Res. 2006;12:1928–1935. doi: 10.1158/1078-0432.CCR-05-1181. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 22.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 23.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 24.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obacz J, Pastorekova S, Vojtesek B, Hrstka R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Mol Cancer. 2013;12:93. doi: 10.1186/1476-4598-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of hypoxia-inducible factor 1alpha (HIF-1alpha) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. J Biol Chem. 2013;288:25244–25253. doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273:11995–11998. doi: 10.1074/jbc.273.20.11995. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Callapina M, Goodall GJ, Brune B. Functional Integrity of Nuclear Factor κB, Phosphatidylinositol 3′-Kinase, and Mitogen-Activated Protein Kinase Signaling Allows Tumor Necrosis Factor α-Evoked Bcl-2 Expression to Provoke Internal Ribosome Entry Site-Dependent Translation of Hypoxia-Inducible Factor 1α. Cancer Res. 2004;64:9041–9048. doi: 10.1158/0008-5472.CAN-04-1437. [DOI] [PubMed] [Google Scholar]
- 30.Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of Hypoxia-inducible Factor-1α by Transcriptional and Translational Mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Meceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009;28:313–324. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 32.Bian CX, Shi Z, Meng Q, Jiang Y, Liu LZ, Jiang BH. P70S6K1 regulation of angiogenesis through VEGF and HIF-1alpha expression. Biochem Biophys Res Commun. 2010;398:395–399. doi: 10.1016/j.bbrc.2010.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karam AK, Santiskulvong C, Fekete M, Zabih S, Eng C, Dorigo O. Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskeleton (Hoboken) 2010;67:535–544. doi: 10.1002/cm.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543–552. doi: 10.1111/j.1349-7006.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regen F, Heuser I, Herzog I, Hellmann-Regen J. Striking growth-inhibitory effects of minocycline on human prostate cancer cell lines. Urology. 2014;83:509.e1–6. doi: 10.1016/j.urology.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Bow H, Hwang LS, Schildhaus N, Xing J, Murray L, Salditch Q, Ye X, Zhang Y, Weingart J, Brem H, Tyler B. Local delivery of angiogenesis-inhibitor minocycline combined with radiotherapy and oral temozolomide chemotherapy in 9L glioma. J Neurosurg. 2014;120:662–669. doi: 10.3171/2013.11.JNS13556. [DOI] [PubMed] [Google Scholar]
- 37.Schmid T, Zhou J, Brune B. HIF-1 and p53: communication of transcription factors under hypoxia. J Cell Mol Med. 2004;8:423–431. doi: 10.1111/j.1582-4934.2004.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Yang N, Katsaros D, Huang W, Park JW, Fracchioli S, Vezzani C, Rigault de la Longrais IA, Yao W, Rubin SC, Coukos G. The oncogene phosphatidylinositol 3'-kinase catalytic subunit alpha promotes angiogenesis via vascular endothelial growth factor in ovarian carcinoma. Cancer Res. 2003;63:4225–4231. [PubMed] [Google Scholar]
- 39.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 40.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 42.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]


