Abstract
Spinal cord injury (SCI) is a devastating condition that produces significant changes in the lifestyle of patients. Many molecular and cellular events are triggered after the initial physical impact to the cord. Two major phases have been described in the field of SCI: an acute phase and late phase. Most of the therapeutic strategies are focused on the late phase because this provides an opportunity to target cellular events like apoptosis, demyelination, scar formation and axonal outgrowth. In this mini-review, we will focus on two agents (tamoxifen and a Src kinase family inhibitor known as PP2) that have been shown in our laboratory to produce neuroprotective (increase cell survival) and/or regenerative (axonal outgrowth) actions. The animal model used in our laboratory is adult female rat (~250 g) with a moderate contusion (12.5 mm) to the spinal cord at the T10 level, using the MASCIS impactor device. Tamoxifen or PP2 was administered by implantation of a 15 mg pellet (Innovative Research of America, Sarasota, FL, USA) or by intraperitoneal injections (1.5 mg/kg, every 3 days), respectively, to produce a long-term effect (28 days). Tamoxifen and the Src kinase inhibitor, PP2, are drugs that in rats with a moderate spinal cord injury promote functional locomotor recovery, increase spared white matter tissue, and stimulate axonal outgrowth. Moreover, tamoxifen reduces the formation of reactive oxygen species. Therefore, these drugs are possible therapeutic agents that have a neuroprotective/regenerative activity in vertebrates with SCI.
Keywords: tamoxifen, Src kinase, PP2, trauma, regeneration, neuroprotection
Introduction
Spinal cord injury (SCI) is a detrimental condition that affects mostly young adult individuals (National SCI Statistical Center, 2014). Patients with this condition are subject to drastic changes in their lifestyle, as well as economic stress. After the trauma to the cord, several events take place at the lesion epicenter and surrounding areas (rostral and caudal). These changes start from the first millisecond to months after the lesion, and this eventually lead to the clinical symptoms that are observed in the patient. Among these symptoms are the loss of body functions such as voluntary muscle movement rendering the patient paraplegic or tetraplegic (depending on the location of the injury), lack of somatosensory information, decreased breathing capacity, limited bowel and bladder control, and in some situations development of chronic pain.
SCI is initiated by the physical impact to the cord that triggers molecular and cellular changes immediately after injury resulting in an acute phase where necrosis, axotomy, damage to blood vessels, ischemia, excitoxicity, increase in the production of free radicals and edema occurs (Hulsebosch, 2002). Inflammation follows necrosis, facilitating axonal degeneration and demyelination at the lesion epicenter (Donnelly and Popovich, 2008). In the chronic phase, further inflammation and demyelination continues, as well as programmed cellular death (apoptosis), reactive gliosis and the formation of a gliotic scar (Hulsebosch, 2002; Fitch and Silver, 2008). Neuroprotective compounds are necessary to reduce the number of dead cells (neurons, astrocytes and/or oligodendrocytes) at the lesion epicenter and surrounding areas, and regenerative drugs are needed to promote axonal outgrowth (by either increase in the sprouting and/or regeneration process) across the damaged region. Axonal outgrowth could be achieved by either promoting or stimulating neurite outgrowth or by reducing or blocking the expression of repulsive/repellent factors at the injury site. Therefore, multi-active drugs are required to target most of the cellular events initiated by the physical insult to the spinal cord and as a result may result in some functional locomotor recovery.
Neuroprotective effect of tamoxifen
The use of estradiol as a neuroprotective hormone is very well known in central nervous system conditions like cerebral stroke, brain trauma and spinal cord injury (Dhandapani and Brann, 2002; Yune et al., 2004; Sribnick et al., 2005; Garcia-Segura and Balthazart, 2009; Arevalo et al., 2010; Etgen et al., 2011; Sirriphorn et al., 2012). This hormone promotes cell survival either by an increase in the expression of anti-apoptotic genes (Segarra and Lee, 2004; Scott et al., 2012) or by a decrease in pro-apoptotic genes (Chaovipoch et al., 2006; Sribnick et al., 2006), resulting in some functional locomotor recovery (Sribnick et al., 2010). Another neuroprotective mechanism proposed for estradiol after injury is by its steroidal structure. Several investigators propose that the phenol hydroxyl ring provides antioxidant and anti-inflammatory activities (Sugioka et al., 1987; Behl et al., 1997; Winterle et al., 2001). However, the mitogenic effect of estradiol in tissues like breast and uterus, deep vein thrombosis or pulmonary embolism restricts the use of this hormone as a therapeutic drug for spinal cord injury patients (Cummings et al., 2002; Sare et al., 2008; Laliberté et al., 2011). Therefore, the consideration of tamoxifen as a neuroprotective and regenerative drug is under investigation in our laboratory and others (Don Carlos et al., 2009; Tian et al., 2009; Ismailoglu et al., 2010; Franco et al., 2013; Guptarak et al., 2014; Mosquera et al., 2014).
Tamoxifen is a selective estrogen receptor modulator (SERM) and an FDA approved drug that interacts with estrogen receptors, producing estrogenic or anti-estrogenic effects. The final outcome depends on the target tissue and the expression of co-activators or co-repressors that interact with the activated estrogen receptor (McDonnel and Wardell, 2010). For this reason, tamoxifen is used mostly for the treatment of cancer, due to its antagonistic activity in tissues like breast and uterus. On the other hand, tamoxifen acts as a neuroprotective drug in pathologies like amyotrophic lateral sclerosis (Traynor et al., 2006), ischemic brain injury (Dhandapani and Brann, 2002; Kimelberg et al., 2003; Mehta et al., 2003; Zhang et al., 2007) and spinal cord injury (Tian et al., 2009; Ismailoglu et al., 2010; Guptarak et al., 2014; Mosquera et al., 2014).
Tamoxifen reduces the edema formation in adult rats after SCI and results in some locomotor recovery (Tian et al., 2009; Ismailoglu et al., 2010; Guptarak et al., 2014; Mosquera et al., 2014). This compound crosses the blood brain barrier (Biegon et al., 1996) and could produce some behavioral improvement through a reduction in several molecular/cellular mechanisms related to the formation of reactive oxygen species, inflammatory molecules or the formation of reactive astrocytes (Zhang et al., 2007; Barreto et al., 2009; Arevalo et al., 2011; Sun et al., 2013). In our laboratory, we observed an improvement in functional locomotor behavior when ovariectomized female rats were treated with tamoxifen. Significant changes were observed in the Basso, Beattie and Bresnahan (BBB) open field test (Mosquera et al., 2014), as well as in the number of animals that crossed the round beam test at 7, 14, 21 and 28 days post injury (Figure 1). The narrow beam crossing test was used to monitor the contribution of equilibrium and balanced posture (mediated through the vestibulospinal and propioception related tracts) on walking. Rats crossed a 3 feet long round bar (2.5 cm diameter) suspended about 15 inches from the ground (Merkler et al., 2001). The percent of rats in each group able to cross the bar after SCI, with or without the use of their hindlimbs, was determined weekly. Estradiol and tamoxifen treated rats showed a significant higher capacity for crossing the round beam at 7, 14, 21 and 28 days post injury than non-treated control group. Fifty percent of the rats in the estradiol (n = 12) and tamoxifen (n = 10) groups were able to cross the round beam without paw positioning but maintaining some balance by the first week. In contrast, less than 0.01 % of the control rats (n = 13) were able to cross the beam. This pattern was kept until the 4th week where 75% of the estradiol-treated rats were able to cross the beam with or without paw positioning and only 0.2% of the control group crossed. The locomotor recovery was associated with an increase in the amount of white mater spared tissue and a decrease in the superoxide activity (Mosquera et al., 2014). Estradiol or tamoxifen may upregulate the expression of glutathione and/or superoxide dismutase proteins, or these multi-active compounds could work as free radical scavengers to reduce reactive oxygen species after trauma to the spinal cord (Prokai and Simpkins, 2007). Post-treatment experiments demonstrated that tamoxifen administration after SCI produced a significant locomotor recovery in the BBB open field test and beam crossing test, and this effect is related to an increase in the amount of spared tissue, increase in neurofilament immunoreactivity caudal to the lesion epicenter and reduction in the reactive gliosis (unpublished results: Colon et al., 2014). Additional mechanisms that may explain the locomotor improvement by tamoxifen administration after SCI could be a reduction in the edema, a diminution in the apoptotic process, a decline in the production of inflammatory cytokines (TNFα & IL-1β), and a decrease of myelin loss and inhibitory proteins (Tian et al., 2009; Ismailoglu et al., 2010; Liu et al., 2010; Guptarak et al., 2014). Similar results with tamoxifen were observed after brain injury and cerebral ischemia (Zhang et al., 2007; Liu et al., 2010; Franco-Rodriguez et al., 2013; Sun et al., 2013). Therefore, tamoxifen (an FDA approved drug) should be considered as a neuroprotective agent to treat spinal cord injury conditions (Figure 2A).
Figure 1.
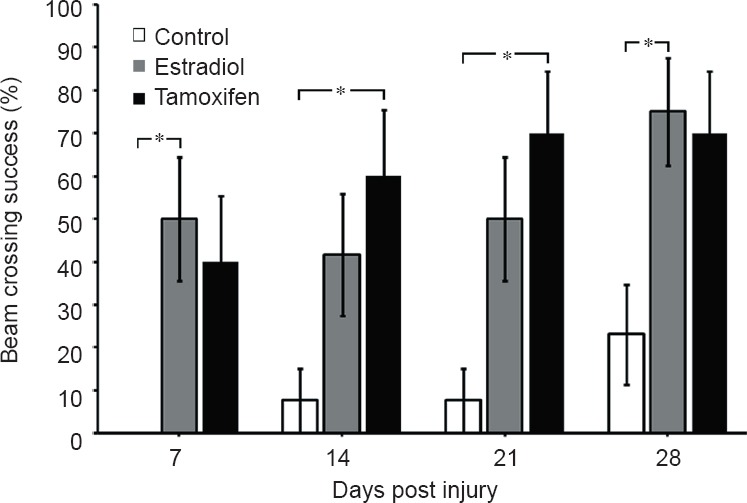
Estradiol and tamoxifen (TAM) improve the behavioral performance of injured rats in the beam crossing test.
Ovariectomized rats were treated with estradiol (3 mg) or tamoxifen (15 mg), either by silastic tubing or commercial pellets, respectively, 1 week prior to spinal cord injury. The injured rats were tested weekly and the number of rats that crossed the narrow beam was analyzed with analysis of varivance followed by Bonferroni post hoc test. Results demonstrated a significance difference between control (n = 13) and treated (estradiol: n = 12; TAM: n = 10) animals (*P < 0.0016).
Figure 2.
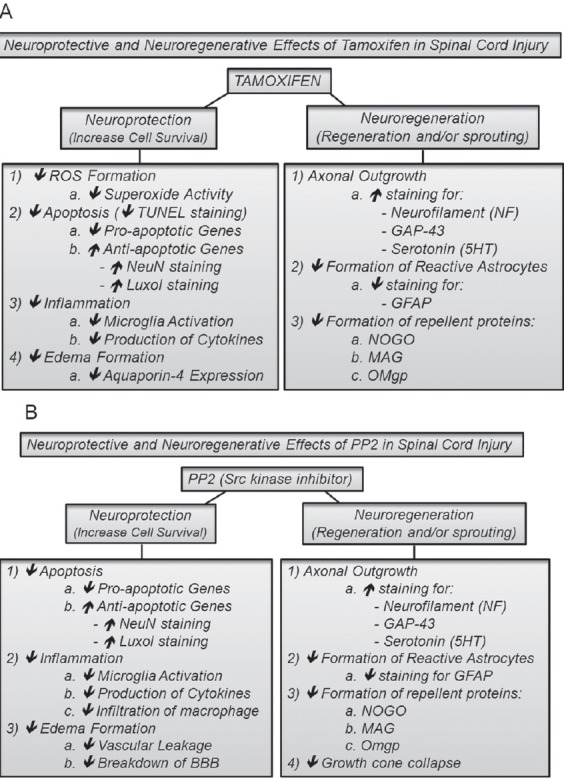
Neuroprotective and neurodegenerative effects of TAM and PP2 in spinal cord injury.
TAM (A) and PP2 (B) are drugs that in a moderate spinal cord injury act as neuroprotective and neuroregenerative agents that promote functional locomotor recovery and increase spared white matter tissue. Additionally, TAM plays an important role as superoxide scavenger. ↑ Increase; ↓ decrease; TAM: tamoxifen; PP2: Src kinase inhibitor; ROS: reactive oxygen species; NF: neurofilament; NeuN: neuronal transcription factor; 5HT: 5-hydroxytryptamine; BBB: blood brain barrier; GAP-43: growth associated protein 43; GFAP: glial fibrillary acidic protein.
Neuroprotective/regenerative effect of PP2
Investigators are attempting to enhance axonal outgrowth after spinal cord injury either by infusing factors that promote/stimulate neurite outgrowth (Lu and Tuszynski, 2008) or by blocking the factors that have a non-permissive/repulsive effect on regeneration or sprouting (Bouquet and Nothias, 2007). Oligodendrocytes and reactive astrocyte produce these repellent factors. Among the repulsive factors are NOGO, MAG, OMgp, chondroitin sulfate and the Eph receptors (Chen et al., 2000; Wilson et al., 2002; Grados-Munro and Fournier 2003; McGee and Strittmatter 2003; Fitch and Silver, 2008). The complex intracellular signaling by which these proteins hold their effects is through activation of kinases that promote growth cone collapse. The aforementioned NOGO-A is activated by Src (Yokoyama et al., 2006) and the signaling cascade activated by EphA receptors after ligand binding also requires Src activation to induce the repulsive activity (Knoll and Drescher, 2004).
The Src-tyrosine kinases (SFKs), is the largest family of non-receptor tyrosine kinases. The SFKs are widely expressed in many cell types and in different subcellular compartments (Thomas and Brugge, 1997). SFKs induce cellular responses associated with proliferation, growth control, survival, differentiation and cytoskeletal arrangements (Thomas and Brugge, 1997; Kalia et al., 2004; Zhao et al., 2009). SFKs can be switched from an inactive to an active state through control of its phosphorylation state, or through protein interactions.
SFKs are the meeting point of several signaling pathways associated with neuropathologies like stroke, Alzheimer's disease, epilepsy and central nervous system trauma (Lennmyr et al., 2004; Jadhav et al., 2007; Liang et al., 2009; Liu et al., 2014). This family of kinases can phosphorylate or mediate the activation of central nervous system proteins such as epidermal growth factor (EGF), extracellular regulated kinase (ERK), Eph, NOGO, myelin associated glycoprotein (MAG) (Thomas and Brugge, 1997; Georgakopoulos et al., 2006; Yokoyama et al., 2006; Slack et al., 2008; Wu et al., 2011; Tanaka et al., 2013). After intracerebral hemorrhage in the striatum, blockade of SFKs with PP1-inhibitor, decreased the number of TUNEL-stained cells and reduced behavioral abnormalities (Ardizzone et al., 2007). In addition, the activation of SFK mediates changes in the blood brain barrier permeability and promotes edema by phosphorylation of metalloproteinase, tight junction proteins and other BBB proteins. The access of SFK inhibitors, like PP1 and PP2, to the endothelial cells from rat brain after its systemic administration inhibits post-ischemic Src activation and vascular leakage, which resulted in the reduction of brain edema (Liang et al., 2009). Moreover, several studies in the adult spinal cord focus in the pain mediated by Src after nerve injury. The induced neuropathic pain by transection of spinal nerve appears to be related to N-methyl-D-aspartate receptor (NMDA) receptor currents potentiated by SFKs (Katsura et al., 2006). The nerve injury induces activation of SFKs in microglia of the dorsal root ganglion but also in the spinal cord. This activation contributes to the development of hypersensitivity to mechanical stimulation that has been associated to an increased ERK phosphorylation in microglia but not with astrocytes or neurons. Another signaling molecule associated with inflammatory hyperalgesia is EphrinB2. EphrinB2 induces tyrosine phosphorylation of the NMDA receptor-2B subunit via SFKs (Slack et al., 2008).
In the spinal cord, SFKs are also involved in the repulsion of the axonal growth cone during development (Thomas and Brugge, 1997; Kalia et al., 2004). SFK have also been associated with primary and secondary damage after spinal cord injury. SFKs, as mentioned before, has been related to the activation of NMDA receptor and inflammatory pain but also to the early inflammatory response mediated by uncontrolled microglia stimulation. Histological evidence from spinal cord tissue treated with 1-(1,1-dimethylethyl)-3-(4-methylphenyl)-1H-pyrazolo[3,4-d] pyrimidin-4-amine (PPI) after 10 minutes of compression show a decrease in the edema, macrophage infiltration and inflammation (Akiyama et al., 2004). SFKs have also been associated with secondary damage after injury (Akiyama et al., 2004). During secondary damage, the activation of SFK mediated by vascular endothelial growth factor (VEGF), leads to stimulation of endothelial cells survival, angiogenesis and edema (Akiyama et al., 2003). In terms of neuroprotection, the inhibition of SFKs reduced and restricted the macrophages infiltration to the lesion area as early as 3 days after injury (Akiyama et al., 2004). In models of cerebral ischemia and surgically induced brain injury, as occur in spinal cord injury, blockade of SFK prevented VEGF-mediated signaling and vascular leakage, reduction in brain edema and decreased breakdown of the blood brain barrier (Jadhav et al., 2007; Liang et al., 2009). Deposition of fibrinogen in the central nervous system after damage to the blood brain barrier, either after a spinal cord transection or contusion, inhibits neurite outgrowth through SFK activation (Schachtrup et al., 2007).
Until now, no study has elucidated the action of SFKs in long-term recovery after spinal cord injury. In our laboratory, we have demonstrated with histological, immunohistochemical and behavioral studies that long-term blockade of SFKs with PP2 in lesioned rats not only produced long-term functional locomotor recovery, but also augmented spared tissue and increased serotonin fibers caudal to the lesion epicenter (Rosas et al., 2014). Animals treated with PP2 after SCI presented higher scores in the BBB open field test and also demonstrated a better performance in the beam crossing test (Rosas et al., 2014). In contrast to other studies related to neuropathic pain, the effect of PP2 in locomotor recovery was not associated with a reactive gliosis response or the amount of microglial cells present at the injury site. We suggest that phosphorylation of ephexin1 by Src kinase after spinal cord injury may be involved in the collapse of axonal growth cones, as has been demonstrated during CNS development after EphA receptor (Knöll and Drescher, 2004), generating a repulsive microenvironment at the injury site. When PP2 was administered to the injured rats the level of SFKs activation was reduced and the activation of ephexin1 decreased, resulting in a better environment for axonal outgrowth. Because SFKs are associated with different events present after spinal cord injury, more studies are necessary to elucidate other key elements upstream or downstream in its signaling cascade that leads to inhibition of locomotor recovery.
Conclusions and future directions
The information discussed previously supports the consideration of tamoxifen and PP2 in SCI conditions. These drugs demonstrated their neuroprotective actirity because of their effect in functional locomotor recovery and increased spared tissue after SCI. Decrease in ROS, inflammation and apoptosis are possible mechanisms regulated by TAM (Figure 2A). However, additional studies are required to determine which genes are activated in these cellular processes. Moreover, if tamoxifen also provides some regenerative activity and the proteins involved in any axonal outgrowth are also unclear. Blockade of Src activation with PP2 demonstrated, in the other hand, some regenerative activity due to an increase in serotonin fibers caudal to the lesion epicenter (Figure 2B). Although some molecular mechanisms have been related to tamoxifen and PP2 activity after SCI, further experiments are warranted to establish the role of these drugs in the activation of anti-apoptotic genes, downregulation of pro-apoptotic proteins, changes in the expression of proteins that may generate a repulsive environment for axonal regeneration and expression of cytoskeletal proteins related to neurite outgrowth. Moreover, in order to determine the future use of tamoxifen or SFK inhibitors (PP1 or PP2) for the treatment of human spinal cord injury, more studies should be aimed to determine the optimal doses and therapeutic window for their use. Finally, the spinal cord injury field should consider the neuroprotective and neuroregenerative effects associated with the concomitant use of both drugs (tamoxifen and PP1/PP2) as a therapy for SCI.
Acknowledgments:
We thank Dr. Laurivette Mosquera for the analysis of the beam crossing data and Mr. Hector Bravo for his comments on the manuscript.
Footnotes
Funding: The project was partially supported by the MBRS-RISE Program (R25 GM061838) and COBRE (5P20-GM103642).
Conflicts of interest: None declared.
References
- Akiyama C, Yuguchi T, Nishio M, Fujinaka T, Taniguchi M, Nakajima Y, Yoshimine T. Src family kinase inhibitor PP1 improves motor function by reducing edema after spinal cord contusion in rats. Acta Neurochir Suppl. 2003;86:421–423. doi: 10.1007/978-3-7091-0651-8_87. [DOI] [PubMed] [Google Scholar]
- Akiyama C, Yuguchi T, Nishio M, Tomishima T, Fujinaka T, Taniguchi M, Nakajima Y, Kohmura E, Yoshimine T. Src family kinase inhibitor PP1 reduces secondary damage after spinal cord compression in rats. J Neurotrauma. 2004;21:923–931. doi: 10.1089/0897715041526230. [DOI] [PubMed] [Google Scholar]
- Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38:1621–1625. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J Neuroendocrinol. 2011;24:183–190. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochimic et Biophys Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernia O, Carrero P, Azcoitia I, Garcia-Segura LM. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain z Effects of aging and prolonged depletion of ovarian hormones. Neuroendocrinol. 2009;150:5010–5015. doi: 10.1210/en.2009-0352. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. jMol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Biegon A, Brewster M, Degani H, Pop E, Somjen D, Kaye AM. A permanently charged tamoxifen derivative displays anticancer activity and improved tissue selectivity in rodents. Cancer Res. 1996;56:4328–4331. [PubMed] [Google Scholar]
- Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16. doi: 10.1007/978-0-387-76715-4_1. [DOI] [PubMed] [Google Scholar]
- Chaovipoch P, Bozak KA, Gernhold LM, West EJ, Chongthammakun S, Foyd CL. 17β-Estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber a B, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Colon JM, Cajigas A, Santiago JM, Torrado AI, Salgado IK, Grafals N, Miranda JD. Society for Neuroscience. Washington, DC: 2014. Tamoxifen treatment promotes locomotor recovery, increases white matter spared tissue and decreases reactive gliosis after chronic spinal cord injury. Poster 315.20/U10. [Google Scholar]
- Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Reprod. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology 34S. 2009;1:113–122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Jover-Mengual T, Zukin RS. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Front Neuroendocrinol. 2011;32:336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Rodriguez NE, Dueñas-Jimenez JM, De la Torre-Valdovinos B, Lopez-Ruiz JR, Hernandez-Hernandez L, Dueñas-Jimenez SH. Tamoxifen favoured the rat sensorial cortex regeneration after a penetrating brain injury. Brain Res Bull. 2013;98:64–75. doi: 10.1016/j.brainresbull.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendrocrinol. 2009;30:5–9. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados-Munro EM, Fournier AE. Myelin-associated inhibitors of axon regeneration. J Neurosci Res. 2003;74:479–485. doi: 10.1002/jnr.10803. [DOI] [PubMed] [Google Scholar]
- Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O. The cáncer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma. 2014;31:268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Ismailoglu O, Oral B, Gorgulu A, Sutcu R, Demir N. Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J Clin Neurosci. 2010;17:1306–1310. doi: 10.1016/j.jocn.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Matchett G, Hsu PK, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–686. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Jin Y, Charniga C, Feustel PJ. Neuroprotective activity of tamoxifen in permanent focal ischemia. J Neurosurg. 2003;99:138–142. doi: 10.3171/jns.2003.99.1.0138. [DOI] [PubMed] [Google Scholar]
- Knöll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberté F, Dea K, Sheng-Duh M, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism?Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18:1052–1059. doi: 10.1097/gme.0b013e3182175e5c. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlstrom H, Terént A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand. 2004;110:175–179. doi: 10.1111/j.1600-0404.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- Liang S, Pong K, Gonzales C, Chen Y, Ling H, Mark RJ, Boschelli F, Boschelli DH, Ye F, Carolina A, Sosa B, Mansour TS, Frost P, Wood A, Pangalos MN, Zaleska MM. Neuroprotective profile of novel src kinase inhibitors in rodent models of cerebral ischemia. J Pharmacol Exp Ther. 2009;331:827–835. doi: 10.1124/jpet.109.156562. [DOI] [PubMed] [Google Scholar]
- Liu da Z, Sharp FR, Van KC, Ander BP, Ghiasvand R, Zhan X, Stamova B, Jickling GC, Lyeth BG. Inhibition of Src family kinases protects hippocampal neurons and improves cognitive function after traumatic brain injury. J Neurotrauma. 2014;31:1268–1276. doi: 10.1089/neu.2013.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tian D, Li Z, Qu W, Zhan Y, Xie M, Yu Z, Wang W, Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol. 2008;209:313–320. doi: 10.1016/j.expneurol.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10:620–628. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Dhandapani KM, De Sevilla LM, Clinton Webb R, Mahesh VB, Brann DW. Tamoxifen, a selective estrogen receptor modulator, reduces ischemic damage caused by middle cerebral artery occlusion in the ovariectomized female rat. Euroendocrinology. 2003;77:44–50. doi: 10.1159/000068332. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera L, Colon JM, Santiago JM, Torrado AI, Melendez M, Segarra AC, Rodriguez-Orengo JF, Miranda JD. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: Their ntioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National SCI, Statistical Center (2014) Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 37:117–118. doi: 10.1179/1079026813Z.000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas OR, Torrado AI, Santiago JM, Rodriguez AE, Salgado IK, Miranda JD. Long-term treatment with PP2 after spinal ord injury resulted in functional locomotor recovery and increased spared tissue. Neural Regen Res. 2014;9:2164–2173. doi: 10.4103/1673-5374.147949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous Vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104:11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Zhang QG, Wang R, Vadlamudi R. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33:85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Lee SJ. Neuroprotective effects of estrogen. In: Legato M, editor. In: Principles of Gender-Specific Medicine. 11. San Diego, CA: Elsevier Science; 2004. pp. 96–103. [Google Scholar]
- Siriphorn A, Dunham KA, Chompoopong S, Floyd CL. Postinjury administration of 17β-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J Comp Neurol. 2012;520:2630–2646. doi: 10.1002/cne.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack S, Battaglia a, Cibert-Goton V, Gavazzi I. EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family inases during inflammatory hyperalgesia. Neuroscience. 2008;156:175–183. doi: 10.1016/j.neuroscience.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury. J Neurosci Res. 2005;82:83–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84:10641075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- Sun X, Ji C, Hu T, Wang Z, Chen G. Tamoxifen as an effective neuroprotectant against early brain injury and learning deficits induced by subarachnoid hemorrhage: possible involvement of inflammatory signaling. J Neuroinflam. 2013;10:157–169. doi: 10.1186/1742-2094-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Fujita Y, Ueno M, Shultz LD, Yamashita T. Suppression of SHP-1 promotes corticospinal tract sprouting and unctional recovery after brain injury. Cell Death Dis. 2013;4:ne567. doi: 10.1038/cddis.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W. Tamoxifen attenuates inflammatory-Mediated damage and improves functional outcome after spinal cord injury in rats. J Neurochem. 2009;109:1658–1667. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, Cudkowicz ME. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–27. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Irizarry-Ramirez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11:229–239. [PubMed] [Google Scholar]
- Winterle JS, Mill T, Harris T, Goldbeck RA. Absolute kinetic characterization of 17β-estradiol as a radical-scavenging, Antioxidant synergist. Arch Biochem Biophis. 2001;392:233–244. doi: 10.1006/abbi.2001.2431. [DOI] [PubMed] [Google Scholar]
- Wu HC, Chang CH, Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. EphrinB2 induces pelvic-urethra reflex potentiation via Src kinase-dependent tyrosine phosphorylation of NR2B. Am J Physiol Renal Physiol. 2011;300:F403–411. doi: 10.1152/ajprenal.00520.2010. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Tezuka T, Hoshina N, Nakazawa T, Yamamoto T. Phosphorylation at Tyr-694 of Nogo-A by Src-family kinases. Biochem Biophys Res Commun. 2006;349:1401–1405. doi: 10.1016/j.bbrc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol. 2007;204:819–827. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Cao X, Wu G, Loh HH, Law P. Neurite outgrowth is dependent of the association of c-Src and lipid rafts. Neurochem Res. 2009;34:2197–2205. doi: 10.1007/s11064-009-0016-7. [DOI] [PubMed] [Google Scholar]


