Spinal cord injury (SCI) is an unexpected event that is both devastating and debilitating, resulting in not just motor and sensory loss, but also autonomic dysfunction of the bladder, bowel and sexual organs. Currently, there are no treatments available to improve outcome following SCI, leaving individuals with permanent and lifelong physical disability. Worldwide it is estimated that more than 500,000 people sustain a SCI each year, with average lifetime cost of paraplegia and quadriplegia estimated at $5 million and $9.5 million respectively. We therefore urgently need effective therapies to improve quality of life following SCI, and this requires a greater understanding of how cell and axonal injury develops after the traumatic event.
Injury to the spinal cord occurs via both primary and secondary injury mechanisms. Primary injury, the mechanical event caused by the initial traumatic insult, is clearly irreversible. However, secondary injury, which involves a cascade of systemic and cellular processes that develop over time after the primary insult, is considered potentially reversible and a target for therapeutic intervention. Edema is a secondary injury factor that has been well documented to contribute to and exacerbate tissue damage following SCI. The development of edema following SCI has been well characterized both within the injury epicentre (Sharma et al., 1991) and in the adjacent segments where a delayed rostrocaudal spread of edema has been demonstrated (Leonard et al., 2013). Importantly, increased edema following injury has been recently shown to raise intrathecal pressure (ITP) at the site of injury (Kwon et al., 2009; Leonard et al., 2015). Such increases in pressure may reduce local tissue blood flow, starving the spinal cord of oxygen and essential substrates, resulting in further secondary injury, cell death and greater functional deficits.
Whilst there are currently no treatments for traumatic SCI, standard clinical management involves surgical stabilisation and decompression of bone fragments. It is also recommended that clinicians maintain mean arterial blood pressure (MABP) in a patient with an acute SCI at 85–90 mmHg with the expectation that the spinal cord will be adequately perfused at these levels (Vale et al., 1997). However, given that spinal cord perfusion pressure (SCPP) is in fact determined by the difference between MABP and ITP, any increase in ITP will ultimately reduce SCPP despite maintained MABP. This knowledge alone highlights the need for a greater understanding of ITP changes following traumatic SCI, and the need for novel approaches aimed at combating elevated pressure.
Increases in intracranial pressure (ICP) following traumatic brain injury (TBI) have long been considered a keystone for clinical management. Indeed, it is widely accepted that elevated ICP significantly contributes to increased mortality and morbidity after TBI, and a variety of approaches including osmotherapy, corticosteroids, hyperventilation and surgery have been introduced in an attempt to limit the swelling and its consequences. The most common surgical intervention in TBI is the performance of a craniotomy in which the dura is opened and a dural substitute inserted to allow the brain to expand beyond the limits normally imposed by an intact dura and skull. Such approaches to ITP management after SCI have not received widespread support, with differences in anatomical structure between the cranium and spinal column being cited. Specifically, it is thought that the longitudinal structure of the spinal cord should allow for dissipation of fluid in both a caudal and rostral direction as opposed to the skull-enclosed brain in TBI, which remains in a “tightly closed box” resulting in herniation of brain tissue rather than dissipation of fluid to the spinal column.
Both experimental and clinical studies have now demonstrated that a significant increase in ITP occurs at the site of SCI, which is not relieved by dissipation of fluid away from the injury site. As an example, our own recent experimental studies have shown that significant and persistent increases in ITP occur in a balloon compression model of traumatic SCI in which the cord is compressed for 5 minutes and then decompressed (Leonard et al., 2015). The initial increase in ITP was associated with space occupying hemorrhage, whilst the more delayed increases at 3 days post-SCI were associated with significantly increased edema development. Given that ITP was found to be maximal at 3 days post-injury, the results suggest that there may be a substantial window of opportunity for therapeutic intervention aimed at reducing this pressure.
The benefits of surgical decompression following SCI have long been debated, with a number of studies showing little or no benefit (Vaccaro et al., 1997). However, within these studies the earliest time point for surgical intervention was 72 hours. Given that edema develops early post injury, reaching highly significant maximal increases by 3 days post-SCI (Leonard et al., 2015), 72 hours post-injury may be too late to have any significant benefit. As such, the timing of surgical decompression has received much attention so as to determine whether more beneficial outcomes could be observed at early versus late surgical decompression times. Vaccaro et al. (1997) found that there is no significant benefit between early and late decompressive surgery following SCI. In contrast, many studies have shown that early decompression significantly increased neurological function both experimentally (Rabinowitz et al., 2008) and clinically (Carlson et al., 2003). Early clinical decompression has also been shown to significantly reduce lesion volume (Carlson et al., 2003) and reduce the presence of neurogenic shock (Tuli et al., 2007). Whilst there is no standard of care regarding the timing of surgical decompression following acute SCI, Fehlings and Perrin (2006) recommend that surgical decompression be performed within 24 hours of injury.
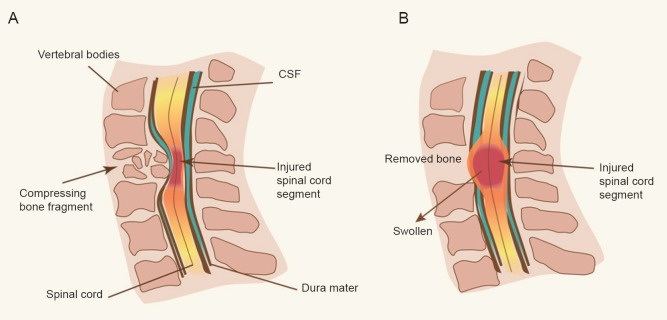
Whilst early surgical decompression has been suggested to improve outcomes following traumatic SCI (Fehlings and Perrin, 2006), the time taken from initial trauma to the surgical procedure can unfortunately be substantial, thus impacting on the potential outcome of patients. Batchelor et al. (2011), employing an experimental model which replicates the clinical condition of bone fragments directly compressing the spinal cord, investigated not just the effects of early versus delayed surgical decompression, but also how injury development can be reduced whilst the cord remains within such a compressive state. Interestingly, it was reported that raised intracanal pressure during a compressive spinal cord state, is one of the most important determinants of neurological recovery following traumatic SCI, with poor long-term behavioral and histological outcomes observed with significantly elevated intracanal pressure. Furthermore, the authors identified that hypothermia may be a potential intervention to reduce intracanal pressure prior to surgical decompression. Whilst this study provides promising improvements in clinical care and importantly “buys time” until surgical decompression can occur, it did not address the continuing edema development that occurs after the surgical decompression. Highlighting the significance of this continuing post-surgical edema, a recent clinical investigation of ITP following traumatic SCI has demonstrated that while a laminectomy sufficiently decompresses the spinal cord, no cerebrospinal fluid (CSF) remains between the swollen spinal cord and surrounding dura on magnetic resonance imaging (Werndle et al., 2014). This suggests that despite surgical decompression, the swollen spinal cord abuts against the non-yielding dura mater, causing resultant increases in ITP and a reduction in spinal cord perfusion (Figure 1).
Figure 1.
Illustration of spinal cord injury in a compressed (A) and decompressed (B) state.
Prior to surgical decompression (A), the cord remains compressed by protruding bone fragments that result in increased intrathecal pressure. However, even following surgical decompression, when the compression bone has been removed (B), there are increases in intrathecal pressure at the injury site given that the cord significantly swells and abuts against the non-yielding dura mater. CSF: Cerebrospinal fluid.
Consistent with this, Kwon et al. (2009) demonstrated that surgical decompression following clinical SCI results in a significant increase in ITP caudal to the injury site. While the cause of this unexpected increase following decompression is unknown, it may be due, in part, to the relief of significant pressure gradients across the injury site while the mechanical obstruction of the spinal cord is in place. Specifically, while the mechanical obstruction exists, there would be greater pressure in the rostral region compared to the caudal. However, once the spinal cord has been decompressed, pressure would equilibrate across the injury site resulting in increased pressure in the caudal region. Alternatively, the continued swelling of the spinal cord may block CSF flow, therefore creating increased pressure over time. Regardless of the cause, ever increasing pressure within the spinal cord despite surgical decompression remains a serious issue, likely resulting in further ischemic tissue damage and ultimately contributing to greater functional deficits.
The focus has thus turned to alternative strategies to prevent the increase in pressure with time following SCI, which initially is associated with haemorrhage although at later time points, is thought to be due to edema formation (Leonard et al., 2015). At the surgical level, one possible novel intervention might be duraplasty. There is certainly evidence in TBI that the performance of a craniotomy with duraplasty helps to alleviate building ICP within the skull. Whether such a strategy might result in real changes in ITP following SCI is unknown. The second approach is pharmacological, where the intervention is targeted at addressing the actual cause of water accumulation rather the symptoms (increased pressure). In this respect, two targets seem particularly attractive. The first is inflammation, where inflammatory factors released either by activated microglia or through neurogenic inflammation, increase the permeability of the blood spinal cord barrier and facilitate vasogenic edema formation (Leonard et al., 2013). Since the increased permeability of the blood spinal cord barrier will result in an osmotic driver for edema formation, eliminating this driver would reduce the development of edema and increased ITP. The second target is the aquaporin water channels, which are both an entry portal for incoming water and an exit channel for edema resolution. Pharmacological agents that either deactivate or activate these water channels at appropriate time points may not only stop water entering the tissue, but may also facilitate exit of water from the tissue.
From the above, it is clear that tissue swelling associated with water accumulation continues despite surgical intervention after SCI. Aside from the effects of swelling on ITP that have been noted above, tissue water accumulation in itself disrupts ion concentrations, action potentials, neurotransmission and metabolism. It is likely that tissue regeneration is also strongly inhibited in such an environment. Targeting the mechanisms of such water accumulation therefore not only reduces increased pressure, thus restoring spinal cord perfusion, but also restores the homeostatic balance that promotes normal physiology, metabolism and any potential tissue regeneration.
AVL is supported by the Neil Sachse Foundation, Australia, a philanthropic organisation supporting research into spinal cord injury.
References
- Batchelor PE, Kerr NF, Gatt AM, Cox SF, Ghasem-Zadeh A, Wills TE, Sidon TK, Howells DW. Intracanal pressure in compressive spinal cord injury: reduction with hypothermia. J Neurotrauma. 2011;28:809–820. doi: 10.1089/neu.2010.1622. [DOI] [PubMed] [Google Scholar]
- Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85-A:86–94. [PubMed] [Google Scholar]
- Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–35. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, Gorelik S, Slobogean GP, Umedaly H, Giffin M, Nikolakis MA, Street J, Boyd MC, Paquette S, Fisher CG, Dvorak MF. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine. 2009;10:181–193. doi: 10.3171/2008.10.SPINE08217. [DOI] [PubMed] [Google Scholar]
- Leonard AV, Thornton E, Vink R. Substance P as a mediator of neurogenic inflammation following balloon compression induced spinal cord injury. J Neurotrauma. 2013;30:1812–1823. doi: 10.1089/neu.2013.2993. [DOI] [PubMed] [Google Scholar]
- Leonard AV, Thornton E, Vink R. The relative contribution of edema and hemorrhage to raised intrathecal pressure following traumatic spinal cord injury. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3543. doi:10.1089/ neu.2014.3543. [DOI] [PubMed] [Google Scholar]
- Rabinowitz RS, Eck JC, Harper CM, Jr, Larson DR, Jimenez MA, Parisi JE, Friedman JA, Yaszemski MJ, Currier BL. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine. 2008;33:2260–2268. doi: 10.1097/BRS.0b013e31818786db. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Winkler T, Stalberg E, Olsson Y, Dey PK. Evaluation of traumatic spinal cord edema using evoked potentials recorded from the spinal epidural space. An experimental study in the rat. J Neurol Sci. 1991;102:150–162. doi: 10.1016/0022-510x(91)90063-d. [DOI] [PubMed] [Google Scholar]
- Tuli S, Tuli J, Coleman WP, Geisler FH, Krassioukov A. Hemodynamic parameters and timing of surgical decompression in acute cervical spinal cord injury. J Spinal Cord Med. 2007;30:482–490. doi: 10.1080/10790268.2007.11754582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, Herbison GJ, Northrup BE. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:2609–2613. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH, Smielewski P, Jamous A, Bell BA, Zoumprouli A, Papadopoulos MC. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42:646–655. doi: 10.1097/CCM.0000000000000028. [DOI] [PubMed] [Google Scholar]