Abstract
During the past 40 years, polybrominated diphenyl ethers (PBDEs) have been widely used as flame retardants and nearly all women have some level of exposure. PBDEs have been isolated from amniotic fluid and cord plasma indicating vertical transmission; however, their effects on pregnancy outcome are largely unknown. Therefore, we quantified PBDE-47, the most common conger in maternal plasma samples collected at the time of labor from women who subsequently had term or preterm birth (PTB). Women were then scored based on whether or not they had very low, low, medium, high or very high peripheral plasma concentrations of PBDE-47. Probit regression analysis suggested that women in the PTB group had a greater chance of scoring higher on this scale (P < 0.001). Women with high (OR = 3.8, CI: 1.6, 9.7; P = 0.003) or very high PBDE-47 concentrations were at greater odds (OR = 5.6, CI: 2.2, 15.2; P < 0.001) for PTB than women with very low levels of PBDE-47. Results became even more significant after adjustment for maternal race, age, and marital status. These findings suggest that high levels of maternal exposure to PBDEs might increase the risk for PTB.
1. Introduction
Polybrominated diphenyl ethers (PBDEs) have been in widespread use as flame retardants in home construction, furniture, clothing, and electronic appliances for decades. They save lives and reduce injury by giving occupants valuable time to extinguish or escape from a spreading fire. However, they are not covalently bound to materials that they are used in. With time, they leach into the environment and have become one of the most prevalent of the persistent organic pollutants (POPs).
Polybrominated diphenyl ethers most commonly enter the body through inhalation or ingestion of PBDE-contaminated dust where they bioaccumulate in lipophilic tissues (Costa and Giordano, 2007, Costa et al., 2008). Their concentrations have been increasing in human tissues since their introduction into consumer products in the 1970s (Schecter et al., 2005, Thomsen et al., 2002, Fängström et al., 2008). Breast-milk and blood concentrations of PBDEs are 10- to 100-fold higher in the United States than in other countries (Costa and Giordano, 2007, Costa et al., 2008), reflecting greater usage, which is often for compliance with strict fire codes (Trudel et al., 2011).
Polybrominated diphenyl ethers may affect human health as endocrine disruptors because of their structural similarity to triiodothyronine (T3) and thyroxine (T4). Site-directed mutagenesis and bioassay studies indicate that PBDEs interfere with the ligand-binding domain of the thyroid hormone receptor (TR) to inhibit the transcription of TR-dependent genes and their biological effects (Ibhazehiebo et al., 2011). Developmental exposure to PBDEs causes hypothyroid-like conditions in pregnancy and increased hyperactivity and learning and memory deficits in the offspring (Branchi et al., 2003, Costa and Giordano, 2007).
Polybrominated diphenyl ethers have been detected in amniotic fluid (Miller et al., 2012), umbilical cord plasma (Vizcaino et al., 2011, Frederiksen et al., 2010,2009b, Kim et al., 2009, Kawashiro et al., 2008, Gómara et al., 2007, Herbstman et al., 2007, Jaraczewska et al., 2006), umbilical cord tissue (Kawashiro et al., 2008), placental tissues (Frederiksen et al., 2009a, Qing Zhang et al., 2008, Gómara et al., 2007, Main et al., 2007), and fetal membranes (Miller et al., 2009). Maternal plasma levels have recently been found to correlate with higher thyroid-stimulating hormone (TSH) levels (Zota et al., 2011). TSH is negatively regulated by T3 and T4, suggesting the reduced bioactivity of these hormones. Overt and subclinical hypothyroidism increase the risk for preterm birth (PTB) (Vissenberg et al., 2012, Stagnaro-Green, 2011). Therefore, we hypothesized that increased exposure to PBDEs might increase the risk of spontaneous PTB.
2. Materials and Methods
2.1. Patients and Sampling
Samples for this study were collected as a part of a larger study that investigated genetic biomarkers for PTB. The parent study was approved by TriStar Nashville, the institutional review board at Centennial Medical Center, and the institutional review board at the University of Texas Medical Branch at Galveston, TX, USA. Written consent was acquired from all patients to use their samples for the original study and to deposit them into a biobank for use in future research projects that would include the current study. All subjects were recruited at Centennial Women’s Hospital in Nashville, TN, USA, between September 2008 and December 2011. Pregnant women between the ages of 18 and 40 were eligible and enrollment occurred at the time of admission for delivery. All subjects had regular uterine contractions at a minimum frequency of two contractions every ten minutes. Gestational age was determined by last menstrual period dating and verified by ultrasound dating.
Maternal blood samples were collected in EDTA tubes at the time of admission for preterm or term labor and transported to the blood on ice. Blood samples were then centrifuged at 1,500 g. Plasma was then separated, aliquoted, and stored at −80°C until assay. Samples from patients whose pregnancies ended in spontaneous PTB without pPROM were randomly selected for the present study as cases. The distribution of gestational age at delivery was: quartile 1, 23–32.6 weeks; quartile 2, 32.6–35.1 weeks; quartile 3, 35.1–36 weeks; quartile 4, 36–37 weeks. Samples from women with term labor and delivery (37 weeks), intact membranes, and no pregnancy-related complications were randomly selected for this study to use as controls (without any matching criteria).
2.2. Immunoassays
Polybrominated diphenyl ether(s) exist as 209 different congers that are usually quantified by high-resolution mass-spectroscopy; however, PBDE-47 is the most abundant conger in the environment. It is well-studied in pregnant and nonpregnant women and concentrations of this particular conger correlate well with total PBDE exposure (Frederiksen et al., 2010). To reduce the cost of performing studies where PBDEs must be quantified, immunoassays have been developed and found to be useful for quantifying PBDE-47 in biological and environmental samples that correlate well with data from mass spectroscopy of the same samples (Shelver et al., 2008, Xu et al., 2009). We used a commercially available version of this immunoassay (Abraxis, Warminster, PA, USA) to quantify PBDE-47 equivalents 25–1000 pg/ml in maternal plasma samples for this project. In this assay, PBDE-47 in the samples competes with horseradish peroxidase-conjugated PBDE for binding sites onto an antibody attached to magnetic beads. Beads are then separated and washed with buffer, incubated in substrate, and color development is monitored by absorbance at 450 nm. Sample concentrations are estimated from a standard curve of 0–1000 pg/ml PBDE-47. PBDE concentrations below the sensitivity of the assay (25 pg/ml) were set equal to the sensitivity of the assay for analysis.
2.3. Statistical analyses
All statistical analyses were performed using the R programming language (www.r-project.org). Comparisons of patient characteristics between groups were made using χ2 or Wilcoxon rank-based tests. When evaluating PBDE-47 levels, a significant number of samples fell above the standard curve (1000 pg/ml), and there was insufficient sample to re-assay them at a lower dilution. Therefore, patient samples were ranked on an ordinal scale of 1–5 based on having very low (135 pg/ml), low (136–199 pg/ml), moderate (200–321 pg/ml), high (322–1000 pg/ml) or very high (>1000 pg/ml) maternal plasma concentrations of PBDE-47. These cutoffs were based on quartiles for PBDE-47 concentrations in a subset of patients from the control group whose PBDE-47 levels were <1000 pg/ml. Ordinal scale values of patients with high PBDEs and low PBDEs were compared using probit regression, as previously described (Faraway 2006). Logistic regression methods were used to compare the potential association of level of PBDE with the risk of PTB. All models were checked for lack-of fit by analysis of deviance (Faraway, 2006) and the analysis was restricted to patients for whom there were complete data. Results are presented as proportions and odds ratios with 95% CI.
3. Results
Basic patient demographics are shown in Table 1. Although both outcome groups were similar with regard to maternal age, African–American women were over-represented in the control group and under-represented in the PTB group. Single women were over-represented among women who delivered at term.
Table 1.
Characteristics of women whose plasma samples were used for this study.
| Maternal Characteristic | Control | PTB | P value |
|---|---|---|---|
| Race | |||
| Caucasian (n) | 65 | 73 | 0.0011 |
| African–American (n) | 132 | 9 | |
| Marital Status | |||
| Married (n) | 84 | 50 | 0.0061 |
| Unmarried (n) | 113 | 32 | |
| Age2 (years) | 27 (18, 43) | 27 (18, 41) | 0.77093 |
| Gestational age at delivery2 (weeks) | 39 (37, 41) | 35 (23, 36) | 0.0013 |
PTB preterm birth
Fisher’s exact test
Median (minimum, maximum)
Wilcoxon Signed Rank test
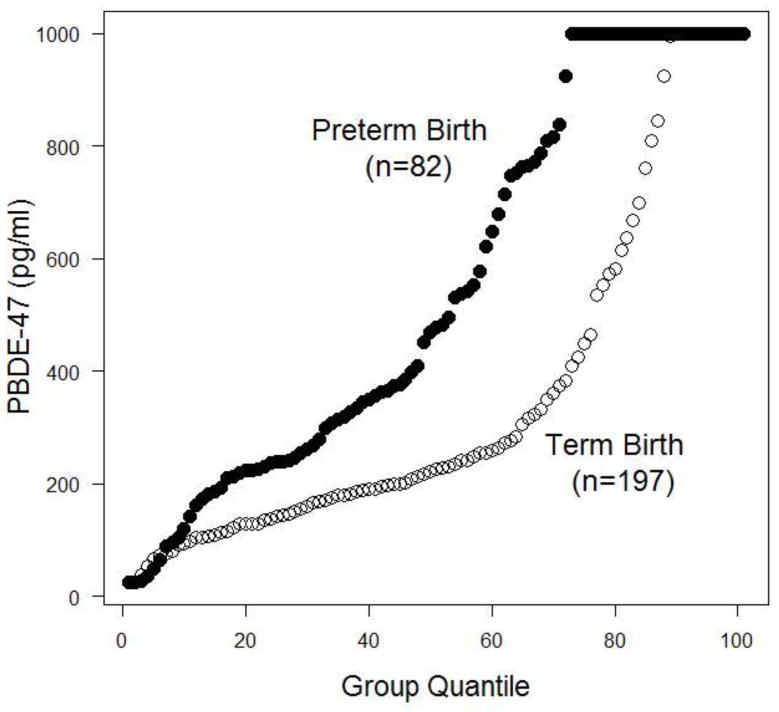
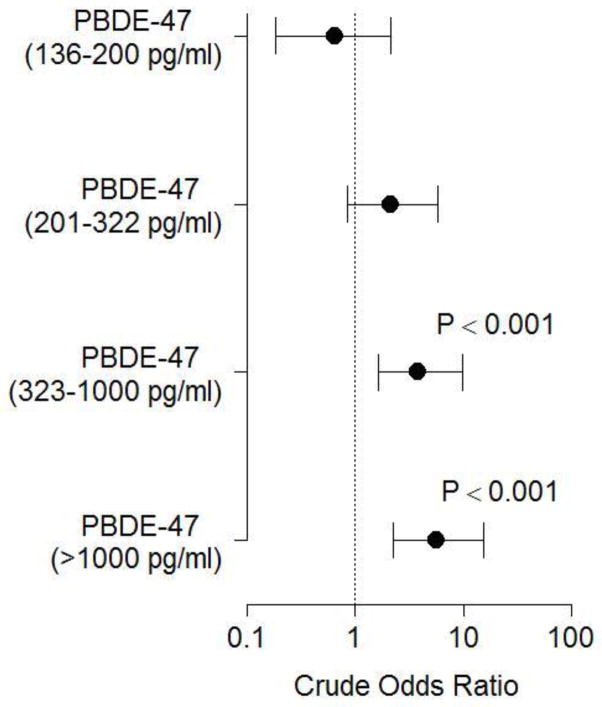
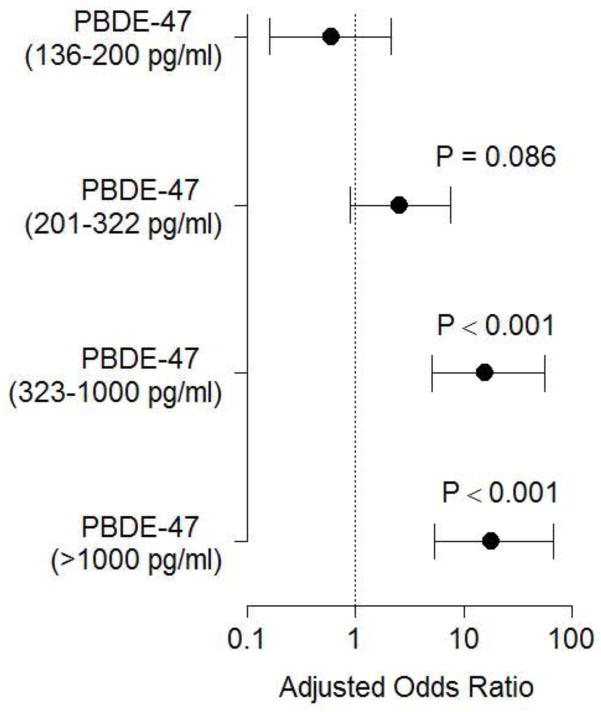
Empirical distributions for PBDE-47 levels are shown in Figure 1, indicating that at nearly each quartile, PBDE concentrations were higher for women who delivered preterm. Number and proportion of women at each PBDE grouping described above are shown in Table 2. Probit analysis suggested that women whose pregnancies ended in PTB might be more likely to be in a higher grouping on this ordinal scale (P < 0.001). Similarly, logistic regression analysis demonstrated that women with increased concentrations of PBDE have a dose-dependent increased odds of PTB that became significant for the high and very high exposure groups (Figure 2). This association remained significant after adjustment for maternal race, marital status, and age (Figure 3).
Figure 1.
Cumulative empirical distribution of maternal plasma polybrominated diphenyl ether (PBDE) concentrations in women who deliver at term or preterm. PBDE levels for each patient are shown, ranked into a group-specific quartile (shown on the x-axis).
Table 2.
Proportion of women delivering term and preterm at each level of polybrominated diphenyl ether(s) (PBDE). Patients in the PTB group were significantly more likely to score higher on this ordinal scale (P=0.001).
| PBDE Level | Control | PTB |
|---|---|---|
| 25–135 pg/ml | 45/197 | 8/82 |
| 136–200 pg/ml | 43/197 | 5/82 |
| 200–321 pg/ml | 42/197 | 16/82 |
| 322–1000 pg/ml | 43/197 | 29/82 |
| >1000 pg/ml | 24/197 | 24/82 |
This manuscript contains original research performed by our group and is not under consideration elsewhere. None of the authors have any financial or other conflicts of interest in the results of this research.
Figure 2.
Effect of PBDE levels on the odds of delivering preterm. Crude odds ratios ± 95% CI are shown. Bars that cross 1 are not statistically significant.
Figure 3.
Effect of PBDE levels on the odds of delivering preterm after adjustment for maternal race, marital status, and age. Adjusted odds ratios ± 95% CI are shown. Bars that cross 1 are not statistically significant.
4. Discussion
We found that women with higher levels of PBDE-47 in their peripheral plasma are more likely to deliver preterm than at term. This finding is consistent with previous cell culture studies where we found that PBDEs increased the production of COX-2 and PGE2 production with unstimulated placental cultures (Peltier et al., 2012). Increased production of prostaglandins in the reproductive tract may lead to preterm labor and premature cervical ripening. PBDE exposure also increased bacteria-stimulated IL-1b, but reduced IL-10 production with placental explants in this study (Peltier et al., 2012). The placenta is rich in macrophages and a recent study with mouse immune cells found that PBDE exposure increased the expression of CD86, MHC-II, and CD80 and CD11b, markers of macrophage activation (Koike et al., 2012). By enhancing macrophage susceptibility, PBDEs may lower the threshold for ascending infections, a common risk factor for PTB, to activate the labor mechanism through proinflammatory cytokines.
Although we suspect that PBDEs increase the risk of PTB by enhancing placental inflammation, most studies to date have focused on the role of PBDEs as thyroid disruptors. PBDEs prevented T3-induced arborization of mouse cerebellum (Ibhazehiebo et al., 2011), suggesting that it might reduce the biological activity of the thyroid hormones. There is some evidence that overt or subclinical hypothyroidism may increase the risk of PTB in women (Stagnaro-Green et al., 2011). In a prospective cohort study of women presenting for prenatal care over a one-year period at a major hospital, women with subclinical hypothyroidism were at more than double the risk for PTB as euthyroid women (Casey et al., 2005). Treatment of clinical (RR = 0.23, CI: 0.1, 0.52) or sub-clinical (RR = 0.31, CI: 0.11, 0.90) hypothyroidism with L-thyroxine significantly reduced the risk for PTB (Vissenberg et al., 2012); however, the mechanism by which the thyroid hormones modulate this risk is unclear.
Thyroid hormones have complex interactions with the immune system that when disrupted may increase the risk of PTB. Inflammatory diseases such as sepsis (Schonberger et al., 1979, Leon-Sanz et al., 1997) or kidney disease are worse in the setting of hypothyroidism (Lin et al., 2012). Administration of T4 reduces the mortality and production of proinflammatory cytokines after cecal ligation and puncture, a common model of bacterial sepsis (Inan et al., 2003) for which thyroidectomized animals were more susceptible (Moley et al., 1984).
The beneficial effects of the thyroid hormones on survival in inflammatory disease may be due to inhibition of the production of proinflammatory cytokines (Vito et al., 2011). Experimental hypothyroidism enhanced streptococcal induction of IL-1β in rats, which was reversed by T4 treatment. Thyroxine supplementation also attenuated bacteria-induced increases in peripheral plasma IL-6, TNF-α, KC, MIF, and IFN-γ in a model of meningitis (Chen et al., 2012). Lymphocytes and/or monocytes isolated from patients with Hashimoto’s thyroiditis had greater secretion of proinflammatory cytokines, including C-reactive protein, TNF-α, IL-2, IFN-γ, MCP-1, IL-6, and IL-1β, than cells from healthy controls. Treatment of these patients for 3–6 months with L-thyroxine reduced the release of these cytokines from immune cells compared with patients who received placebo (Krysiak and Okopien, 2011). Additional work with animal and cell culture models will be valuable for identifying the mechanism(s) by which PBDEs increase the risk of PTB and for evaluating the role of thyroid hormones more fully.
A strength of our study is the use of an immunoassay that permitted the analysis of a large number of samples. The use of a well-defined cohort of patients and extensive use of samples from African–American women, who are at the highest risk for PTB, are further advantages. Our study is not without limitations, however. The participants were limited to samples that were available in our freezer at the time and do not constitute a random sample drawn from the population. This makes it difficult to estimate clinically useful statistics such as specificity and sensitivity (which were 58.8 and 65.2% respectively for PBDE-47 concentrations > 322 pg/ml) and impossible to estimate positive and negative predictive values, which use estimates of PTB prevalence. There were also insufficient quantities of sample to re-assay samples that were above the standard curve. This necessitated the use of ordinal and logistic regression methods that are considerably less powerful. The effect sizes, however, were sufficiently large that the study was not affected by this limitation. Although we found evidence that risk for PTB may be influenced by PBDE exposure, we did not have sufficient numbers of patients at earlier gestational ages to determine if there is a dose-dependent relationship between PBDE levels and the severity of PTB. When we split the PTB group into very preterm (<34 weeks) and moderate (34–36 weeks and six days) PTB we obtained similar trends to the above analyses (the unadjusted analysis did not reach statistical significance, however), with no obvious difference in effect size for high and very high levels of PBDE exposure for very and moderate PTBs. Also, we do not have sufficient numbers of cases to determine how PBDEs may have modified the risk for clinical or histological chorioamnionitis inducing PTB. All of these limitations are being addressed by our current studies, which use prospectively collected blood samples from asymptomatic women. As with all epidemiological studies, the potential effects of unmeasured confounders are difficult to quantify.
In summary, we found that PBDE exposure increased the odds of PTB. However, further work with prospectively collected samples, animal models, and cell cultures are needed, to confirm these findings and identify potential mechanism(s) for these persistent organic pollutants found in nearly all pregnant women that may increase the risk of PTB.
Highlights.
Polybrominated diphenyl ethers (PBDEs) may enhance placental inflammation
Nearly 100% of women have some exposure to PBDEs in pregnancy
Aberrant inflammation is a common cause of preterm birth
How PBDEs may affect risk for preterm birth is unclear
We found that greater exposure to PBDEs increases the odds of preterm birth
Acknowledgments
Drs. Peltier, Getahun, and Menon were funded in part by the National Institute of Environmental Health Sciences (NIEHS) of the National Institute of Health (1R01ES023116-01). The opinions expressed are solely the responsibility of the authors and do not necessarily reflect the official views of the NIEHS.
Footnotes
Conflict of Interest Statement
This manuscript contains original research performed by our group and is not under consideration elsewhere. None of the authors have any financial or other conflicts of interest in the results of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sjolinder M, Wang X, Altenbacher G, Hagner M, Berglund P, Gao Y, Lu T, Jonsson AB, Sjolinder H. Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection. PLoS One. 2012;7:e41445. doi: 10.1371/journal.pone.0041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- Fängström B, Athanassiadis I, Odsjö T, Norén K, Bergman A. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980–2004. Mol Nutr Food Res. 2008;52:187–193. doi: 10.1002/mnfr.200700182. [DOI] [PubMed] [Google Scholar]
- Faraway J. Extending the linear model with R: generalized, linear, mixed effects and non-parametric regression models. Chapman & Hall/CRC; New York: 2006. [Google Scholar]
- Frederiksen M, Thomsen M, Vorkamp K, Knudsen LE. Patterns and concentration levels of polybrominated diphenyl ethers (PBDEs) in placental tissue of women in Denmark. Chemosphere. 2009a;76:1464–1469. doi: 10.1016/j.chemosphere.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs–a review of levels and sources. Int J Hyg Environ Health. 2009b;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Thomsen C, Frøshaug M, Vorkamp K, Thomsen M, Becher G, Knudsen LE. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health. 2010;213:233–242. doi: 10.1016/j.ijheh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Gómara B, Herrero L, Ramos JJ, Mateo JR, Fernández MA, García JF, González MJ. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41:6961–6968. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115:1794–1800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119:168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Koyuncu A, Aydin C, Turan M, Gokgoz S, Sen M. Thyroid hormone supplementation in sepsis: an experimental study. Surg Today. 2003;33:24–29. doi: 10.1007/s005950300004. [DOI] [PubMed] [Google Scholar]
- Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ. 2006;372:20–31. doi: 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Kawashiro Y, Fukata H, Omori-Inoue M, Kubonoya K, Jotaki T, Takigami H, Sakai S, Mori C. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr J. 2008;55:1071–1084. doi: 10.1507/endocrj.k08e-155. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee YJ, Lee E, Patra N, Lee J, Kwack SJ, Kim KB, Chung KK, Han SY, Han JY, Lee BM, Kim HS. Exposure assessment of polybrominated diphenyl ethers (PBDEs) in umbilical cord blood of Korean infants. J Toxicol Environ Health A. 2009;72:1318–1326. doi: 10.1080/15287390903212436. [DOI] [PubMed] [Google Scholar]
- Koike E, Yanagisawa R, Takigami H, Takano H. Brominated flame retardants stimulate mouse immune cells in vitro. J Appl Toxicol. 2012;33:1451–1459. doi: 10.1002/jat.2809. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2011;96:2206–2215. doi: 10.1210/jc.2010-2986. [DOI] [PubMed] [Google Scholar]
- Leon-Sanz M, Lorente JA, Larrodera L, Ros P, Alvarez J, Esteban AE, Landin L. Pituitary-thyroid function in patients with septic shock and its relation with outcome. Eur J Med Res. 1997;2:477–482. [PubMed] [Google Scholar]
- Lin YC, Lin YC, Chen TW, Yang WC, Lin CC. Abnormal thyroid function predicts mortality in patients receiving long-term peritoneal dialysis: a case-controlled longitudinal study. J Chin Med Assoc. 2012;75:54–59. doi: 10.1016/j.jcma.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast Michigan. Environ Sci Technol. 2009;43:3042–3046. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417–418:294–298. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley JF, Ohkawa M, Chaudry IH, Clemens MG, Baue AE. Hypothyroidism abolishes the hyperdynamic phase and increases susceptibility to sepsis. J Surg Res. 1984;36:265–273. doi: 10.1016/0022-4804(84)90097-0. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33:745–749. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Zhang J, Kang Sun X, Sheng Jiang Y, Zhou J, Bin Wang L, Yi Ye Z, Kui Fang D, bin Wang G. Levels of PCDD, PCB and PBDE compounds in human placenta tissues. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:911–918. [PubMed] [Google Scholar]
- Schecter A, Päpke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schonberger W, Grimm W, Gempp W, Dinkel E. Transient hypothyroidism associated with prematurity, sepsis, and respiratory distress. Eur J Pediatr. 1979;132:85–92. doi: 10.1007/BF00447374. [DOI] [PubMed] [Google Scholar]
- Shelver WL, Parrotta CD, Slawecki R, Li QX, Ikonomou MG, Barcelo D, Lacorte S, Rubio FM. Development of a magnetic particle immunoassay for polybrominated diphenyl ethers and application to environmental and food matrices. Chemosphere. 2008;73:S18–S23. doi: 10.1016/j.chemosphere.2007.01.088. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A. Overt hyperthyroidism and hypothyroidism during pregnancy. Clin Obstet Gynecol. 2011;54:478–487. doi: 10.1097/GRF.0b013e3182272f32. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A, Akhter E, Yim C, Davies TF, Magder L, Petri M. Thyroid disease in pregnant women with systemic lupus erythematosus: increased preterm delivery. Lupus. 2011;20:690–699. doi: 10.1177/0961203310394894. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ Sci Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Trudel D, Scheringer M, von Goetz N, Hungerbuhler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol. 2011;45:2391–2397. doi: 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- Vissenberg R, van den Boogaard E, van Wely M, van der Post JA, Fliers E, Bisschop PH, Goddijn M. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2012;18:360–373. doi: 10.1093/humupd/dms007. [DOI] [PubMed] [Google Scholar]
- Vito PD, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Lopez-Espinosa MJ, Llop S, Rebagliato M, Ballester F. Polybromodiphenyl ethers in mothers and their newborns from a non-occupationally exposed population (Valencia, Spain) Environ Int. 2011;37:152–157. doi: 10.1016/j.envint.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Xu T, Cho IK, Wang D, Rubio FM, Shelver WL, Gasc AME, Li J, Li QX. Suitability of a magnetic particle immunoassay for the analysis of PBDEs in Hawaiian euryhaline fish and crabs in comparison with gas chromatography/electron capture detection-ion trap mass spectrometry. Environ Pollut. 2009;157:417–422. doi: 10.1016/j.envpol.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]