Abstract
In this study, we investigated the effects of streptozotocin-induced type 1 diabetes on antioxidant-like protein-1 immunoreactivity, protein carbonyl levels, and malondialdehyde formation, a marker for lipid peroxidation, in the hippocampus. For this study, streptozotocin (75 mg/kg) was intraperitoneally injected into adult rats to induce type 1 diabetes. The three experimental parameters were determined at 2, 3, 4 weeks after streptozotocin treatment. Fasting blood glucose levels significantly increased by 20.7–21.9 mM after streptozotocin treatment. The number of antioxidant-like protein-1 immunoreactive neurons significantly decreased in the hippocampal CA1 region, but not the dentate gyrus, 3 weeks after streptozotocin treatment compared to the control group. Malondialdehyde and protein carbonyl levels, which are modified by oxidative stress, significantly increased with a peak at 3 weeks after malondialdehyde treatment, and then decreased 4 weeks after malondialdehyde treatment. These results suggest that neurons in the hippocampal CA1 region, but not the dentate gyrus, are susceptible to oxidative stress 3 weeks after malondialdehyde treatment.
Keywords: nerve regeneration, hippocampus, dentate gyrus, lipid peroxidation, type 1 diabetes, malondialdehyde, neurons, neural regeneration
Introduction
Diabetes is the most prevalent metabolic disorder in humans and affects approximately 8.3% of the adult population worldwide (Shi and Hu, 2014). Diabetes is characterized by high blood glucose levels over a prolonged period. This hyperglycemia produces many clinical symptoms because of the resultant damage to various organs, including the brain. In the brain, diabetes reduces the ability of neurons to adapt to oxidative and metabolic stress (Kapogiannis and Mattson, 2011). In addition, diabetes can cause stroke and worsen episodic memory impairment (Messier, 2005) and synaptic plasticity deficits. Increasing evidence shows that oxidative stress is an important pathway through which several diseases, including diabetes, exert their deleterious effects (Rains and Jain, 2011).
There is a growing body of evidence demonstrating that the glycation toxicity caused by hyperglycemia elicits the generation of excess reactive oxygen species (ROS) and nitric oxide in neurons. Brain tissue is highly susceptible to ROS because it has a high rate of oxidative metabolic activity, high concentration of polyunsaturated fatty acids in membrane lipids, and low antioxidant capacity (Fukudome et al., 2008; Nishikawa et al., 2000). Once a radical is formed, propagation can lead to increasing levels of damage. The highly reactive hydroxyl radical, in turn, can cause lipid peroxidation and DNA damage (Robb-Gaspers and Connor, 1997). In diabetic patients, oxidative and nitrosative stress is increased, which can damage cellular proteins, lipids, and DNA, thereby inhibiting their normal functions and disturbing homeostasis within the neurons, ultimately resulting in cell death (Paradies et al., 2011).
Antioxidant-like protein-1 (AOP-1) was first identified as a molecule exhibiting a sequence similar to that of mouse MER5 (Tsuji et al., 1995). AOP-1 has two conserved catalytic cysteine residues with 93.3% homology to the C22 subunit of Salmonella typhimurium alkyl hydroperoxide reductase (Chae et al., 1994; Tsuji et al., 1995), a mitochondrial antioxidant protein. AOP-1 can scavenge ROS with thioredoxin-dependent peroxidase and is expressed in mitochondria (Shih et al., 2001) and in the cytoplasm (Feng et al., 2007). In addition, there is growing evidence that AOP-1 is associated with various biological processes such as cell proliferation, differentiation, apoptosis, and gene expression (Fujii and Ikeda, 2002).
However, there have been no comprehensive studies on the effects of streptozotocin (STZ)-induced type 1 diabetes on AOP-1 expression and oxidative stress-induced protein modification. Therefore, in the present study, we investigated the spatial and temporal changes of AOP-1 expression and oxidative stress-induced protein modification in the hippocampus of rats with STZ-induced type 1 diabetes.
Materials and Methods
Experimental animals
7-week-old male Wistar rats, weighing 190–200 g, were purchased from Orient Bio, Inc. (Seongnam, South Korea). They were housed at 23°C with 60% humidity and a 12-hour light-dark cycle, with free access to food and tap water. Animal handling and care conformed to guidelines established to comply with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996), and were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University. All of the experiments and procedures were designed to minimize the number of animals used and the suffering caused.
Induction of type 1 diabetes and experimental design
The rats were randomly and evenly divided into control and STZ groups. STZ (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.1 M sodium citrate buffer (pH 4.3). Following 1 week of acclimation to the vivarium, diabetes was induced by a single intraperitoneal injection of 75 mg/kg of STZ. For the control group, sodium citrate buffer was administered intraperitoneally in the same volume used for STZ. After 72 hours, fasting blood glucose levels were monitored and rats with blood glucose levels of > 8.00 mM in the STZ group were utilized for the study and divided into three time point subgroups with fifteen rats in each subgroup.
Evaluation of blood glucose levels and tissue processing
Blood was sampled by a “tail nick” at 9–11 a.m. using a 27 G needle before sacrifice, and glucose in blood was analyzed by using an Accu-chek glucometer (Roche, Mannheim, Germany). For immunohistochemical analysis, control and STZ-treated rats at 2, 3 and 4 weeks after STZ treatment (n = 5 per group) were anesthetized by intraperitoneal injection of 30 mg/kg Zoletil 50® (Virbac, Carros, France) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The whole brains were removed and post-fixed in the same fixative for 6 hours. Brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Brains were serially sectioned (30 μm) in the coronal plane using a cryostat (Leica, Wetzlar, Germany). Sections were collected in 6-well plates containing PBS for further processing.
Detection of AOP-1 immunoreactivity
To obtain accurate immunohistochemical data, the free- floating sections were carefully processed under the same conditions. Tissue sections were selected between –3.00 and –4.08 mm posterior to the bregma with reference to the rat brain atlas (Paxinos and Watson, 2007) for each rat. Ten sections, 90 μm apart from each other, were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 minutes and 10% normal goat serum in 0.05 M PBS for 30 minutes. They were then incubated with mouse anti-AOP-1 (1:1,000; Sigma) overnight at room temperature and subsequently treated with biotinylated goat anti-mouse IgG and a streptavidin-peroxidase complex (1:200; Vector, Burlingame, CA, USA). Sections were then visualized by reaction with 3,3′-diaminobenzidine tetrachloride (Sigma) in 0.1 M Tris-HCl buffer (pH 7.2) and mounted on gelatin-coated slides. Sections were mounted in Canada Balsam (Kanto, Tokyo, Japan) following dehydration.
The number of AOP-1-immunoreactive cells in each section of the hippocampal CA1, 3 region and dentate gyrus was counted using an image analysis system (Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA) equipped with a computer-based CCD camera. The cell counts from all sections of all rats were averaged.
Indirect measures of oxidative stress
To elucidate the effects of STZ on protein modification, the carbonylation of proteins by oxidative stress was determined in control and STZ-treated rats at 2, 3 and 4 weeks after STZ treatment (n = 5 in each group) by the Levine method (Levine et al., 1990). The color intensity of the supernatant was measured using a spectrophotometer (Agilent, Sata Clara, CA, USA) at 370 nm against 2 M HCl. Carbonyl content was calculated by using the molar extinction coefficient (21 × 103 L/mol•cm), and results were expressed as nmol/mg protein.
The effects of STZ on lipid peroxidation levels in control and STZ-treated rats at 2, 3 and 4 weeks after STZ treatment (n = 5 in each group) were assessed by measuring malondialdehyde (MDA) formation by using the Bioxytech MDA-586 kit (Oxis Research, Portland, OR, USA). Briefly, bilateral hippocampi were dissected out using a surgical blade and homogenized in 20 mM PBS (pH 7.4) containing 5 mM butylated hydroxytoluene. After centrifugation of the homogenates at 3,000 × g for 10 minutes at 4°C, the supernatant was collected. For each reaction, 10 μL of probucol and 640 μL of diluted R1 reagent (1:3 of methanol:N-methyl-2-phenylindole) were added and mixed with 150 μL of 12 N HCl. Each reaction was incubated at 45°C for 60 minutes and centrifuged at 10,000 × g for 10 minutes. The supernatant was collected and MDA formation was determined by measuring the absorbance at 586 nm. MDA data were normalized to the protein concentration.
Statistical analysis
The data shown here are expressed as the mean ± SEM and were analyzed by using GraphPad Prizm 5.0 software (GraphPad Software Inc., La Jolla, CA, USA) and differences between groups were statistically analyzed by one-way analysis of variance followed by Bonferroni's post-hoc analysis.
Results
Changes in blood glucose levels
In the control group, the mean fasting blood glucose level was 5.85 mM. The blood glucose level significantly increased to 20.70, 21.8, and 21.9 mM at 2, 3, and 4 weeks after STZ treatment, respectively, compared to that in the control group (P < 0.05). The blood glucose levels did not show any significant changes between STZ-treated groups (Figure 1).
Figure 1.
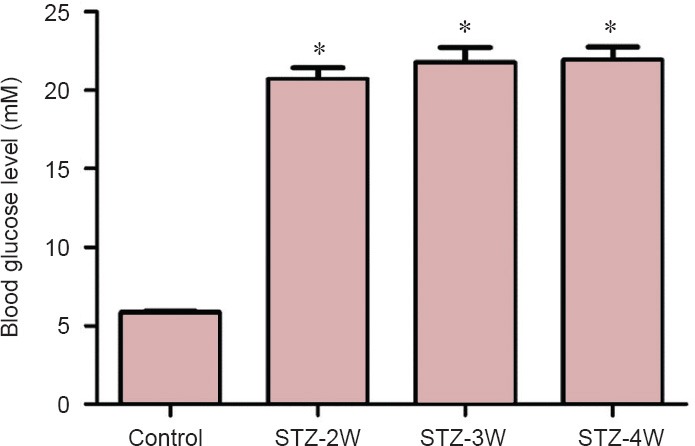
Fasting blood glucose levels in control and streptozotocin (STZ)-treated rats.
Fasting blood glucose level was increased in rats after STZ treatment. Differences among the means were analyzed statistically by one-way analysis of variance followed by Bonferroni's post-hoc analysis (n = 15 per group; *P < 0.05, vs. control group). The bars indicate the standard error of the mean (SEM). STZ-2W, STZ-3W and STZ-4W mean 2, 3, and 4 weeks after STZ treatment, respectively.
Changes in AOP-1 immunoreactivity
In the CA1 region in all groups, AOP-1 immunoreactivity was mainly detected in non-pyramidal cells (Figure 2A–D). Two weeks after STZ treatment, the number of AOP-1 immunoreactive neurons significantly decreased to 24.4% of that in the control group (Figure 2B, E). Three to four weeks after STZ treatment, the number of AOP-1 immunoreactive neurons detected in the CA1 region was similar to that at 2 weeks after STZ treatment (Figure 2C–E).
Figure 2.
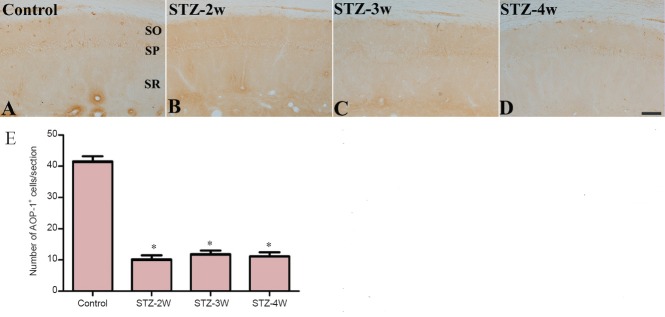
Antioxidant-like protein-1 (AOP-1) immunoreactivity in the hippocampal CA1 region of control and streptozotocin (STZ)-treated rats.
AOP-1 immunoreactivity was decreased in rats after STZ treatment. (A–D) Photomicrographs of immunohistochemistry for AOP-1 immunoreactivity in the hippocampal CA1 region of controls (A) and rats treated with STZ for 2 (B), 3 (C) and 4 weeks (D). AOP-1 immunoreactivity is found in non-pyramidal cells of the CA1 region. SO: Stratum oriens; SP: stratum pyramidale; SR: stratum radiatum. Scale bar: 100 μm. (E) Number of AOP-1-immunoreactive cells per section from each group. Differences between groups were analyzed statistically by oneway analysis of variance followed by Bonferroni's post-hoc analysis (n = 5 per group; *P < 0.05, vs. control group). The bars indicate the standard error of the mean (SEM). STZ-2W, STZ-3W, and STZ-4W mean treatment with STZ for 2, 3 and 4 weeks, respectively.
In the CA3 region in the control group, AOP-1 immunoreactivity was observed in the non-pyramidal cells (Figure 3A). Two weeks after STZ treatment, the number of AOP-1 immunoreactive neurons significantly decreased to 78.3% of that in the control group (Figure 3B and E). Three to four weeks after STZ treatment, the number AOP-1 immunoreactive neurons significantly decreased to 41.4% and 39.4% of that in the control group, respectively (Figure 3C–E).
Figure 3.
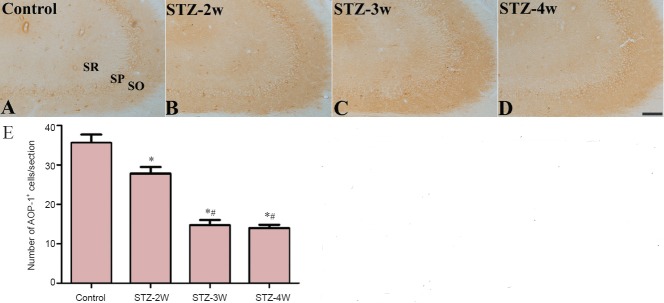
Antioxidant-like protein-1 (AOP-1) immunoreactivity in the hippocampal CA3 region of control and streptozotocin (STZ)-treated rats.
AOP-1 immunoreactivity was decreased in rats after STZ treatment. (A–D) Photomicrographs of immunohistochemistry for AOP-1 immunoreactivity in the hippocampal CA1 region of controls (A) and rats treated with STZ for 2 (B), 3 (C) and 4 weeks (D). AOP-1 immunoreactivity is found in non-pyramidal cells of the CA3 region. SO: Stratum oriens; SP: stratum pyramidale; SR: stratum radiatum. Scale bar: 100 μm. (E) Number of AOP-1-immunoreactive cells per section from each group. Differences between groups were analyzed statistically by one-way analysis of variance followed by Bonferroni's post-hoc analysis (n = 5 per group; *P < 0.05, vs. control group; #P < 0.05, vs. STZ-2W group). The bars indicate the standard error of the mean (SEM). STZ-2W, STZ-3W, and STZ-4W mean treatment with STZ for 2, 3 and 4 weeks, respectively.
In the dentate gyrus of the control group, AOP-1 immunoreactivity was detected in the interneurons in the polymorphic layer (Figure 4A). Two weeks after STZ treatment, the number of AOP-1 immunoreactive neurons decreased, although a significant difference was not observed between groups (Figures 4B and E). Three to four weeks after STZ treatment, the number of AOP-1 immunoreactive neurons in the dentate gyrus was similar to that in the control group (Figure 4C–E).
Figure 4.
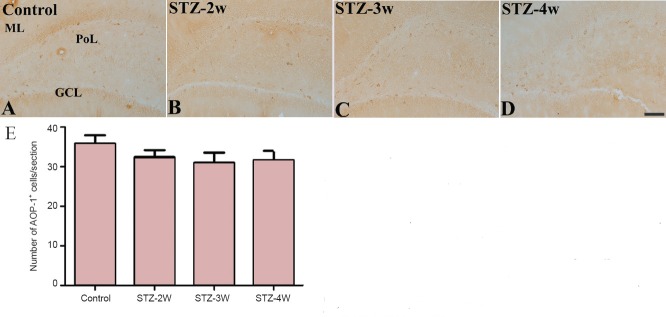
Changes in antioxidant-like protein-1 (AOP-1) immunoreactivity in the hippocampal dentate gyrus of rats after strep tozotocin (STZ) treatment.
No obvious change in AOP-1 immunoreactivity was observed in rats after STZ treatment. (A–D) Photomicrographs of immunohistochemistry for AOP- 1 immunoreactivity in the hippocampal dentate gyrus in controls (A) and rats treated with STZ for 2 (B), 3 (C) and 4 weeks (D). AOP-1 immunoreactivity is found in interneurons in the polymorphic layer (PoL) of the dentate gyrus. ML: Molecular layer; GCL: granule cell layer. Scale bar: 100 μm. (E) Number of AOP-1-immunoreactive cells per section of each group. Differences between groups were analyzed statistically by one-way analysis of variance followed by Bonferroni's post-hoc analysis (n = 5 per group). There are no significant differences in the number of AOP-1 immunoreactive cells between groups. The bars indicate the standard error of the mean (SEM). STZ-2W, STZ-3W, STZ- 4W mean treatment with STZ for 2, 3 and 4 weeks, respectively.
Changes in MDA and protein carbonyl levels
In the control group, the MDA and protein carbonyl levels in the hippocampal homogenates were 25.4 and 1.82 nmol/mg protein. MDA and protein carbonyl levels increased 2 weeks after STZ treatment; however, there were no significant differences between the groups. Three weeks after STZ treatment, MDA and protein carbonyl levels had significantly increased by 1.98 and 2.20 fold compared to those in the control group, respectively. Four weeks after STZ treatment, MDA and protein carbonyl levels had significantly decreased compared to 3 weeks after STZ treatment. However, MDA levels were significantly higher 4 weeks after STZ treatment compared to those in the control group (Figure 5).
Figure 5.
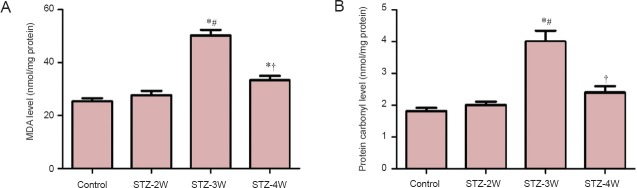
Malondialdehyde (MDA) level (A) and protein carbonyl level modified by oxidative stress (B) in the hippocampi of control rats and rats that received streptozotocin (STZ) treatment for 2, 3 and 4 weeks (STZ-2W, STZ-3W, STZ-4W, respectively).
Both of these two indices were increased in rats after STZ treatment. Differences between groups were analyzed statistically by one-way analysis of variance followed by Bonferroni's post-hoc analysis (n = 5 per group; *P < 0.05, vs. control group; #P < 0.05, vs. STZ-2W group; †P < 0.05, vs. STZ- 3W group). The bars indicate the standard error of the mean (SEM).
Discussion
The STZ-induced rat model of type 1 diabetes has been used extensively in pathophysiological studies of diabetes (Gispen and Biessels, 2000). STZ treatment induces spatial learning impairment in rats (Biessels et al., 1998; Kamal et al., 2000) and moderate cognitive impairment in both humans (Desrocher and Rovet, 2004) and animal models of type 1 diabetes (Biessels et al., 1996). In the present study, we induced type 1 diabetes with 75 mg/kg STZ in rats because administration of 75 mg/kg STZ influences the Km values and Vmax of tryptophan-5-hydroxylase in the brain (Herrera et al., 2005). Reactive oxygen species, which are formed during oxidative stress, induce cellular and molecular abnormalities in the hippocampus of STZ-treated rats. In addition, the accumulation of ROS causes an imbalance between their production and removal by the antioxidant system, which finally leads to an increase in DNA oxidation and lipid peroxidation in neurons.
We first measured blood glucose levels 3 days after STZ treatment and excluded rats showing low fasting blood glucose levels lower than 8.00 mM because some of these rats did not show any significant increase in blood glucose levels after STZ treatment. At 2–4 weeks after STZ treatment, the blood glucose levels were higher than 20 mM. We used these rats for AOP-1 immunohistochemistry and for biochemical analysis. AOP-1 immunoreactivity was predominantly observed in the non-pyramidal cells of the CA1, 3 regions or neurons of the dentate gyrus. This result is consistent with our previous study where AOP-1 was primarily found in non-pyramidal cells through double immunofluorescent labeling for AOP-1 and glutamic acid decarboxylase 67 or parvalbumin, which are markers for GABAergic neurons (Hwang et al., 2005). The number of AOP-1 immunoreactive neurons decreased in the hippocampal CA1, 3 regions, but not in the dentate gyrus, 2–4 weeks after STZ treatment. These spatial and temporal changes of AOP-1 suggest that AOP-1 immunoreactive neurons in the hippocampal CA1 region are relatively vulnerable to STZ damage, whereas AOP-1 immunoreactive neurons in the dentate gyrus are relatively resistant to STZ damage. This result was supported by previous studies showing that terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive neurons are predominantly found in the hippocampal CA1 region in 8-month-old rats with spontaneous type 1 diabetes (Li et al., 2002), whereas TUNEL-positive cells and cleaved capase-3 levels are detected at similar levels in the dentate gyrus between controls and the rats treated with STZ for 4 weeks (Guo et al., 2014). In addition, STZ treatment produces chromatin aggregates and clumps within the nucleus and swollen mitochondria in the pyramidal cells of the hippocampal CA1 region (Ye et al., 2011). In contrast, no apoptotic changes occur in neurons of the dentate gyrus within 4 weeks after STZ treatment (Guo et al., 2014).
Next, we observed the lipid peroxidation and protein carbonyl modification induced by ROS in the hippocampal homogenates. MDA and protein carbonyl levels were significantly increased 3 weeks after STZ treatment; this result is closely related to the reduced number of AOP-1 immunoreactive neurons observed 3 weeks after STZ treatment. Furthermore, this result is consistent with our previous study demonstrating that lipid peroxidation based on 4-hydroxy-2-nonenal immunoreactivity was significantly increased 3 weeks after STZ treatment (Yi et al., 2011). Similarly, diabetic patients also show high levels of lipid peroxidation (Bonnefont-Rousselot et al., 2000; Robertson, 2004). The hyperglycemia induced by STZ causes increased production of ROS resulting in protein glycosylation and glucose auto-oxidation (Bonnefont-Rousselot et al., 2000; Robertson, 2004). It has been reported that MDA levels are significantly increased 4 weeks after STZ treatment (Samarghandian et al., 2013; De Morais et al., 2014). However, in the present study, we observed a significant reduction in MDA and protein carbonyl levels 4 weeks after STZ treatment, although the MDA levels in this group were still significantly higher than those in the control group. This difference may be associated with the dosage of STZ used in the study. In the present study, we used a higher dose of STZ (75 mg/kg), whereas previous studies used 50–60 mg/kg STZ. This discrepancy may have resulted in differences in the peak time of lipid peroxidation after STZ treatment in hippocampal homogenates.
Neurons are particularly susceptible to oxidative stress because they utilize large amounts of oxygen and glucose and possess large amounts of polyunsaturated fatty acids and transition metal ions, but are relatively deficient with regard to antioxidant defense systems (Halliwell and Gutteridge, 1990). Lipid peroxidation of the neuronal membrane results in cellular dysfunction by disturbing ionic gradient receptors and transport functions (Nohl, 1993; Franco et al., 2006). The increase in lipid peroxidation and protein carbonyls modification by ROS may be closely associated with neuronal death in the hippocampal CA1 region 4 weeks after STZ treatment (Li et al., 2002; Ye et al., 2011). STZ treatment significantly increases the hippocampal densities of cannabinoid 1 receptor protein and of specific cannabinoid 1 receptor binding sites in the nerve terminals and total membrane at 30 days after STZ treatment, whereas cannabinoid 1 receptor mRNA expression is decreased by 25% (Duarte et al., 2007). The cannabinoid 1 receptor is predominantly expressed in the central nervous system and aggravates aging-related brain damage (Bilkei-Gorzo, 2012).
In conclusion, STZ significantly increases the lipid peroxidation and protein carbonyl modification through increased ROS generation 3 weeks after STZ treatment. In addition, STZ treatment significantly reduces the number of AOP-1-immunoreactive neurons in the hippocampal CA1 region but not the dentate gyrus. Changes in AOP-1 immunoreactivity, lipid peroxidation, and protein carbonyl modification 3 weeks after STZ treatment may be associated with selective neuronal damage in the hippocampal CA1 region.
Footnotes
Funding: This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (MEST), Republic of Korea, No. 2010-0007712.
Conflicts of interest: None declared.
Copyedited by Sethy NK, Zanoveli JM, Ponce-Lopez T, Li CH, Song LP, Zhao M
References
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci. 2012;367:3326–3341. doi: 10.1098/rstb.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26:163–176. [PubMed] [Google Scholar]
- Chae HZ, Chung SJ, Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- De Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, da Cunha JM, Zanoveli JM. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res. 2014;258:52–64. doi: 10.1016/j.bbr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10:36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- Duarte JMN, Nogueira C, Mackie K, Oliveira CR, Cunha RA, Köfalvi A. Increase of cannabinoid CB1 receptor density in the hippocampus of streptozotocin-induced diabetic rats. Exp Neurol. 2007;204:479–484. doi: 10.1016/j.expneurol.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Feng Y, Liu DQ, Wang Z, Liu Z, Cao HQ, Wang LY, Shi N, Meng XM. AOP-1 interacts with cardiac-specific protein kinase TNNI3K and down-regulates its kinase activity. Biochemistry (Mosc) 2007;72:1199–1204. doi: 10.1134/s0006297907110053. [DOI] [PubMed] [Google Scholar]
- Franco R, Bortner CD, Cidlowski JA. Potential roles of electrogenic ion transport and plasma membrane depolarization in apoptosis. J Membr Biol. 2006;209:43–58. doi: 10.1007/s00232-005-0837-5. [DOI] [PubMed] [Google Scholar]
- Fujii J, Ikeda Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002;7:123–130. doi: 10.1179/135100002125000352. [DOI] [PubMed] [Google Scholar]
- Fukudome D, Matsuda M, Kawasaki T, Ago Y, Matsuda T. The radical scavenger edaravone counteracts diabetes in multiple low-dose streptozotocin-treated mice. Eur J Pharmacol. 2008;583:164–169. doi: 10.1016/j.ejphar.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels G-J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Wang SH, Yuan Y, Li FF, Ye KP, Huang Y, Xia WQ, Zhou Y. Vulnerability for apoptosis in the hippocampal dentate gyrus of STZ-induced diabetic rats with cognitive impairment. J Endocrinol Invest. 2014;37:87–96. doi: 10.1007/s40618-013-0030-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Herrera R, Manjarrez G, Hernandez J. Inhibition and kinetic changes of brain tryptophan-5-hydroxylase during insulin-dependent diabetes mellitus in the rat. Nutr Neurosci. 2005;8:57–62. doi: 10.1080/10284150400027115. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Hua L, Yoo KY, Kim DW, Kang TC, Choi SY, Won MH, Kim DH. Anti-oxidant-like protein 1 is altered in non-pyramidal cells and expressed in astrocytes in gerbil hippocampal CA1 region after transient forebrain ischemia. Brain Res. 2005;1062:111–119. doi: 10.1016/j.brainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Duis SE, Gispen WH. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia. 2000;43:500–506. doi: 10.1007/s001250051335. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and 745 type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26:26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br Med Bull. 1993;49:653–667. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int. 2011;58:447–457. doi: 10.1016/j.neuint.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Amsterdam: Elsevier Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb-Gaspers SJ, Connor JR. Oxidative stress-induced cell damage in the CNS: A proposal for a final common pathway. In: Connor JR, editor. In: Metals and oxidative damage in neurological disorders. New York: Plenum Press; 1997. pp. 341–351. [Google Scholar]
- Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Borji A, Delkhosh MB, Samini F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J Pharm Pharm Sci. 2013;16:352–362. doi: 10.18433/j3zs3q. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- Shih SF, Wu YH, Hung CH, Yang HY, Lin JY. Abrin triggers cell death by inactivating a thiol-specific antioxidant protein. J Biol Chem. 2001;276:21870–21877. doi: 10.1074/jbc.M100571200. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Copeland NG, Jenkins NA, Obinata M. Mammalian antioxidant protein complements alkylhydroperoxide reductase (ahpC) mutation in Escherichia coli. Biochem J. 1995;307:377–381. doi: 10.1042/bj3070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wang F, Yang RH. Diabetes impairs learning performance and affects the mitochondrial function of hippocampal pyramidal neurons. Brain Res. 2011;1411:57–64. doi: 10.1016/j.brainres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011;36:117–128. doi: 10.1007/s11064-010-0280-6. [DOI] [PubMed] [Google Scholar]


