Abstract
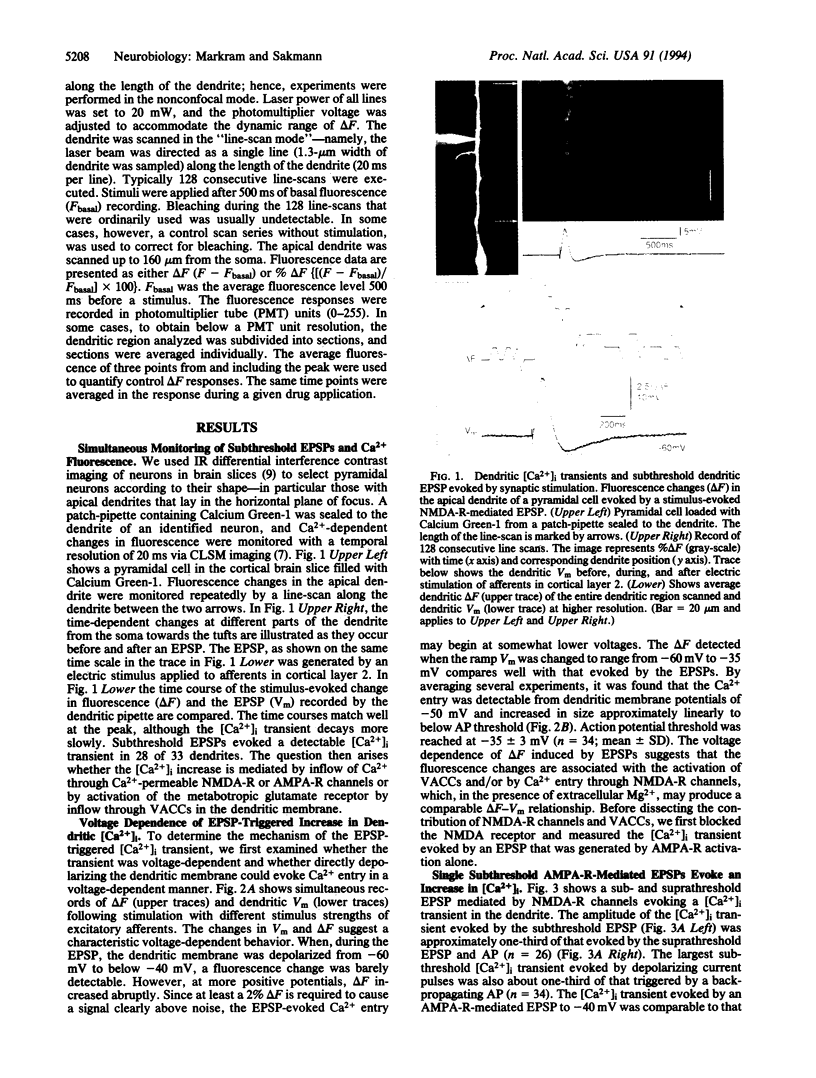
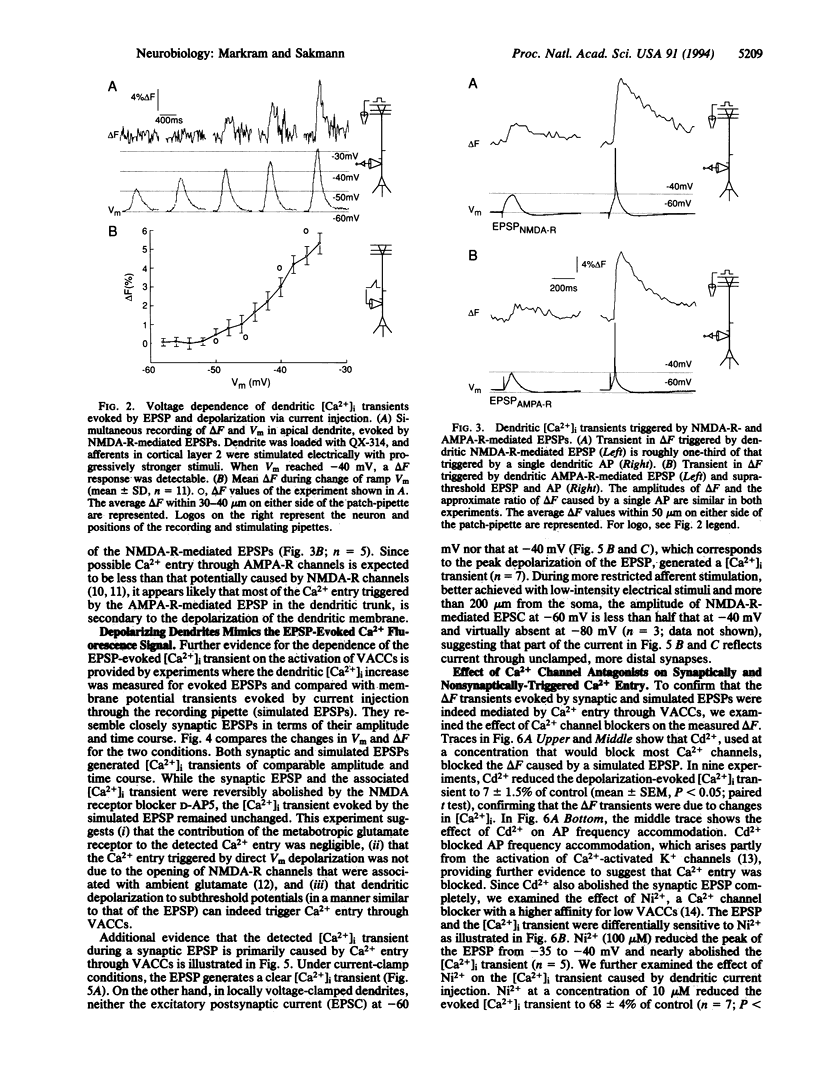
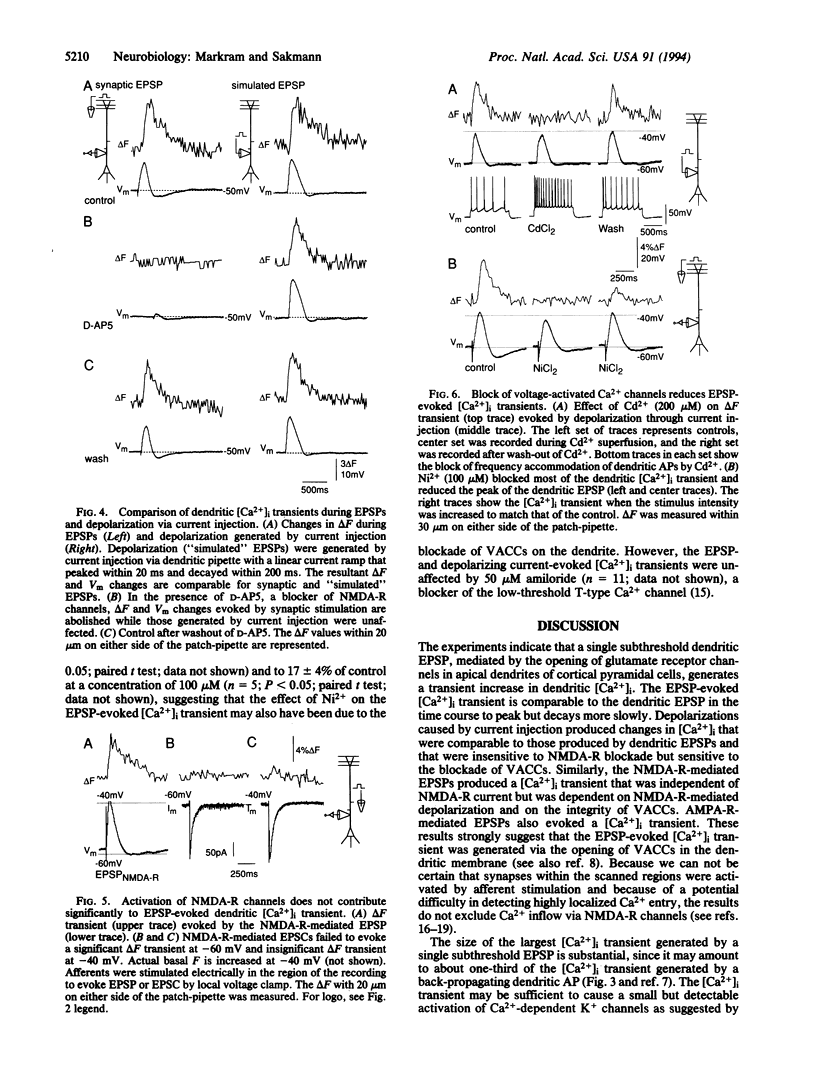
Simultaneous recordings of membrane voltage and concentration of intracellular Ca2+ ([Ca2+]i) were made in apical dendrites of layer 5 pyramidal cells of rat neocortex after filling dendrites with the fluorescent Ca2+ indicator Calcium Green-1. Subthreshold excitatory postsynaptic potentials (EP-SPs), mediated by the activation of glutamate receptor channels, caused a brief increase in dendritic [Ca2+]i. This rise in dendritic [Ca2+]i was mediated by the opening of low-voltage-activated Ca2+ channels in the dendritic membrane. The results provide direct evidence that dendrites do not function as passive cables even at low-frequency synaptic activity; rather, a single subthreshold EPSP changes the dendritic membrane conductance by opening Ca2+ channels and generating a [Ca2+]i transient that may propagate towards the soma. The activation of these Ca2+ channels at a low-voltage threshold is likely to influence the way in which dendritic EPSPs contribute to the electrical activity of the neuron.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford S., Frenguelli B. G., Schofield J. G., Collingridge G. L. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol. 1993 Sep;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai Y., Friedman A., Connors B. W., Gutnick M. J. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993 Jan-Feb;3(1):26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991 Feb;65(2):371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Gutnick M. J. Intracellular Calcium and Control of Burst Generation in Neurons of Guinea-Pig Neocortex in Vitro. Eur J Neurosci. 1989 Jul;1(4):374–381. doi: 10.1111/j.1460-9568.1989.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Guthrie P. B., Segal M., Kater S. B. Independent regulation of calcium revealed by imaging dendritic spines. Nature. 1991 Nov 7;354(6348):76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Hamill O. P., Prince D. A. Sodium channels in dendrites of rat cortical pyramidal neurons. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2473–2477. doi: 10.1073/pnas.86.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D., Chapman B., Stryker M. P. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H., Ross W. N., Jaffe D., Callaway J. C., Lasser-Ross N., Lisman J. E., Johnston D. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992 Dec;9(6):1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Nicoll A., Larkman A., Blakemore C. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. J Physiol. 1993 Aug;468:693–710. doi: 10.1113/jphysiol.1993.sp019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. J., Petrozzino J. J., Nicoll R. A., Connor J. A. The role of Ca2+ entry via synaptically activated NMDA receptors in the induction of long-term potentiation. Neuron. 1993 Nov;11(5):817–823. doi: 10.1016/0896-6273(93)90111-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Regehr W., Kehoe J. S., Ascher P., Armstrong C. Synaptically triggered action potentials in dendrites. Neuron. 1993 Jul;11(1):145–151. doi: 10.1016/0896-6273(93)90278-y. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989 Nov 10;246(4931):815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., Zhou Z., Konnerth A., Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993 Jul;11(1):133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Segal M., Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol. 1992 Mar;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994 Jan 6;367(6458):69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Hagihara K., Sato H., Hata Y. NMDA receptors in the visual cortex of young kittens are more effective than those of adult cats. Nature. 1987 Jun 11;327(6122):513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]