Abstract
Notch pathway activation maintains neural stem cells in a proliferating state and increases nerve repair capacity. To date, studies have rarely focused on changes or damage to signal transduction pathways during cerebral hemorrhage. Here, we examined the effect of acupuncture in a rat model of cerebral hemorrhage. We examined four groups: in the control group, rats received no treatment. In the model group, cerebral hemorrhage models were established by infusing non-heparinized blood into the brain. In the acupuncture group, modeled rats had Baihui (DU20) and Qubin (GB7) acupoints treated once a day for 30 minutes. In the DAPT group, modeled rats had 0.15 μg/mL DAPT solution (10 mL) infused into the brain. Immunohistochemistry and western blot results showed that acupuncture effectively inhibits Notch1 and Hes1 protein expression in rat basal ganglia. These inhibitory effects were identical to DAPT, a Notch signaling pathway inhibitor. Our results suggest that acupuncture has a neuroprotective effect on cerebral hemorrhage by inhibiting Notch-Hes signaling pathway transduction in rat basal ganglia after cerebral hemorrhage.
Keywords: nerve regeneration, acupuncture, cerebral hemorrhage, immunohistochemistry, western blot assay, Notch1, Hes1, rats, DAPT, neural stem cells, NSFC grant, neural regeneration
Introduction
Cerebral hemorrhage is non-traumatic, and caused by rupture of blood vessels in brain parenchyma. The most common causes are hypertension, cerebral arteriosclerosis, and intracranial vascular malformations. Cerebral hemorrhage is often induced by exertion and emotion, and most patients show sudden onset during activity. A large number of studies have shown that cerebral hemorrhage causes neuronal apoptosis (Chamnanvanakij et al., 2002; Riggs et al., 2005; Gao et al., 2009), which aggravates the brain damage and causes contralateral limb dysfunction. Therefore, to reduce the degree of brain injury and promote functional recovery, it is important to inhibit neuronal apoptosis (Guan et al., 2013). Previous studies have shown that acupuncture reduces damage by effectively promoting growth, repair, and restoration of the nervous system on the injury side (Feng et al., 2013; Li et al., 2013; Nam et al., 2013), although the underlying mechanism is not fully understood.
The Notch signaling pathway is an evolutionarily conserved signal transduction pathway involved in regulating cell proliferation, differentiation, and apoptosis in almost all tissues and organs (Monahan et al., 2009; Guo et al., 2010; Yalcin-Ozuysal et al., 2010; Fernandez-Valdivia et al., 2011; Gianni-Barrera et al., 2011). Despite this, few studies have focused on the Notch signaling pathway in the central nervous system. Recently, it has been shown that related gene and protein expression in the Notch signal transduction pathway is present in the central nervous system (Yagi et al., 2012). Notch1, a Notch homologue, is a highly conserved cell membrane receptor protein, which can directly regulate gene transcription and inhibit or promote cell differentiation, proliferation, and apoptosis (Miele, 2006). Notch1 protein is widespread in embryonic neural tissue, but decreases rapidly after birth until it is no longer detected (Zhang et al., 2012). However, Notch1 protein expression is detected in adult neural stem cells from the subventricular zone of the lateral ventricles, the retina, and hippocampal dentate gyrus (Zhang et al., 2012).
The main role of the Notch signaling pathway is in proliferation and differentiation of stem cells, facilitation of inhibitory cell differentiation signals via “bypass inhibition”, and following signal transmission, initiation of family protein binding between cell membrane Notch receptors and its ligands, Delta/Serrate/Lag-2 (DSL) and CBF-1/Suppressor of hairless/Lag (CSL), on neighboring cells. During central nervous system development, Notch-Hes signaling inhibits neural stem cell differentiation into neuronal and glial cells by maintaining them in an undifferentiated state with self-renewing capacities. Binding of the Notch receptor to its ligand, activates the Notch signaling pathway and inhibits neuronal differentiation, improves the internal environment, promotes endogenous neural stem cell proliferation, and enhances nerve repair (Chapouton et al., 2010; Falk et al., 2012). In vitro experiments show that during cerebral ischemic injury, the Notch signal transduction pathway upregulates endogenous Notch1 receptor expression (Guo et al., 2013).
To date, studies have seldom focused on changes in the Notch signaling pathway during cerebral hemorrhagic injury, and there are no consistent conclusions on the effects. Thus, in this study, we used a rat model of cerebral hemorrhage to investigate the effect of acupuncture on Notch1 and Hes1 expression in brain tissue. Our aim was to determine if acupuncture promotes restoration of brain injury after cerebral hemorrhage by increasing Notch1 and Hes1 expression, which may provide a theoretical foundation for acupuncture treatment during cerebral hemorrhage.
Materials and Methods
Animals
Healthy specific-pathogen-free male Wistar rats (n = 156), aged 50 days and weighing 350 ± 20 g were supplied by the Harbin Veterinary Research Institute in China (Certification No. SCXK (Hei) 20100027). All rats were housed at 22 ± 2°C with humidity of 50 ± 5% and noise < 60 dB.
Establishment of a cerebral hemorrhage model
In accordance with a previous method (Rosenberg et al., 1990), rats were intraperitoneally anesthetized with 10% chloral hydrate (350 mg/kg) (Royalton, Dalian, Liaoning Province, China), and fixed in the prone position to a stereotaxic apparatus. Rats were positioned with the anterior and posterior fontanelles in the same horizontal plane (determined by the upper incisor tooth hook plane being 2.4 mm lower than the plane between the ears). The median scalp was shaved and sterilized, and a median incision approximately 1 cm long was made. The periosteum was stripped using a bone stripper, and the anterior fontanelle and coronal suture exposed. A round hole (1.0 mm diameter) was drilled using a dental drill (3.5 mm right and 0.2 mm posterior to bregma) until the dural surface was reached. The tail was disinfected with alcohol and 3 cm cut from the caudal end. Blood (50 μL) was obtained using a microinjector fixed on the stereotaxic apparatus, which entered vertically approximately 6 mm along the hole. Non-heparinized blood (50 μL) was infused into the caudate putamen at 25 μL/min. The needle was maintained in place for 5 minutes, and simultaneously, gauze was used to bandage the tail wound. Finally, the skull wound was blocked with zinc phosphate cement and the scalp sutured.
Screening rat models
In accordance with Berderson's score (Berderson et al., 1986), the specific method for assessing cerebral hemorrhage models was: rats were lifted by their tails, until they were 10 cm higher than the desktop; the paw of a normal rat will be straight.
Berderson score:

After model establishment, the rats had regained consciousness, according to appropriate signs and symptoms, rats with intracranial hematoma were considered successful models of cerebral hemorrhage.
Experimental groups
The rats were randomly divided into control (n = 12), model (n = 48), acupuncture (n = 48), and N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT) (Notch inhibitor) (n = 48) groups. The model, acupuncture and DAPT groups were equally subdivided into four subgroups according to different time points (1, 3, 7, and 14 days) after model establishment. The control group was only observed at day 1. Rats in the control group did not receive any treatment, and were directly decapitated and samples collected. In the model group, samples were collected at 1, 3, 7, and 14 days after model establishment. In the acupuncture group, rats received acupuncture 12 hours after model establishment, and once every 24 hours thereafter. The acupuncture method was as follows: rats were fixed on the acupuncture board. The Baihui (DU20) (specific location: head between the middle ears) and Qubin (GB7) (specific location: leading edge of ear root) acupoints were treated on the affected side using a 1.3 inch stainless steel needle (Huatuo, Suzhou, Jiangsu Province, China). The needling depth was 1.0 inch, and the needle was maintained in place for 30 minutes. During this time, the needle was twisted three times for 5 minutes each, at a speed of 200 cycles per minute. Hippocampal specimens were collected at 1, 3, 7, and 14 days after acupuncture. In the DAPT group, a round hole (3.6 mm diameter) was drilled using a dental drill (1.4 mm right and 0.8 mm posterior to bregma) 30 minutes before model establishment. Within 5 minutes, 10 μL DAPT solution (0.15 μg/μL) was injected using a microsyringe (Hamilton, Bonaduz, Switzerland) via the lateral ventricle. The needle was maintained in place for 5 minutes. Hippocampal specimens were obtained at 1, 3, 7, and 14 days after modeling.
Specimen collection
Rats were intraperitoneally anesthetized using an overdose of chloral hydrate at appropriate time points after surgery. The chest was opened rapidly and the heart exposed. Catheterization from the left ventricle into the aortic root was performed. A small incision was made in the right atrial appendage for an exit hole. The ventricle was perfused using approximately 200 mL each of 4°C saline and 4% paraformaldehyde/0.1 M phosphate buffer. Samples were dehydrated, paraffin embedded, and then sliced into 6 μm-thick sections for hematoxylin-eosin staining and immunohistochemistry. Some rats were directly decapitated after anesthesia overdose, and basal ganglia tissue immediately stored in liquid nitrogen at −80°C for western blot assay.
Immunohistochemistry
Notch1 and Hes1 expression in the basal ganglia was determined by immunohistochemistry. After paraffin embedding and dehydration, sections were placed in 3% fresh H2O2 solution at 25°C for 10 minutes to block endogenous peroxidase, and then washed in 0.01 M PBS three times for 2 minutes each. Sections were immersed in 0.01 M citrate buffer (pH 6) and heated twice in the microwave for 5 minutes each time, with 10 minute intervals. After naturally cooling to 25°C, sections were incubated with primary antibody (rabbit Notch1 or Hes1 polyclonal antibodies; 1:200; NeoMarkers, Fremont, CA, USA) overnight at 4°C, and washed with 0.01 M PBS three times for 2 minutes each. Sections were then incubated with biotinylated secondary antibody (horseradish peroxidase-goat anti-rabbit IgG; NeoMarkers) at 37°C for 30 minutes, washed with 0.01 M PBS three times for 2 minutes each, visualized with 3,3′-diaminobenzidine (Golden Bridge Biotechnology Co., Ltd., Beijing, China), counterstained with hematoxylin, mounted with neutral gum, and then observed under an optical microscope (Olympus, Tokyo, Japan).
Image analysis
Data were analyzed using Motic Med 6.0 pathological image analysis system (Motic Med Industrial Group Co., Ltd., Xiamen, Fujian Province, China). Each section was randomly observed and counted at 400× magnification. Total positive cell number was calculated.
Western blot assay
Basal ganglia tissue was extracted using the RIPA lysis buffer protein BCA kit (Biomiga, San Diego, CA, USA) to determine total protein concentration. Total protein loaded from each sample was 50 μg/well. Samples were electrophoresed and transferred to membranes. Film was blocked with 5% skim milk in phosphate Tween buffer at 4°C overnight. Samples were incubated with primary antibody (rabbit Notch1, polyclonal antibody; 1:1,000; Epitomics, Burlingame, CA, USA) for 16–24 hours at 4°C. Membranes were then washed with Tris-buffered saline with Tween 20, and incubated in secondary antibody (goat anti-rabbit IgG; 1:1,500; Epitomics) for 1 hour at room temperature. Gray values for specific bands were measured using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed by the first author using SPSS 18.0 software (SPSS, Chicago, IL, USA), with values expressed as the mean ± SD. Inter-group data were compared by one-way analysis of variance and the least significant difference test. Values of P < 0.05 were considered statistically significant.
Results
Notch1 protein expression in rat basal ganglia tissue
In each group, Notch1 protein expression was visible in the basal ganglia at 1, 3, 7, and 14 days after modeling. Compared with the control group, Notch1 protein expression was significantly higher in the model group at 1, 3, and 7 days (P < 0.01). Furthermore, compared with the model group, Notch1 protein expression was significantly lower in the DAPT group at each time point, particularly at 3 days (P < 0.01) although significant differences were still detected at 7 and 14 days (P < 0.05). At all time points, Notch1 protein expression was significantly lower in the acupuncture group than in the model group (P < 0.01), and was inhibited in the DAPT and acupuncture groups (P > 0.05; Table 1, Figure 1).
Table 1.
Notch1 protein expression (obtained from gray values) in the basal ganglia of rats with intracerebral hemorrhage
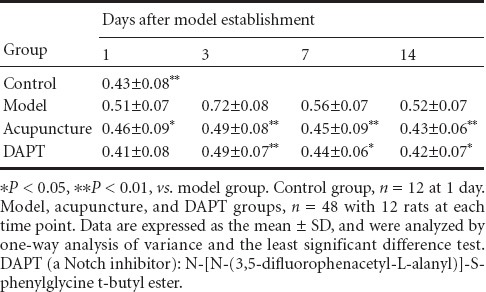
Figure 1.
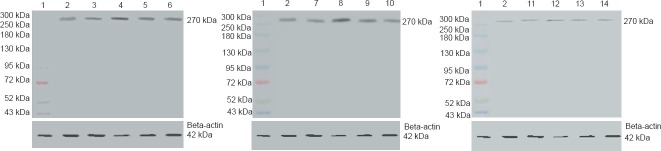
Notch1 protein expression in the basal ganglia of rats with intracerebral hemorrhage (western blot assay).
Compared with the control group, Notch1 protein expression was higher in the model group at 1, 3, and 7 days. Compared with the model group, Notch1 protein expression was lower in the DAPT(a Notch inhibitor) group at each time point, particularly at 3 days, and lower in the acupuncture group at all time points. 1: Marker; 2: control group; 3–6: model group (1, 3, 7, and 14 days after modeling); 7–10: acupuncture group (1, 3, 7, and 14 days after modeling); 11–14: DAPT group (1, 3, 7, and 14 days after modeling). DAPT: N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester.
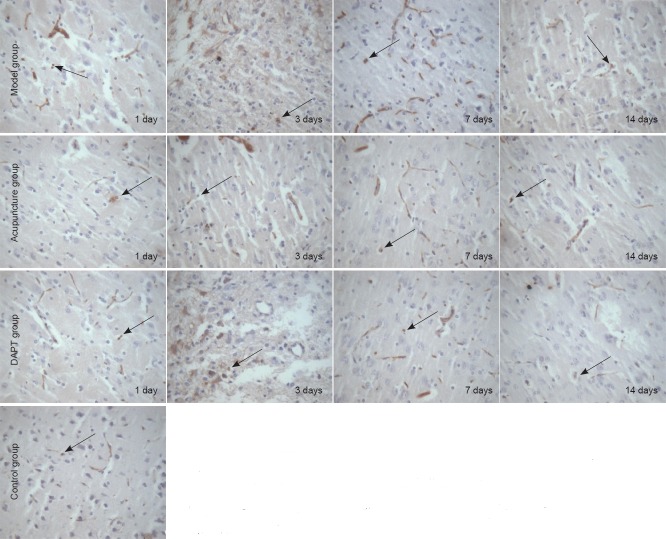
Notch1 immunoreactivity in rat basal ganglia tissue
Notch1-immunoreactive cells were widely distributed in the cytoplasm and nucleus. Weak Notch1 immunoreactivity was observed in the control group. Notch1-immunoreactive cell number was significantly higher in the model group than in the control group at various time points (P < 0.05), and significantly lower in the DAPT group than the model group at 1, 3 (P < 0.01), and 7 (P < 0.05) days. Notch1-immunoreactive cell number was lower in the acupuncture group than in the model group at 1, 3, and 7 days (P < 0.05). No significant difference was detected between the acupuncture and DAPT groups (P > 0.05; Table 2, Figure 2).
Table 2.
Notch1 immunoreactivity (cell count, × 400 magnification) in the basal ganglia of rats with cerebral hemorrhage
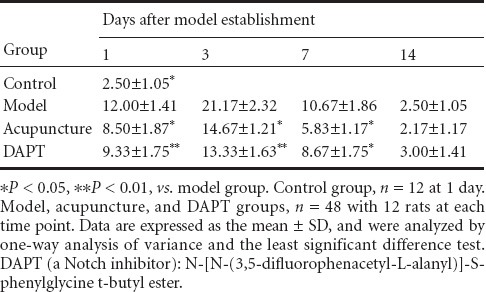
Figure 2.
Notch1 immunoreactivity in the basal ganglia of rats with cerebral hemorrhage and acupuncture treatment (immunohistochemical staining, × 400 magnification).
Notch1-immunoreactive cell number was higher in the model group than the control group at various time points after cerebral hemorrhage induction. Notch1-immunoreactive cell number was lower in the DAPT (a Notch inhibitgor) and acupuncture groups than the model group at 1, 3, and 7 days. DAPT: N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester. Arrows show Notch1-immunoreactive cells.
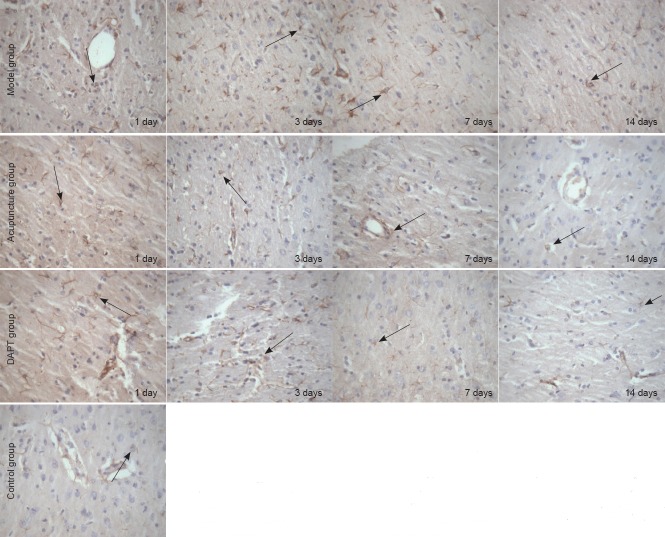
Hes1 immunoreactivity in rat basal ganglia tissue
Hes1-immunoreactive cells were mainly expressed in vascular endothelial and glial cells. Hes1 immunoreactivity was observed in the control group. At various time points, Hes1-immunoreactive cell number was significantly increased in the model group compared with the control group (P < 0.05). Moreover, Hes1-immunoreactive cell number was significantly lower in the acupuncture group than the model group at all time points (P < 0.05). Hes1 immunoreactivity was suppressed in the DAPT group, with significant differences between DAPT and model groups at 3, 7, and 14 days (P < 0.05). Hes1 immunoreactivity was inhibited in both the acupuncture and DAPT groups, but no significant difference was detected at any time point (P > 0.05; Table 3, Figure 3).
Table 3.
Hes1 immunoreactivity (cell count, × 400 magnification) in the basal ganglia of rats with cerebral hemorrhage
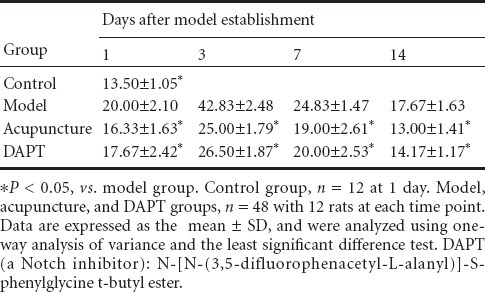
Figure 3.
Hes1 immunoreactivity in the basal ganglia of rats with cerebral hemorrhage (immunohistochemical staining, × 400).
Hes1-immunoreactive cell number was higher in the model group than the control group at various time points after cerebral hemorrhage induction. Hes1-immunoreactive cell number was lower in the acupuncture group than the model group at various time points. DAPT, a Notch inhibitor, suppressed Hes1 immunoreactivity at various time points after cerebral hemorrhage induction. DAPT: N-[N-(3,5- difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester.
Discussion
Neural stem cells can self-renew and undergo multi-directional differentiation. Under certain conditions, they differentiate into neuronal or glial cells. The Notch signaling pathway is thought to be a main factor for embryonic neural stem cell growth regulation, playing a “side restrain” role (Artavanis-Tsakonas et al., 1999; Struhl, 1999; Shi et al., 2008). Specifically, the Notch signaling pathway indirectly maintains neural stem cell differentiation and self-renewal by inhibiting neuronal and glial cell differentiation (Androutsellis-Theotokis et al., 2006). Using chicken embryos, researchers have shown Notch's “side restrain", with the activated Notch signaling system inhibiting neuronal differentiation. Notch signaling pathway activation causes restraint of neural stem cell differentiation, and instead they proliferate. (Wakamatsu et al., 2000).
Our western blot results showed that Notch1 expression increased significantly at 1 day after modeling, peaked at 3 days, reduced at 7 days, and then obviously decreased and was close to normal levels at 14 days. Notch1 expression was visibly lower in the acupuncture and DAPT groups than the model group at various time points. DAPT is an inhibitor of the Notch signaling pathway. In vivo and in vitro experiments show that DAPT application blocks the Notch signaling pathway and causes G1/S phase cell cycle delay in human embryonic stem cells, inducing neuronal cell differentiation and significantly reducing the time required for neural maturation (Borghese et al., 2010). In the present study, we found no significant difference between the acupuncture and DAPT groups, indicating that acupuncture plays a similar role to DAPT, and inhibits Notch1 and Hes1 expression and indirectly maintains neural stem cell self-renewal. Our immunohistochemical staining results are consistent with the observed western blot trends at 1 and 3 days. Notch1 immunoreactivity in the acupuncture group was significantly weaker than the model group at 7 days, and also weaker than the DAPT group, suggesting that acupuncture inhibits Notch signaling pathway expression. The Notch-Hes signaling pathway effect on neural stem cells, as well as our western blot and immunohistochemistry results, suggests that acupuncture may inhibit neuronal differentiation of neural stem cells by inhibiting the Notch-Hes signaling pathway and maintaining neural stem cell proliferation. This assumption needs further research, but offers new research directions for neural stem cell regeneration and repair.
Acknowledgments:
We are very grateful to all the staff of Harbin Medical University in China for their help.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81273824, 30772840; Ministry of Education Doctoral Fund in China, No. 20102327110003; the Natural Science Foundation of Heilongjiang Province in China, No. ZD201204; and Special funds for Technological Innovation Research of Harbin, China, No. 2012RFXXS062
Conflicts of interest: None declared.
Copyedited by James R, Wysong S, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bac SK, Kittappa R, McKay RD. Notchsinalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brustle O. Inhibition of Notch signaling in human embryonic stem cell-deribed neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and invivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Chamnanvanakij S, Margraf LR, Burns D, Perlman JM. Apoptosis and white matter injury in preterm infants. Pediatr Dev Pathol. 2002;5:184–189. doi: 10.1007/s10024001-0205-0. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Skupien P, Hesl B, Coolen M, Moore JC, Madelaine R, Kremmer E, Faus-Kessler T, Blader P, Lawson ND, Bally-Cuif L. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R, Falk A, Dyson MR, Melidoni AN, Parthibam K, Young JL, Roake W, McCafferty J. Generation of anti-notch antibodies and their application in blocking notch signalling in neural stem cells. Methods. 2012;58:69–78. doi: 10.1016/j.ymeth.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen J. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappaB-mediated neuronal cell apoptosis in cerebral ischemia- reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Takeuchi H, Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS, Jafar-Nejad H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138:1925–1934. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Liu W, Sun ZD, Zhao SG, Liu XZ. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009 doi: 10.1186/1471-2202-10-7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A, Kandel JJ. Notch: a key regulator of tumor angiogenesis and metastasis. Histol Histopathol. 2012;27:151–156. doi: 10.14670/hh-27.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni-Barrera R, Trani M, Reginato S, Banfi A. To sprout or to split? VEGF, Notch and vascular morphogenesis. Biochem Soc Trans. 2011;39:1644–1648. doi: 10.1042/BST20110650. [DOI] [PubMed] [Google Scholar]
- Guan XL, Hou LC, Yang H, Wang FX, He M. Glutamate in rat brain hemorrhage in brain water content and apoptosis changes and basic fibroblast growth factor. Zhongfeng yu Shenjing Jibing Zazhi. 2013;30:325–328. [Google Scholar]
- Guo D, Li C, Teng Q, Sun Z, Li Y, Zhang C. Notch1 overexpression promotes cell growth and tumor angiogenesis in myeloma. Neoplasma. 2013;60:33–40. doi: 10.4149/neo_2013_005. [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhao NJ, Lin L, Zhang MF, Zheng ZH. Notch1 gene on human U251 glioma cell proliferation and cycle. Zhongguo Bingli Shengli Zazhi. 2010;26:1115–1119. [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Q. Acupuncture therapy for stroke patients. Int Rev Neurobiol. 2013;111:159–179. doi: 10.1016/B978-0-12-411545-3.00008-0. [DOI] [PubMed] [Google Scholar]
- Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- Monahan P, Rybak S, Raetzman LT. The Notch target gene Hes1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386–4394. doi: 10.1210/en.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam MH, Ahn KS, Choi SH. Acupuncture stimulation induces neurogenesis in adult brain. Int Rev Neurobiol. 2013;111:67–90. doi: 10.1016/B978-0-12-411545-3.00004-3. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutictarget in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- Riggs JE, Libell DP, Brooks CE, Hobbs GR. Impact of institution of a stroke program upon referral bias at a rural academic medical center. J Rural Health. 2005;21:269–271. doi: 10.1111/j.1748-0361.2005.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G. Greenwald l Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–524. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by Notch-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth migration and invasion, and induces apoptosis via inactivation of Akt-mTOR and NF-κB signaling pathways. J Cell Biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- Yagi H, Saito T, Yanagisawa M, Yu RK, Kato K. Lewis X-carrying N-glycans regulate the proliferation of mouse embryonic neural stem cells via the Notch signaling pathway. J Biol Chem. 2012;287:24356–24364. doi: 10.1074/jbc.M112.365643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin-Ozuysal O, Fiche M, Guitierrez M, Wagner KU, Raffoul W, Brisken C. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 2010;17:1600–1612. doi: 10.1038/cdd.2010.37. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Huang GW, Liu H, Chang H, Wilson JX. Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutr Neurosci. 2012;15:55–60. doi: 10.1179/1476830511Y.0000000025. [DOI] [PubMed] [Google Scholar]