Retinal ganglion cells (RGCs) are responsible for propagating signals derived from visual stimuli in the eye to the brain, along their axons within the optic nerve to the superior colliculus, lateral geniculate nucleus and visual cortex of the brain. Damage to the optic nerve either through trauma, such as head injury, or degenerative disease, such as glaucoma causes irreversible loss of function through degeneration of non-regenerating RGC axons and death of irreplaceable RGCs, ultimately leading to blindness (Berry et al., 2008). The degeneration of RGCs and their axons is due to the loss of the necessary source of retrogradely transported neurotrophic factors (NTFs) being hindered by axonal injury. NTFs are survival factors for neurons and play a pivotal part in axon regeneration. Stem cells particularly mesenchymal stem cells (MSCs) have been shown to possess a natural intrinsic capacity for paracrine support, releasing multiple signalling molecules including NTFs. By transplanting MSCs into the vitreous, they are positioned adjacent to the injured retina to provide paracrine-mediated therapy for the retinal neuronal cells (Johnson et al., 2010a; Mead et al., 2013). Additionally, MSCs may be pre-differentiated into supportive glial-like cells, such as Schwann cells, which could further increase their potential for paracrine support of injured neurons (Martens et al., 2013). Thus, MSCs have received considerable attention as a new cellular therapy for both traumatic and degenerative eye disease, acting as an alternative source of NTFs, protecting injured RGCs and promoting regeneration of their axons (Figure 1).
Figure 1.
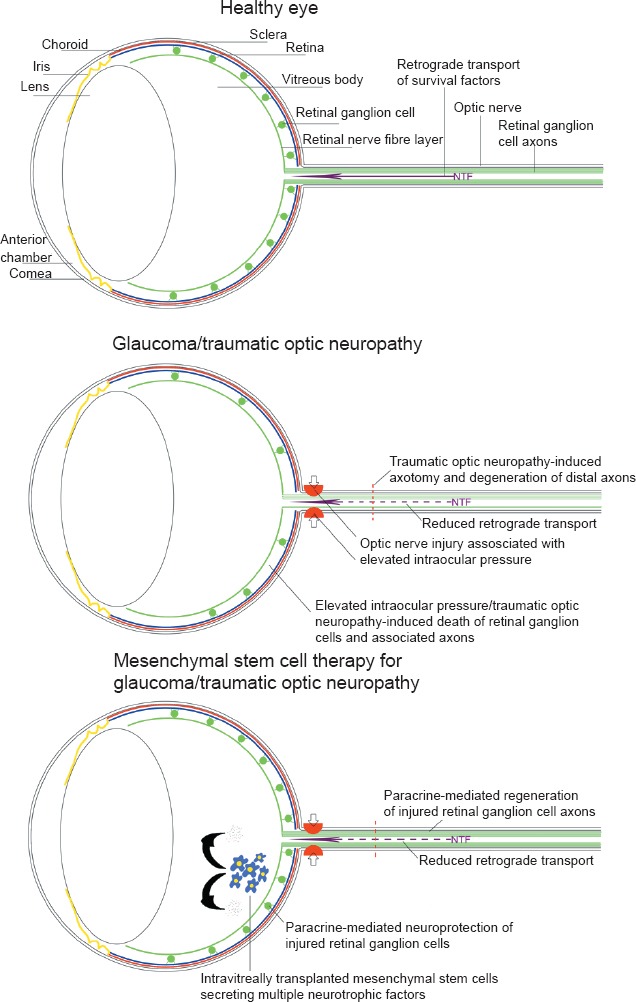
Schematic diagram demonstrating the effects of glaucoma and traumatic optic neuropathy on the eye and the potential of mesenchymal stem cells as a therapy.
Bone marrow mesenchymal stem cells: Bone marrow mesenchymal stem cells (BMSCs) were the first MSCs to gather interest as a cellular therapy for ocular disease. Following transplantation into the vitreous of a rat model of glaucoma, BMSCs increased the number of surviving RGCs by 10–20% (Yu et al., 2006; Johnson et al., 2010a). In a model of traumatic optic nerve injury, BMSCs increased the survival of RGCs by 15–20% 8–28 days after transection/crush of the optic nerve (Levkovitch-Verbin et al., 2010; Mead et al., 2013; Mesentier-Louro et al., 2014) and increased the number of regenerating axons found at distances 100–1,200 μm distal to the lesion site by 2-fold compared to control animals receiving dead cells (Mead et al., 2013; Mesentier-Louro et al., 2014). In both models, the BMSCs survived but showed no sign of differentiating into neuronal or glial phenotypes, thus leading to the conclusion that the neuroprotective effects elicited were through paracrine-mediated effects, either direct signalling between the grafted stem cells and the injured RGCs, or activation of retinal glia by the stem cells and glia-mediated neuroprotection/axogenesis.
Dental pulp stem cells: We are interested in exploring the use of dental pulp stem cells (DPSCs) as an alternative source of stem cells for cellular therapy for the eye (Mead et al., 2013, 2014). DPSCs are neural crest-derived cells that can be isolated from adult teeth, an easily accessible source. Previous PCR-based gene expression studies suggested that, like BMSCs, DPSCs secrete multiple NTFs. In our most recent study using an in vitro co-culture system using axotomised RGC, we compared human-derived DPSCs, BMSCs and adipose-derived mesenchymal stem cells (ADSCs) for their potential to protect and regenerate injured RGCs (Mead et al., 2014). Like BMSCs and DPSCs ADSCs secrete multiple different NTFs; however, their efficacy as a treatment for the eye is unknown. We cultured human-derived MSCs with injured rat retinal cells and assessed their neuroprotective and neuritogenic potential, and the role of specific NTFs including platelet-derived growth factor (PDGF) which was recognised as an important BMSC-derived factor for RGC neuroprotection (Johnson et al., 2013). In co-culture, we administered a variety of different Fc-fusion protein inhibitors to selectively block particular receptors and assess the changes in neuroprotective and neuritogenic effects elicited by the MSCs. This study highlighted several important points: firstly, human-derived DPSCs were the most neuroprotective and neuritogenic, followed by BMSCs and ADSCs, respectively; secondly, a variety of NTFs were identified to play a significant role in the neuroprotection/neuritogenesis seen, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), as well as other NTFs such as glial cell line-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF) and PDGF-AA/AB/BB; thirdly, the neuritogenic properties of the MSCs were strongly inhibited by Fc-TrkAr, suggesting NGF plays an important role in MSC-mediated axon regeneration. Finally, using Fc-PDGFA/Br inhibitors, our study underscored the important role of DPSC/MSC-derived PDGF-AA and PDGF-AB/BB in retinal neuroprotection confirming a previous study using BMSCs (Johnson et al., 2013). We substantiated our findings using ELISA analyses on conditioned media from MSCs, confirming the secretion of NTFs by the MSCs with significantly higher quantities from DPSCs (Mead et al., 2014). We also performed a PCR array on the MSCs which indicated a diverse NTF profile of the three MSC populations. The distinct NTF profiles of DPSCs, BMSCs and ADSCs underlined the fact that the source of MSC is critical for determining the effectiveness of a planned cellular therapy. The PCR array data also revealed a previously unconsidered, and relatively unknown, factor, VGF-neuropeptide, which was expressed at considerably higher titres in DPSCs than BMSCs or ADSCs. At the time of our studies, very little was known about the neuroprotective/neuroregenerative properties of VGF. Thus, we ventured to investigate the effects of the recombinant VGF-neuropeptide on injured retinal cultures and elucidated that this new factor presented a potent neuroprotective effect (Mead et al., 2014). Considering this novel finding as well as the recently demonstrated importance of FGF-2 in BMSC-mediated neuroprotection of RGCs (Mesentier-Louro et al., 2014), it is very plausible that other neuroprotective/axogenic trophic molecules may be residing in the cocktail of the MSC secretome. Our study using primary human-derived MSCs corroborate our previous findings using rat primary cells that DPSCs were more potent in their in vitro RGC neuroprotection and RGC neuritogenesis which corresponded with their secretion of significantly higher levels of NGF, BDNF and NT-3 than BMSCs (Mead et al., 2013). DPSCs were also more effective in an in vivo model of optic nerve/RGC injury whereby DPSCs promoted a significantly greater increase in RGC survival and a further 2-fold increase in the number of regenerating axons found at distances 100–1,200 μm distal to the lesion site after intravitreal transplantation compared with BMSCs (Mead et al., 2013). This remarkable ability of DPSCs/MSCs to promote axon regeneration of RGCs after intravitreal transplantation has recently been corroborated by another study (Mesentier-Louro et al., 2014).
The question is whether it is possible to further enhance the neurotrophic property of DPSCs/MSCs, and hence their therapeutic potential for nerve repair. In a recent study DPSCs that were differentiated into Schwann cells, a supportive glial cell of the peripheral nervous system, were shown to have significantly higher levels of secreted NTFs (Martens et al., 2013) compared to undifferentiated cells. The effectiveness of differentiating stem cells into glia prior to treating the injured nervous system was evaluated by culturing the cells with injured dorsal root ganglion cells, a neuron found in the peripheral nervous system of the spinal cord. The authors demonstrated a significant increase in survival and neuritogenesis of dorsal root ganglion cells and also showed myelination of the growing neurites by DPSC-derived Schwann cells, in comparison to undifferentiated DPSCs. Although this was only an in vitro study in the peripheral nervous system, it is tempting to speculate that the elevated NTF secretion and subsequent neuroprotection of differentiated DPSC-derived Schwann cells may represent a more efficacious therapy for traumatic and degenerative eye disease and nerve repair.
Engraftment of stem cells in the retina: One interesting observation is the surprising ability for MSCs to survive in vivo when transplanted in the eye, with multiple studies detecting cells months after transplantation (Johnson et al., 2010a; Mead et al., 2013), which may be attributed to the immunoprivileged environment of the eye. However, despite this survival, MSCs were restricted to the vitreous, failing to engraft into the retina. A previous study identified that the barrier to engraftment is the activated glia which may prevent the injected stem cells migrating into the retina (Johnson et al., 2010b). It may be argued that the NTF-secreting MSCs would be more efficacious if in the same retinal microenvironment as the RGCs and even that the MSC survival following transplantation would be more pronounced if embedded in the cellular retina rather than clustered in the vitreous. Therefore, as well as enhancing the neurotrophic profile of MSCs by potentially differentiating them into glia, increasing the propensity for MSCs to engraft within the retina may possibly increase the neuroprotective and axogenic effects further. Further studies are warranted to clarify the most suitable stem cell injection site for retinal neural therapy.
Conclusions: Although we have performed an in depth comparison of three common human-derived MSC types and identified DPSCs as the most efficacious cell type for RGC neuroprotection and axon regeneration, further studies are required to confirm the relative (pre)clinical efficacy of the different human-derived stem cells in vivo and therefore the most “advantageous” MSCs for ocular repair. Noteworthy, early clinical trials have recently started to test the safety of BMSCs for retinal and optic nerve damage (www.clinicaltrials.gov/show/NCT01920867). Based on our recent findings, we propose DPSCs as a novel and advantageous MSC type for retinal neuroprotection and repair (Mead et al., 2013, 2014).
BM was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) (No. BB/F017553/1) and the Rosetrees Trust.
Ann Logan, Martin Berry and Wendy Leadbeater were co-authors on the original paper.
References
- Berry M, Ahmed Z, Lorber B, Douglas M, Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008;26:147–174. [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010a;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci. 2010b;51:960–970. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Dekorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2013;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2013;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueiredo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9:e110722. doi: 10.1371/journal.pone.0110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006;344:1071–1079. doi: 10.1016/j.bbrc.2006.03.231. [DOI] [PubMed] [Google Scholar]


