Abstract
Diffusion tensor imaging is a sensitive way to reflect axonal necrosis and degeneration, glial cell regeneration and demyelination following spinal cord injury, and to display microstructure changes in the spinal cord in vivo. Diffusion tensor imaging technology is a sensitive method to diagnose spinal cord injury; fiber tractography visualizes the white matter fibers, and directly displays the structural integrity and resultant damage of the fiber bundle. At present, diffusion tensor imaging is restricted to brain examinations, and is rarely applied in the evaluation of spinal cord injury. This study aimed to explore the fractional anisotropy and apparent diffusion coefficient of diffusion tensor magnetic resonance imaging and the feasibility of diffusion tensor tractography in the evaluation of complete spinal cord injury in rats. The results showed that the average combined scores were obviously decreased after spinal cord transection in rats, and then began to increase over time. The fractional anisotropy scores after spinal cord transection in rats were significantly lower than those in normal rats (P < 0.05); the apparent diffusion coefficient was significantly increased compared with the normal group (P < 0.05). Following spinal cord transection, fractional anisotropy scores were negatively correlated with apparent diffusion coefficient values (r = –0.856, P < 0.01), and positively correlated with the average combined scores (r = 0.943, P < 0.01), while apparent diffusion coefficient values had a negative correlation with the average combined scores (r = –0.949, P < 0.01). Experimental findings suggest that, as a non-invasive examination, diffusion tensor magnetic resonance imaging can provide qualitative and quantitative information about spinal cord injury. The fractional anisotropy score and apparent diffusion coefficient have a good correlation with the average combined scores, which reflect functional recovery after spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, spinal cord transection, average combined score, magnetic resonance imaging, diffusion tensor imaging, fractional anisotropy, apparent diffusion coefficient, fiber tractography, neural regeneration
Introduction
Spinal cord injury (SCI) is a kind of severe impairment in the central nervous system, which usually causes sensory, motor and autonomic disorders to varying degrees (Van Goethem et al., 2005; Zhang et al., 2013; Ma et al., 2014). At present, SCI can be evaluated through two indicators: histopathological findings and spinal cord function scores. The former is an invasive examination that cannot be used in the long term in individuals, thus restricting its clinical application. The latter is prone to be influenced by subjective factors. Therefore, it is crucial that we find a feasible method for the evaluation of SCI and functional restoration, as well as long-term observation in the clinic.
Magnetic resonance imaging (MRI) is a commonly used method for the diagnosis of SCI and relevant disease (Boldin et al., 2006; Bozzo et al., 2011). Conventional MRI is better than CT examination in visualizing the morphology of the spinal cord and the variation of signals, but it is difficult to assess functional status and fine structure using T1, T2 and other parameters (Kulkarni et al., 1988; Schwartz and Hackney, 2003; Ducreux et al., 2006). The development of diffusion tensor imaging (DTI) allows a more sensitive diagnosis of SCI than conventional MRI examination (Hesseltine et al., 2006; Chang et al., 2010; Rajasekaran et al., 2010; Song et al., 2011; Kerkovskύ et al., 2012; Mulcahey et al., 2012b). Fiber tractography (FT) contributes to the visualization of white matter fibers, structural integrity and the damage of the fiber bundle (Basser and Jones, 2002; Fujiyoshi et al., 2013). Currently, DTI has been widely used in brain examination (Jiang et al., 2010; Young et al., 2010; Van der Eerden et al., 2013; Clemm von Hohenberg et al., 2014; Hasan et al., 2014; Jia et al., 2014; Sternberg et al., 2014), but has rarely been reported in the detection of SCI (Brennan et al., 2013).
DTI is a non-invasive means of examination (Vedantam et al., 2014) that can provide objective information about the degree of SCI accurately, qualitatively and quantitatively through a long-term follow-up observation. After parameter optimization and image post-processing, DTI can exclude the artifacts caused by cerebrospinal fluid and respiration, and is expected to provide strong support for the clinical diagnosis and treatment of SCI (Song et al., 2002; Mukherjee, 2005; Kamble et al., 2011). Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) are the common parameters in DTI. FA is an indicator of the quantitative assessment of the displacement of water molecules depending on their direction, and reflects the ratio of the anisotropic component in the diffusion tensor. ADC is used to quantitatively assess the diffusion of water molecules, independent of direction (Moseley et al., 1991; Pierpaoli and Basser, 1996; Andre and Bammer, 2010; Cheran et al., 2011; Buchy et al., 2012; Zatorre et al., 2012; Han et al., 2013; Jones et al., 2013; Tseng et al., 2013; Abhinav et al., 2014). Based on the findings of DTI, FT can display the spatial directivity and integrity of the fiber bundle (Cohen-Adad et al., 2008; Hobert et al., 2013; Rao et al., 2013; Kelley et al., 2014).
The ultimate goal of SCI treatment is to completely restore the normal physiological function of the spinal cord, so the function of the injured spinal cord is attracting more attention. Animals are often used to simulate the pathological process of human SCI (Pillai et al., 2011; Griffin et al., 2013). As the hindlimb may spontaneously restore motor function after complete SCI (de Leon et al., 1998; Traub, 2001), the Basso-Beattie-Bresnahan (BBB) scale score has also been found to spontaneously increase after injury (Basso et al., 1995, 1996), but this score is very subjective, so the BBB scale fails to detect the prognosis (Dietz, 2003; Chen et al., 2004). Guertin (2004) found that qualitative methods such as BBB scoring are mainly used to determine incomplete SCI, and accordingly proposed a semi-quantitative scoring method for the evaluation of complete SCI in 2004: the average combined scores (ACOS). The hindlimb movements of rats in an open space were observed and counted at random within 1 minute, including nonbilaterally alternating movements (NBA) and bilaterally alternating movements (BA). The range of motion was assessed using two-dimensional kinematic analysis and then integrated for semi-quantitative analysis of the rat hindlimb functional restoration (Guertin, 2004).
DTI has a high sensitivity and objectivity, and may provide more accurate prognostic indicators (Mukherjee, 2005; Mulcahey et al., 2012a). Therefore, this study aimed to explore the feasibility of DTI and FT in the evaluation of functional recovery in rats with complete spinal cord transection injury, especially their correlation with ACOS.
Materials and Methods
Establishment of spinal cord transection model
Twelve 8-week-old adult male Sprague-Dawley rats, weighing 250–300 g, were provided by the Experimental Animal Center of Xi’an Jiaotong University of China (specific pathogen-free grade; production license No. SCXK (Shaan) 2007-001, user license No. SYXK (Shaan) 2007-003). The experimental design was approved by the Medical Ethics Committee of Xi’an Jiaotong University in China. All operations were carried out under anesthesia to minimize the pain rats suffered during the experiment. In brief, after rats were anesthetized with 25 mg/kg of 2% sodium pentobarbital via intraperitoneal injection, they were fixed in a prone position and placed on a sterile operating table, where their dorsal skin was disinfected. Taking the T10 segment as the center, a 2-cm longitudinal incision was made, then the skin, fascia, paraspinal muscles and soft tissue were bluntly dissected, and the wounds were covered with sterile gauze to stop bleeding. The T9 and T10 spinous processes and the T10 vertebral plate were removed, fully exposing the T10 segments. A surgical needle was inserted into the spinal cord through the ventral side of spinal cord. Then the needle was gently lifted and the spinal cord was rapidly transected using a thin blade. Complete transection of the spinal cord was defined as a success upon the appearance of the surgical thread pulled out. Immediately after spinal cord transection, rats exhibited tail wagging, hindlimb retraction and flutter, and eventually flaccid paralysis. The wounds were repeatedly washed with saline and gentamicin, then sutured. After the SCI model was established, rats were divided into separate cages at 18–26°C and 40–70% humidity, below 85 db noise, allowing free access to food. Artificial urination was performed three times daily.
Detection of hindlimb motor function
Hindlimb ACOS rating was performed 24 hours before injury, and 6 hours, 1–5 weeks after injury. Hindlimb movement is mainly based on the motion of one or two joints. NBA is primarily unilateral movement, such as rapid trembling of the claw, kicking, twitching or cramps; BA refers to a series of flexion and extension movements alternating in the bilateral hindlimbs. The range of motion was obtained from treadmill exercise and recorded as 0: no action occurs; 1: the maximum range of motion is less than half of the normal range of motion; 2: the maximum range of motion is equal to or greater than half of the normal range of motion. Score = the times of NBA within 1 minute + (the times of BA within 1 minute × 2)] × range of motion. Finally, all the scores were averaged. A higher score indicated better motor function (Guertin, 2004). Because of differences in circadian activity in rats, all scores were recorded after 8:00 p.m.
MRI and DTI examination
After hindlimb motor function scores were calculated, the spinal cord in the rats was detected using a conventional 3.0 T MRI scanner and a 3.0 T DTI scanner.
Conventional 3.0 T MRI scan
Using a 3.0 T Signa MRI machine (GE Medical Systems, Milwaukee, WI, USA), a phased array spinal cord coil was customized. Conventional three-dimensional T2WI (repetition time (TR) 2,600 ms, echo time (TE) 120 ms) and T1WI (TR 560 ms, TE 11.3 ms) scans were performed on the spinal cord. The thickness of both sagittal sections and cross-sections = 1.5 mm, the space = 0, field of view (FOV) = 8, acquisition matrix = 320 × 224.
3.0 T DTI scan
The 3.0 T DTI scan was performed using a 3.0 T Signa MRI machine (GE Medical Systems) at the same loci with a conventional MRI scan. The diffusion-weighted coefficient (b value) was 1,000 s/mm2, the diffusion-sensitive gradient was 15 different directions, TR = 3,500 ms, TE = 87.5 ms, thickness = 2.4 mm, space = 0, FOV = 10, acquisition matrix = 64 × 64. All the data were input into a separate workstation, Advantage Windows 4.2 (GE Healthcare). Based on the FA map, the region of interest (the spinal cord tissue) was placed in the inferior medulla and the inferior oblongata. The morphological reconstruction of the spinal cord nerve fiber bundle was plotted using FuncTool software (GE Healthcare). The ROI was selected by two independent testers, and the ADC and FA values were obtained.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) and data are expressed as the mean ± SD. Differences were tested using analysis of variance, and pairwise comparisons among each time point were conducted using the least significant difference method. A P < 0.05 level was considered statistically significant. Pearson correlation analysis was applied to analyze FA, ADC and ACOS, and P < 0.01 as the criterion of statistical significance.
Results
General condition of a spinal cord transection rat model
All rats regained consciousness from anesthesia within 2 hours after spinal cord transection injury, and presented with complete paraplegia, no activity in the hindlimb and tail, and voiding dysfunction. No defecation disorder was found. Rats had no response to acupuncture and were in good general condition. There were no deaths during the experiment.
Hindlimb motor function score of a spinal cord transection rat model
The ACOS was significantly decreased after spinal cord transection (P < 0.05), and gradually increased with time (P < 0.05; Table 1).
Table 1.
Fractional anisotropy (FA), apparent diffusion coefficient (ADC) (10-3 mm2/s) and average combined scores (ACOS) of rats before and after spinal cord transection injury
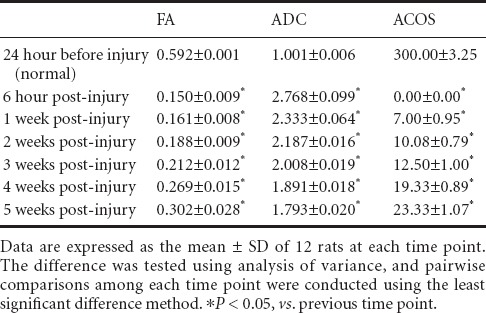
MRI and FT images of a spinal cord transection rat model
Conventional MRI findings showed that the spinal cord was smooth with uniform signals at 24 hours before spinal cord injury, with high signs in cerebrospinal fluid observed on T2-weighted images. Conventional MR T1-weighted images showed obvious reduction of the signals at the transection site with a clear boundary 6 hours post-injury, but the center was unclear. At 1 week post-injury, low signals were visible at the injury site; at 5 weeks post-injury, the signals at the injury site were still lower than those in the normal spinal cord. The overall signal did not change significantly. Conventional MR T2-weighted images showed spinal cord transection clearly at 6 hours post-injury, but cerebrospinal fluid was not visualized; at 1 week post-injury, the transection was unclear with low signals; at 5 weeks post-injury, low signals at the transection site were more common, and the signals were lower than those in normal spinal cord tissue (Figure 1).
Figure 1.
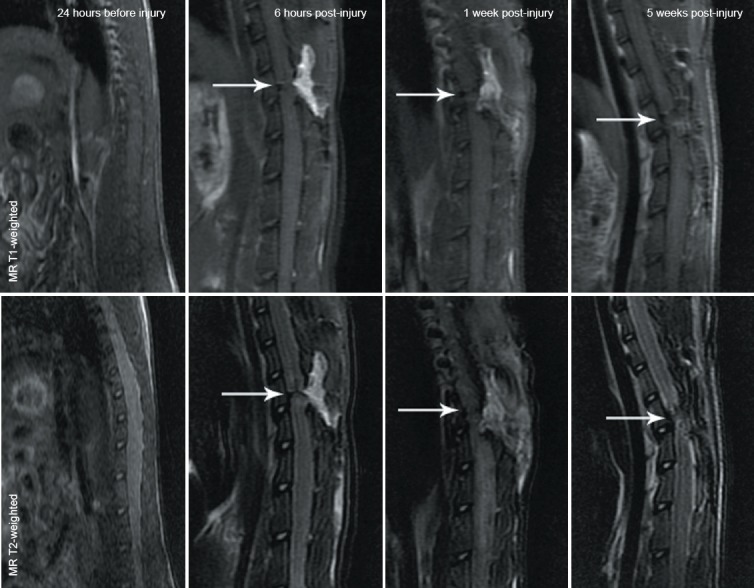
MRI images of the spinal cord in a rat model of spinal cord transection.
Conventional MR T1- and T2-weighted images show that the signals at the injured spinal cord were decreased after spinal cord transection injury occurred, and the low signals were more common as the time post-injury increased. White arrows refer to the spinal cord injury site.
All rats had a clear spinal cord structure and showed no obvious image distortion at 24 hours before injury, as detected by DTI. FT images showed that the white matter fiber bundle was intact and traveled with normal morphology in the normal spinal cord. At 6 hours after spinal cord injury, the fibers at the injury site were disorderly, but no transection was visible. At 1 week post-injury, obvious transected nerve fibers were observed. At 5 weeks post-injury, spinal cord nerve fibers were fractured and disorderly, and the transection injury had deteriorated, but a small amount of nerve fibers were still visible (Figure 2).
Figure 2.
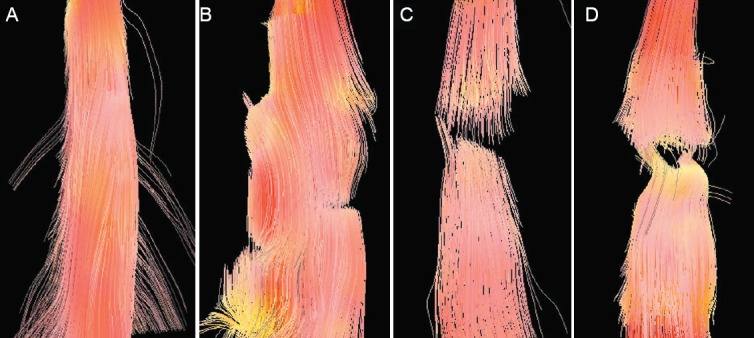
Fiber tractography images of the spinal cord in a rat model of spinal cord transection.
(A) 24 hours before spinal cord injury, i.e., normal spinal cord; (B) 6 hours post-injury; (C) 1 week post-injury; (D) 5 weeks post-injury. Spinal cord nerve fibers were arranged in a disorderly fashion and the transection gap was widened along with the post-injury time.
Changes of FA values and ADC values of a rat model of spinal cord transection
FA values significantly decreased (P < 0.05), while the ADC value increased significantly (P < 0.05) after spinal cord transection. As the post-injury time, FA values gradually increased (P < 0.05) and the ADC value decreased (P < 0.05; Table 1).
Correlation between DTI and hindlimb function score in a rat model of spinal cord transection
Pearson correlation analysis showed that FA values were negatively correlated with ADC values in the rat model of spinal cord transection (r = –0.856, P < 0.01; Figure 3A), which was consistent with our expectations because of the similar formula of FA and ADC values (Cortez-Conradis et al., 2013). FA values had a positive and linear correlation with ACOS (r = 0.943, P < 0.01; Figure 3B); ADC values were negatively correlated with ACOS and the correlation was linear (r = –0.949, P < 0.01; Figure 3C).
Figure 3.
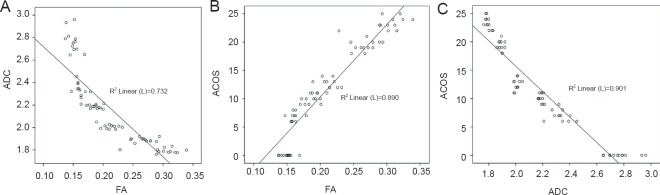
Correlation between diffusion tensor imaging and hindlimb function scores in a rat model of spinal cord transection (Pearson correlation analysis).
(A) Fractional anisotropy (FA) value was negatively correlated with the apparent diffusion coefficient (ADC) value, and the correlation was linear (r = –0.856, P < 0.01). (B) FA values were positively correlated with the average combined score (ACOS) values and the correlation was linear (r = 0.943, P < 0.01). (C) ADC values had a negative and linear correlation with ACOS (r = –0.949, P < 0.01).
Discussion
Spinal DTI technology
DTI was first used in 1994 to measure the diffusion size and direction of nerve cells, and provide image information about the diffusion of water molecules for the three-dimensional reconstruction of white matter fiber bundles. There are two important parameters in DTI, namely FA and ADC. FA values reflect the degree of spatial displacement of water molecules and are associated with the direction that the water molecules diffuse; a higher FA value indicates a stronger anisotropy. The ADC value is an indicator of the diffusion displacement of water molecules, independent of the diffusion direction. Based on these two parameters, DTI can be used to visualize the direction and displacement of water molecule diffusion (Chen et al., 2013). Therefore, DTI can sensitively reflect axonal necrosis and degeneration, glial cell regeneration and demyelination after spinal cord injury occurs, and display changes of the microstructure of the spinal cord in vivo (Fenyes and Narayana, 1999; Foong et al., 2000).
DTI and FT assessment of spinal cord transection injury
Spinal cord transection may cause a series of changes, such as the destruction of cell membranes and myelin, the disappearance of the water molecule diffusion barrier, a reduction in diffusion resistance, and an increase in diffusion displacement (Li et al., 2013; Xu et al., 2013). Because of the presence of axonal fracture, the diffusion of water molecules has no direction, thus decreasing the anisotropy. In this study, ADC values significantly increased and FA values significantly reduced after SCI, showing an obvious correlation. As astrocytes are involved in the inflammation process, gradually forming a glial scar and constructing a physical barrier at the injury site, the degree of the diffusion of water molecules reduces. The proliferating glial cells at the injury site encase the regenerating axons, and partially increase the diffusion anisotropy of water molecules. Our findings showed decreased ADC values and increased FA values following SCI, which was consistent with previous work reporting the reduction of FA values at the injury site in clinical trials (Shanmuganathan et al., 2008; Petersen et al., 2012).
Conventional MRI and FT failed to clearly display spinal cord transection at 6 hours post-injury, possibly because of postoperative bleeding and nearness to the stump, but FA values significantly decreased. At 1 week post-injury, spinal cord transection was still unclear on MRI, while FT images visualized fiber bundle breakage and gaps clearly. At 5 weeks post-injury, the MRI low signal area was expanded, and the FT image showed that spinal cord nerve fibers were arranged in a disorderly fashion with the transection gap widened, but a small amount of nerve fibers were visible. Therefore, conventional MRI can display SCI, but cannot visualize more details; there was no significant difference at 6 hours and at 5 weeks post-injury. FT imaging has obvious superiority over conventional MRI in visualizing spinal cord fibers.
DTI and hindlimb function score
The ultimate goal of SCI treatment is to achieve the complete recovery of spinal cord functions, therefore motor function after SCI in animals has been regarded as an indicator of assessing therapeutic efficacy. The commonly used assessment methods include the BBB score, the combined behavior scores (CBS) and the ACOS. The BBB scoring system is initially proposed to evaluate hindlimb functional restoration after thoracic SCI in rats (Basso et al., 1995; Basso et al., 1996), and is currently used in the evaluation of complete transection and hemisection of the spinal cord (Lu et al., 2002; Qin et al., 2006). The CBS, established in 1985, is a scale covering a wide range of sensory, motor and reflex functions, but it is difficult to promote (Gale et al., 1985). The subsequent modifications make up for some deficiencies in the CBS, such as the application of sports, toe extension and touchdown reflection, contraction reflexion, turning back reflexion, inclined plate tests and swimming tests (Hara et al., 2000). Some qualitative methods such as BBB and CBS are primarily applicable to the detection of incomplete SCI, and insensitive to evaluate complete SCI. Guertin et al. (2004) proposed the ACOS semi-quantitative scoring method. To better evaluate the hindlimb functional recovery in SCI rats, we used the ACOS system, which is more sensitive and comprehensive (Zhang et al., 2007). Comparing FA values, ADC values and ACOS after SCI, we found that FA values and ADC values had a good correlation with the ACOS, which is prone to an objective assessment of the spinal cord transection model in rats.
In summary, DTI can detect SCI earlier than conventional MRI. DTI has more precise parameters and good correlation with neurological function scores, thus it can be used as a quantitative and objective assessment method of recovery after SCI.
Footnotes
Funding: This study was financially supported by a grant from the Shaanxi Provincial Science and Technology Research and Development Project, No. 2013K12-20-08.
Conflicts of interest: None declared.
Copyedited by Jackson C, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Abhinav K, Yeh FC, Pathak S, Friedlander RM, Fernandez-Miranda JC. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: a review. Biochim Biophys Acta. 2014;1842:2286–2297. doi: 10.1016/j.bbadis.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Andre JB, Bammer R. Advanced diffusion-weighted magnetic resonance imaging techniques of the human spinal cord. Top Magn Reson Imaging. 2010;21:367–378. doi: 10.1097/RMR.0b013e31823e65a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis-a technical review. Nmr Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976) 2006;31:554–559. doi: 10.1097/01.brs.0000201274.59427.a4. [DOI] [PubMed] [Google Scholar]
- Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma. 2011;28:1401–1411. doi: 10.1089/neu.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Cowin GJ, Kurniawan ND, Ruitenberg MJ. Longitudinal assessment of white matter pathology in the injured mouse spinal cord through ultra-high field (16.4 T) in vivo diffusion tensor imaging. Neuroimage. 2013;82:574–585. doi: 10.1016/j.neuroimage.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Buchy L, Luck D, Czechowska Y, Malla A, Joober R, Lepage M. Diffusion tensor imaging tractography of the fornix and belief confidence in first-episode psychosis. Schizophr Res. 2012;137:80–84. doi: 10.1016/j.schres.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Chang Y, Jung TD, Yoo DS, Hyun JK. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma. 2010;27:2033–2040. doi: 10.1089/neu.2009.1265. [DOI] [PubMed] [Google Scholar]
- Chen JC, Li XC, Wan Q, Sun CP, He JX, Meng QH, Hong GB. Magnetic resonance diffusion tensor imaging of a sciatic nerve traction injury model and its pathologic correlation. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:7278–7283. [Google Scholar]
- Chen XR, You SW, Jin DD. Study on the BBB scale of hindlimb movements recovery in rats of spinal cord injury. Zhongguo Jizhu Jizui Zazhi. 2004;14:547–549. [Google Scholar]
- Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, Gullapalli RP. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011;28:1881–1892. doi: 10.1089/neu.2010.1741. [DOI] [PubMed] [Google Scholar]
- Clemm von Hohenberg C, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton ME. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2014;40:895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Adad J, Benali H, Hoge RD, Rossignol S. In vivo DTI of the healthy and injured cat spinal cord at high spatial and angular resolution. Neuroimage. 2008;40:685–697. doi: 10.1016/j.neuroimage.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Cortez-Conradis D, Favila R, Isaac-Olive K, Martinez-Lopez M, Rios C, Roldan-Valadez E. Diagnostic performance of regional DTI-derived tensor metrics in glioblastoma multiforme: simultaneous evaluation of p, q, L, Cl, Cp, Cs, RA, RD, AD, mean diffusivity and fractional anisotropy. Eur Radiol. 2013;23:1112–1121. doi: 10.1007/s00330-012-2688-7. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Dietz V. Spinal cord pattern generators for locomotion. Clin Neurophysiol. 2003;114:1379–1389. doi: 10.1016/s1388-2457(03)00120-2. [DOI] [PubMed] [Google Scholar]
- Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006;27:214–216. [PMC free article] [PubMed] [Google Scholar]
- Fenyes DA, Narayana PA. In vivo diffusion tensor imaging of rat spinal cord with echo planar imaging. Magn Reson Med. 1999;42:300–306. doi: 10.1002/(sici)1522-2594(199908)42:2<300::aid-mrm12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyoshi K, Konomi T, Yamada M, Hikishima K, Tsuji O, Komaki Y, Momoshima S, Toyama Y, Nakamura M, Okano H. Diffusion tensor imaging and tractography of the spinal cord: from experimental studies to clinical application. Exp Neurol. 2013;242:74–82. doi: 10.1016/j.expneurol.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Griffin JF, Cohen ND, Young BD, Eichelberger BM, Padua A, Purdy D, Levine JM. Thoracic and lumbar spinal cord diffusion tensor imaging in dogs. J Magn Reson Imaging. 2013;37:632–641. doi: 10.1002/jmri.23862. [DOI] [PubMed] [Google Scholar]
- Guertin PA. Semiquantitative assessment of hindlimb movement recovery without intervention in adult paraplegic mice. Spinal Cord. 2004;43:162–166. doi: 10.1038/sj.sc.3101701. [DOI] [PubMed] [Google Scholar]
- Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain. 2013;136:2952–2965. doi: 10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Hara M, Takayasu M, Watanabe K, Noda A, Takagi T, Suzuki Y, Yoshida J. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg. 2000;93:94–101. doi: 10.3171/spi.2000.93.1.0094. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Wilde EA, Miller ER, Kumar Patel V, Staewen TD, Frisby ML, Garza HM, McCarthy JJ, Hunter JV, Levin HS, Robertson CS, Narayana PA. Serial atlas-based diffusion tensor imaging study of uncomplicated mild traumatic brain injury in adults. J Neurotrauma. 2014;31:466–475. doi: 10.1089/neu.2013.3085. [DOI] [PubMed] [Google Scholar]
- Hesseltine SM, Law M, Babb J, Rad M, Lopez S, Ge Y, Johnson G, Grossman RI. Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am J Neuroradiol. 2006;27:1189–1193. [PMC free article] [PubMed] [Google Scholar]
- Hobert MK, Stein VM, Dziallas P, Ludwig DC, Tipold A. Evaluation of normal appearing spinal cord by diffusion tensor imaging, fiber tracking, fractional anisotropy, and apparent diffusion coefficient measurement in 13 dogs. Acta Vet Scand. 2013;55:36. doi: 10.1186/1751-0147-55-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Wang Y, Huang X, Kuang W, Wu Q, Lui S, Sweeney JA, Gong Q. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci. 2014;39:170–177. doi: 10.1503/jpn.130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Chopp M. MRI evaluation of white matter recovery after brain injury. Stroke. 2010;41:S112-S113. doi: 10.1161/STROKEAHA.110.595629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do's and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kamble RB, Venkataramana NK, Naik AL, Rao SV. Diffusion tensor imaging in spinal cord injury. Indian J Radiol Imaging. 2011;21:221–224. doi: 10.4103/0971-3026.85372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Harel NY, Kim CY, Papademetris X, Coman D, Wang X, Hasan O, Kaufman A, Globinsky R, Staib LH, Cafferty WB, Hyder F, Strittmatter SM. Diffusion tensor imaging as a predictor of locomotor function after experimental spinal cord injury and recovery. J Neurotrauma. 2014;31:1362–1373. doi: 10.1089/neu.2013.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkovský M, Bednarík J, Dušek L, Šprláková-Puková A, Urbánek I, Mechl M, Válek V, Kadanka Z. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 2012;37:48–56. doi: 10.1097/BRS.0b013e31820e6c35. [DOI] [PubMed] [Google Scholar]
- Kulkarni MV, Bondurant FJ, Rose SL, Narayana PA. 1.5 tesla magnetic resonance imaging of acute spinal trauma. Radiographics. 1988;8:1059–1082. doi: 10.1148/radiographics.8.6.3205929. [DOI] [PubMed] [Google Scholar]
- Li ZC, Wang WJ, Zhang JG, Zhao L, Zhao H. Granulocyte-colony stimulating factor improves motor function of rats with spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:7110–7116. [Google Scholar]
- Lu J, Féron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- Ma C, Xu Z, Wang ZQ, Deng SY. Interleukin-17 expression in the injured site of a rat model of complete spinal cord transection. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2824–2829. [Google Scholar]
- Moseley ME, Kucharczyk J, Asgari HS, Norman D. Anisotropy in diffusion-weighted MRI. Magn Reson Med. 1991;19:321–326. doi: 10.1002/mrm.1910190222. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. Diffusion tensor imaging and fiber tractography in acute stroke. Neuroimaging Clinics of North America. 2005;15:655–665. doi: 10.1016/j.nic.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Mulcahey M, Samdani A, Gaughan J, Barakat N, Faro S, Betz RR, Finsterbusch J, Mohamed FB. Diffusion tensor imaging in pediatric spinal cord injury: preliminary examination of reliability and clinical correlation. Spine. 2012a;37:E797–803. doi: 10.1097/BRS.0b013e3182470a08. [DOI] [PubMed] [Google Scholar]
- Mulcahey MJ, Samdani A, Gaughan J, Barakat N, Faro S, Betz RR, Finsterbusch J, Mohamed FB. Diffusion tensor imaging in pediatric spinal cord injury: preliminary examination of reliability and clinical correlation. Spine (Phila Pa 1976) 2012b;37:E797–803. doi: 10.1097/BRS.0b013e3182470a08. [DOI] [PubMed] [Google Scholar]
- Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012;29:1556–1566. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Heidemann RM, Kumar P, Shanbhag N, Lanz T, Dittmar MS, Sandner B, Beier CP, Weidner N, Greenlee MW, Schuierer G, Bogdahn U, Schlachetzki F. Comprehensive small animal imaging strategies on a clinical 3 T dedicated head MR-scanner; adapted methods and sequence protocols in CNS pathologies. PLoS One. 2011;6:e16091. doi: 10.1371/journal.pone.0016091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin DX, Zou XL, Luo W, Zhang W, Zhang HT, Li XL, Zhang H, Wang XY, Wang TH. Expression of some neurotrophins in the spinal motoneurons after cord hemisection in adult rats. Neurosci Lett. 2006;410:222–227. doi: 10.1016/j.neulet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S, Kanna RM, Karunanithi R, Shetty AP. Diffusion tensor tractography demonstration of partially injured spinal cord tracts in a patient with posttraumatic brown sequard syndrome. J Magn Reson Imaging. 2010;32:978–981. doi: 10.1002/jmri.22320. [DOI] [PubMed] [Google Scholar]
- Rao JS, Zhao C, Yang ZY, Li SY, Jiang T, Fan YB, Li XG. Diffusion tensor tractography of residual fibers in traumatic spinal cord injury: a pilot study. J Neuroradiol. 2013;40:181–186. doi: 10.1016/j.neurad.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, Hackney DB. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol. 2003;184:570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. Am J Neuroradiol. 2008;29:655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, Dong TF, Li TS. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20:422–428. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EJ, Lipton ML, Burns J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am J Neuroradiol. 2014;35:439–444. doi: 10.3174/ajnr.A3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD. Could plasticity of inhibition pattern pattern generators? Nat Neurosci. 2001;4:223–224. doi: 10.1038/85048. [DOI] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eerden AW, Khalilzadeh O, Perlbarg V, Dinkel J, Sanchez P, Vos PE, Luyt CE, Stevens RD, Menjot de Champfleur N, Delmaire C, Tollard E, Gupta R, Dormont D, Laureys S, Benali H, Vanhaudenhuyse A, Galanaud D, Puybasset L. White matter changes in comatose survivors of anoxic ischemic encephalopathy and traumatic brain injury: comparative diffusion-tensor imaging study. Radiology. 2013;270:506–516. doi: 10.1148/radiol.13122720. [DOI] [PubMed] [Google Scholar]
- Van Goethem JM, Maes M, özsarlak ö, van den Hauwe L, Parizel P. Imaging in spinal trauma. Eur Radiol. 2005;15:582–590. doi: 10.1007/s00330-004-2625-5. [DOI] [PubMed] [Google Scholar]
- Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Diffusion tensor imaging of the spinal cord: insights from animal and human studies. Neurosurgery. 2014;74:1–8. doi: 10.1227/NEU.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cheng LM. Neurotrophic factors and spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:369–374. [Google Scholar]
- Young RJ, Brennan N, Fraser JF, Brennan C. Advanced imaging in brain tumor surgery. Neuroimag Clin N Am. 2010;20:311–335. doi: 10.1016/j.nic.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ji SR, Wu CY, Fan XH, Zhou HJ, Liu GL. Observation of locomotor functional recovery in adult complete spinal rats with BWSTT using semiquantitative and qualitative methods. Spinal Cord. 2007;45:496–501. doi: 10.1038/sj.sc.3102013. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Li Y, Cao Y, Dong MY, Lü G. Effect of hyperbaric oxygen preconditioning on expression of Bcl-2 and Bax after spinal cord injury. ZhongguoZuzhi Gongcheng Yanjiu. 2013;17:8018–8023. [Google Scholar]


