Seasonal and pandemic influenza virus infections cause substantial mortality and morbidity. At least 18 500 confirmed deaths occurred in the first year of the 2009 H1N1 influenza A pandemic, but modeling estimates suggest that over 200 000 respiratory deaths and an additional 85 000 cardiovascular deaths occurred worldwide, with the majority in Africa and Southeast Asia.1 Like most infectious diseases, influenza virus morbidity and mortality are skewed toward the very young (<2 years), the elderly (>65 years) and pregnant women, conditions in which key elements of the immune system necessary to curtail influenza are compromised.
Influenza is contracted through inhaled aerosol droplets of infectious virus spread from infected individuals. Not unexpectedly, severe disease is more common among persons with underlying medical conditions that limit physiologic reserve, particularly as related to the lungs and cardiovascular system. Diseases of the lung, such as asthma, chronic obstructive pulmonary disease and cystic fibrosis, are thus overrepresented among patients hospitalized with complications of influenza. The virus is cytotoxic for lung epithelial cells, which produce a variety of pro-inflammatory cytokines in response to infection, including type 1 interferons, interleukin (IL)-1, tumor necrosis factor-α and IL-6, thus setting in motion the activation of innate and adaptive responses necessary to restrict viral replication. In addition to interferons, control of the virus is ultimately dependent on a combination of CD8 T cells and CD4-dependent neutralizing antibodies, bespeaking a widespread interaction with the host immune system. Mortality has been linked with extensive lung damage leading to bacterial pneumonia and superinfections, particularly in the elderly, and to hyper-cytokine states that lead to widespread endothelial activation and organ failure, which can complicate infection with more recently acquired avian strains.2
Asthma is more common among patients hospitalized with acute influenza infection. Although afflicted patients are generally younger, asthma involves the airways, which are targeted by the virus, and is frequently treated with steroids, which have impact on host immune responses. As such, severe illness would not be unexpected among hospitalized asthma patients with influenza infection. In this issue of Immunology and Cell Biology, McCullers and colleagues3 became intrigued by epidemiologic studies of the 2009 flu pandemic suggesting that asthma, although a risk factor for hospitalization, was not a risk factor for more severe disease, as established by residence in the intensive care unit or mortality (references therein). Although not precisely investigated by these studies, and complicated by differing thresholds for vaccination and the early use of anti-viral agents (which might mistakenly be withheld from pregnant individuals while being targeted to patients with a history of asthma, for example) and by the heterogeneous nature of asthma,4 the topic remains incompletely explored. Indeed, patients with allergic rhinitis inoculated intranasally with rhinovirus demonstrated attenuated cold symptoms if allergic inflammation was induced before viral inoculation, consistent with attenuating effects of allergy on respiratory viral disease.5
The authors use a model of inhalational fungal exposure involving pre-sensitization of C57BL/6 mice with Aspergillus extract followed by three weekly inhalations of live Aspergillus conidia. Conidia challenge 2 weeks later causes acute allergic lung disease, which peaks after 7 days, with eosinophil infiltration, goblet cell hyperplasia and elevations in immunoglobulin E. By 4 weeks the mice develop peribronchial hyperplasia and hypertrophy along with subepithelial fibrosis, consistent with tissue remodeling. With this model, the investigators compared H1N1 influenza virus administered during the acute as compared with the more subacute remodeling phase of allergic inflammation. Although mice infected during the remodeling phase displayed a course of infection not different from non-sensitized mice, mice infected during the peak of allergic inflammation showed more rapid viral clearance, a reduced interferon response and higher levels of insulinlike growth factor-1, which the authors suggest may have a role in sustaining epithelial integrity. Thus, their data suggest that the allergic lung may be more resistant to the pro-inflammatory features of influenza virus infection, perhaps accounting for the epidemiologic findings.
Although not investigated by the authors, IL-22 has proven critical in maintaining epithelial integrity in a variety of model lung infections, including influenza. A member of the IL-10 family of cytokines, IL-22 activates epithelial production of antibacterial peptides, enhances mucus production, facilitates proliferative repair and protects stem cell niches from destruction during inflammation; fibrosis is also repressed.6 Although generally reparative, the proliferative effects of IL-22 are context dependent, and can mediate pathologic states in psoriasis and certain cancer models. Whereas the receptor is present on epithelia and induced further by inflammation, the cytokine is produced by lymphocytes. Although produced by adaptive T cells, including γδ- and αβ-T cells, interest has recently focused on IL-22 production by a population of innate lymphocytes, now designated Group 3 innate lymphoid cells, or ILC3.7 ILC3 are prevalent in the intestines but their tissue accumulation in other organs in response to inflammation remains incompletely explored. ILC3 and other IL-22-producing cells express the transcription factor RORγt, as well as IL-23 and aryl hydrocarbon receptors; ligation of these receptors leads to elaboration of IL-22. The primary source of IL-23 may be dendritic cells, suggesting that the positioning, maturation and activation of pulmonary dendritic cells in the setting of allergic or remodeled tissues may also be fruitful areas for further study. Another innate cell population in the lung, now designated ILC2, becomes activated during allergic immunity and contributes to allergic inflammation and chronic remodeling.8 Intriguingly, lung ILC2 mediated protection from influenza-induced epithelial damage through release of the epidermal growth factor, amphiregulin.9 Activated effector cells, like Th2 cells present in tissues during acute allergic inflammation, can promote activation of innate and innate-like lymphocytes,8 perhaps underpinning some of the protective effects seen by the authors (Figure 1).
Figure 1.
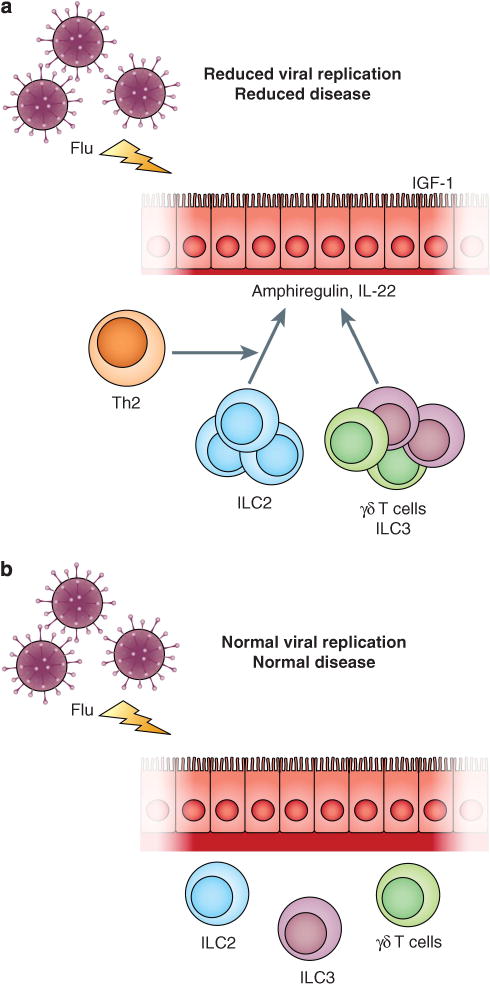
Allergic inflammation and the outcome of influenza virus infection. (a) During acute allergic inflammation, effector Th2 cells contribute cytokines that increase activation and expansion of resident innate RORγt+ lymphoid cells in lung, including γδ-T cells and ILC3, which produces IL-22, a cytokine implicated in epithelial homeostasis in response to injury. Resident ILC2 also releases amphiregulin that may enhance EFGR-mediated preservation of epithelial integrity, thus allowing epithelial cells to increase protective levels of insulin-like growth factor-1 (IGF-1), which may also contribute to the preservation of epithelial function. (b) During the chronic remodeled phase of allergic lung disease (increased amounts of red collagen), effector Th2 cells are no longer active, and do not contribute to expansion of resident innate and innate-like lymphoid populations. The loss of reparative cytokines like IL-22, amphiregulin and IGF-1 leads to increased viral replication and longer duration of disease.
The study by Samarasinghe et al., together with increasing studies of innate cell subpopulations whose contributions continue to be explored, create additional hypotheses for understanding how diverse tissue states might influence host responses to acute perturbations, such as influenza. Further studies of roles for innate lymphoid cells in diverse human lung diseases of inflammation and fibrosis are ongoing, and have created excitement in many fields seeking to understand their contributions to immunity and pathogenesis. The current study emphasizes the importance of using human epidemiologic studies to create testable hypotheses in model systems, such that data generated can be taken back to the clinic and ultimately lead to improvements in human health.
Acknowledgments
This study was supported in part by the grant HL107202 from the National Institutes of Health, the SABRE Center at UCSF and HHMI.
References
- 1.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng P-Y, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modeling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 2.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJD, Chau TNB, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samarasinghe AE, Woolard SN, Boyd KL, Hoselton SA, Schuh JM, McCullers JA. The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunol Cell Biol. 2014;92:449–459. doi: 10.1038/icb.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2008;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila PC, Abisheganaden JA, Wong H, Liu J, Yagi S, Schnurr D, et al. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. J Allergy Clin Immunol. 2000;105:923–932. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 6.Rutz S, Eidenschenk C, Ouyang W. Il-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoidresident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24:707–712. doi: 10.1016/j.coi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, et al. Innate lymphoid cells promote lung tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]


