Abstract
Recent studies have shown that induced expression of endogenous antioxidative enzymes thr-ough activation of the antioxidant response element/nuclear factor erythroid 2-related factor 2 (Nrf2) pathway may be a neuroprotective strategy. In this study, rat cerebral cortical neurons cultured in vitro were pretreated with 10 μM curcumin or post-treated with 5 μM curcumin, respectively before or after being subjected to oxygen-glucose deprivation and reoxygenation for 24 hours. Both pretreatment and post-treatment resulted in a significant decrease of cell injury as indicated by propidium iodide/Hoechst 33258 staining, a prominent increase of Nrf2 protein expression as indicated by western blot analysis, and a remarkable increase of protein expression and enzyme activity in whole cell lysates of thioredoxin before ischemia, after ischemia, and after reoxygenation. In addition, post-treatment with curcumin inhibited early DNA/RNA oxidation as indicated by immunocytochemistry and increased nuclear Nrf2 protein by inducing nuclear accumulation of Nrf2. These findings suggest that curcumin activates the expression of thioredoxin, an antioxidant protein in the Nrf2 pathway, and protects neurons from death caused by oxygen-glucose deprivation in an in vitro model of ischemia/reperfusion. We speculate that pharmacologic stimulation of antioxidant gene expression may be a promising approach to neuroprotection after cerebral ischemia.
Keywords: nerve regeneration, brain injury, curcumin, ischemia/reperfusion injury, oxidative stress, primary cell culture, cortical neurons, oxygen-glucose deprivation, pretreatment, post-treatment, NSFC grant, neural regeneration
Introduction
Cerebral ischemia/reperfusion is characterized by an increase in oxidative stress and a wide variety of cellular alterations, which lead to delayed neuron cell injury, including impaired energy metabolism (Li et al., 2012). The extent of neuroprotection offered by antioxidants may be limited. However, the pharmacologic activation of multiple endogenous antioxidant defense systems may constitute an alternative (Eghwrudjakpor and Allison, 2010), combined therapeutic approach.
Nuclear factor erythroid 2-related factor 2 (Nrf2) belongs to the cap-n-collar family of transcription factors. These factors share a highly conserved basic leucine zipper structure (Hayes and McMahon, 2001; Lau et al., 2013). Nrf2 is normally bound to its suppressor, Keap1, in cytoplasm, but is unbound under certain conditions. Free Nrf2 translocates to the nucleus. Once in the nucleus, Nrf2 binds to antioxidant response element and drives the transcription of downstream genes. Antioxidant response element is an enhancer of many phase II antioxidant enzymes genes, such as heme oxygenase-1 (Choi and Alam, 1996; Linares et al., 2013), glutathione S-transferase (Hayes et al., 2000), sulfiredoxin (Soriano et al., 2008), thioredoxin (Tanito et al., 2007), and reduced nicotinamide adenine dinucleotide phosphate: quinine oxidoreductase 1 (Nioi et al., 2003). Among these, thioredoxin is an important reducer of oxidative stressors because of its ability to protect against H2O2-induced apoptosis. Its inhibition promotes oxidative stress and cell injury (Yoshida et al., 2005). The Nrf2/antioxidant response element pathway can be activated by a variety of small thiol-active molecules as well as many dietary phytochemicals such as sulforaphane and curcumin (Balogun et al., 2003; Katsori et al., 2011). Curcumin, the principle coloring agent present in the rhizomes of Curcuma longa (zingiberaceae), has been used for centuries as a traditional Chinese medicine for the treatment of a variety of medical conditions. Curcumin possesses a broad range of pharmacological activities including antioxidant (Suryanarayana et al., 2007; Antonio and Druse, 2008), anti-inflammatory (Biswas et al., 2005; Lim et al., 2005) and anti-cancer activities (Singh and Khar, 2006; Sa and Das, 2008; Basile et al., 2009). As an anticarcinogen, an important target of curcumin is the Keap1 protein, which normally binds and sequesters Nrf2 in the cytoplasm. Curcumin can directly act on Keap1 to release Nrf2, which then translocates to the nucleus, where it heterodimerizes with small Maf proteins and binds to antioxidant response elements, inducing the expression of a large number of cytoprotective genes (Kang et al., 2008).
A previous in vivo study has demonstrated the potential of curcumin to protect against cerebral ischemia/reperfusion injury (Zhao et al., 2008, 2010). However, the mechanisms by which curcumin directly protects neurons against insults such as ischemia/reperfusion injury remain unclear. The objective of this study was to assess the ability of curcumin to induce expression of the antioxidative protein thioredoxin and to evaluate the antioxidant effects of curcumin against oxidative stress-induced death owing to transient oxygen-glucose deprivation (OGD) as an in vitro model of ischemia/reperfusion.
Materials and Methods
Animals
The experimental protocol used in this study was approved by the Ethics Committee for Animal Experimentation and experiments were conducted according to the Guidelines for Animal Experiments of Chongqing Medical University (Chongqing, China). A total of 145 Sprague-Dawley new-born rats (1 day old, male or female, weighing 7–9 g) of specific pathogen-free grade were supplied by the Laboratory Animal Center, Chongqing Medical University, China (license No. SCXK (Yu) 2007-0001). All reagents were obtained from Sigma Chemical (St. Louis, MO, USA) except where otherwise specified.
Primary culture of rat cortical neurons
Cortical neurons were prepared from the brains of 1-day-old Sprague-Dawley rats, as described previously (Ge et al., 2007). The cells were plated onto poly-L-lysine-coated well plates (Sigma) or glass coverslips at a density of 2 × 106 cells/cm2. Cells were grown in plating medium (consisting of 89% high-glucose DMEM, 10% fetal bovine serum, 1% penicillin-streptomycin; Gibco, Grand Island, NY, USA). After 24 hours, the medium was changed to fresh neurobasal medium (Gibco) supplemented with 2% B27 (Gibco) and 1% penicillin/streptomycin, and then refreshed every 2–3 days. Cultures were incubated at 37°C in a 95% air/5% CO2 in a humidified incubator (Thermo3111, Waltham, MA). Experiments were performed at 5–6 days in vitro.
OGD and curcumin treatment
Rat cortical neurons were deprived of O2 and glucose by changing the culture medium to glucose-free DMEM (Gibco) as previously described (Wang et al., 2009; Xiang et al., 2010). After washing the neurons twice with glucose-free DMEM, they were incubated in an anaerobic chamber at 37°C for 1 hour (Forma model 3131, Thermo Scientific, Marietta, OH, USA) equilibrated with 94% N2, 1% O2 and 5% CO2. At the end of the OGD, the medium was replaced with neurobasal medium supplemented with 2% B27 and neurons were incubated in normal conditions (95% air and 5% CO2, 37°C) for 24 hours of recovery. Control experiments were performed with neurons maintained under identical conditions before, during, and after OGD except for omission of OGD treatment.
For measurements of protein immunoreactivity and enzyme activity, rat cortical neurons were cultured in neurobasal medium supplemented with 2% B27 and 1% penicillin/streptomycin.
For the preconditioning experiments, the following groups were studied. In group 1, cortical neurons were exposed to 0.01% dimethyl sulfoxide for 24 hours without OGD/reoxygenation (control group, n = 4). In group 2, cortical neurons were exposed to curcumin (10 μM) for 24 hours without OGD/reoxygenation (control curcumin group, n = 4). In group 3, cortical neurons were exposed to dimethyl sulfoxide for 24 hours followed by 60 minutes of OGD and 24 hours of reoxygenation (OGD/R + vehicle group, n = 4). In group 4, cortical neurons were exposed to curcumin (10 μM) for 24 hours followed by OGD/reoxygenation (OGD/R + curcumin group, n = 4).
For the postconditioning experiments, there were four groups. Curcumin (5 μM) or vehicle (dimethyl sulfoxide, 0.01%) was added to rat cortical neurons cultured for 1 hour after the ischemic insult at the beginning of reoxygenation and maintained for 24 hours, as the OGD/R + curcumin group (n = 4) and vehicle-treated group (OGD/R + vehicle group, n = 4), respectively. In the control curcumin group (n = 4) and control group (n = 4), cortical neurons were treated identically except that they were not exposed to OGD.
Propidium iodide/Hoechst 33258 staining for cell injury
Neuronal cell injury was assessed using the membrane impermeable fluorescent dye propidium iodide at 10 μg/mL to label dead/apoptotic cells, and the cell permeable fluorescent dye Hoechst 33258 was used to label all cells. After OGD/reoxygenation, cortical neurons cultured on coverslips were exposed to 10 μg/mL propidium iodide for 20 minutes, then fixed in 4% paraformaldehyde for 20 minutes at room temperature, and washed three times in PBS. Cells were incubated with Hoechst 33258 for 30 minutes at room temperature. Fluorescence was observed using a 20× objective lens under a fluorescence microscope (Olympus BX51, Tokyo, Japan) and images were captured using an Olympus camera (Tokyo, Japan). Simultaneous staining of cortical cultures by both propidium iodide and Hoechst was considered a quantitative measure of the extent of cell injury. The number of double-positive cells is expressed as a percentage of Hoechst-stained cells.
Oxidative modification of nucleic acids
Immunocytochemistry was performed on cortical neuron cultures on coverslips as previously described with a few modifications (Danilov and Fiskum, 2008). After 24 hours of reoxygenation, the coverslips were rinsed twice with 0.05 mM PBS and fixed in 4% paraformaldehyde for 20 minutes at room temperature. They were incubated for 30 minutes in PBS containing 10% normal goat serum, then incubated with primary anti-8-hydroxydeoxyguanosine antibody (Biosynthesis Biotechnology Co., Ltd., Beijing, China) at a 1:300 dilution in PBS + 0.3% Triton X-100 at 4°C. After 24 hours, cells were rinsed with PBS and incubated with FITC secondary antibody (1:200) for 20 minutes at room temperature. Then, the sections were counterstained with Hoechst 33258 to facilitate counting of the total number of neurons. Finally, the neurons were examined under a fluorescence microscope (Olympus BX51, Tokyo, Japan). Three independent images were obtained from each individual coverslip, and analyzed in the computer-assisted image analyzer described above using MetaMorph software (Universal Imaging Corp, USA). The relative fluorescence intensity of each image was calculated by dividing the integrated intensity by the number of cells present in the field. Data represent the average values of three images.
Isolation of cytosolic/nuclear fractions
After 24-hour reoxygenation, primary cortical neurons were scraped from culture dishes, resuspended in cold Buffer A (including 10 mM HEPES-KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride) and kept on ice for 10 minutes. Then 25 μL of 10% Nonidet P40 (v/v) was added to the cell suspension. Samples were then centrifuged at 12,000 × g for 5 minutes at 4°C. The resultant supernatant was removed as the cytosolic fraction. Pellets were resuspended in 80 μL of Buffer B (including 20 mM HEPES-KOH pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride) and kept on ice for 20 minutes for high salt extraction. After a final 2 minute centrifugation at 12,000 × g at 4°C, the supernatant containing the nuclear fraction was collected and stored at −70°C. The protein concentrations were measured using a Lowry DC kit with bovine serum albumin used as a concentration standard (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Samples of cortical neurons were analyzed for Nrf2 and thioredoxin protein levels. The lysates and nuclear samples were treated with 50 mM dithiothreitol and NuPage 4 × LDS loading buffer (Invitrogen, Carlsbad, CA, USA) prior to heating at 70°C for 10 minutes. The samples were separated by sodium dodecyl sulfate-poly-acrylamide gel electrophoresis. Each lane was loaded with 30 μg of total protein. After sodium dodecyl sulfate-poly-acrylamide gel electrophoresis, the proteins were transferred onto a polyvinylidene fluoride membrane. The membranes were washed in Tris-buffered saline containing 0.05% Tween-20 (TBST) followed by blocking for 1 hour using 5% non-fat milk in TBST at room temperature. This was followed by incubation overnight at 4°C with primary rabbit anti-thioredoxin polyclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-Nrf2 polyclonal antibody (1:1,000; Abcam), mouse anti-β-actin monoclonal antibody (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or rabbit anti-histone H3 monoclonal antibody (1:5,000; Abcam). The membranes were then washed with TBST and incubated for 1 hour at room temperature in horseradish peroxidase-conjugated rabbit anti-goat (Santa Cruz Biotechnology), goat anti-rabbit (Santa Cruz Biotechnology), or goat anti-mouse antibodies (Santa Cruz Biotechnology) at a 1:5,000 dilution for 1 hour at room temperature. The washed blots were then treated with enhanced chemiluminescence detection reagent (Amersham Bioscience, UK). Films were scanned using an imaging densitometer (Bio-Rad), and the results were quantified using Quantity One 1-D analysis software (Bio-Rad, Richmond, CA, USA). The expression levels of Nrf2 and thioredoxin were corrected to the internal loading control, and fold changes were expressed relative to the levels in the control group.
Thioredoxin enzyme activity assay
Thioredoxin activity was determined using an insulin disulfide reduction assay (Yamamoto et al., 2003). In brief, 40 μg of cellular protein extract was pre-incubated at 37°C for 15 minutes with 2 μL activation buffer (100 mM HEPES, 2 mM EDTA, 1 g/L bovine serum albumin, and 2 mM dithiothreitol) to reduce thioredoxin deactivation. After the addition of 20 μL of reaction buffer (100 mM HEPES, 2.0 mM EDTA, 0.2 mM nicotinamide adenine dinucleotide phosphate, and 140 μM insulin), the reaction was started by addition of mammalian thioredoxin reductase (1 μL, Sigma), or water to controls, and samples were incubated for 30 minutes at 37°C. The reaction was terminated by adding 125 μL of stopping solution (0.2 M Tris-HCl, 10 M guanidine-HCl, and 1.7 mM 3-carboxy-4-nitrophenyl disulfide). The optical density value was read on a Multiskan Spectrum microplate spectrophotometer (Thermo Scientific, USA) at 412 nm.
Immunocytochemistry
Cortical neurons were cultured in chamber slides to 70% confluence. After exposure of the cells to curcumin, they were fixed in 4% paraformaldehyde for 30 minutes at room temperature and washed with PBS. The fixed cells were permeabilized in PBS containing 0.2% Triton X-100 and 5% bovine serum albumin for 90 minutes at room temperature. This was followed by exposure to the primary antibody (rabbit polyclonal anti-Nrf2 antibody; 1:500) at 4°C overnight. After washing the cells in PBS, a secondary antibody linked to Cy3 (goat anti-rabbit lgG) was added along with 4,6-diamidino-2-phenylindole (2 g/mL; nuclear staining) for 90 minutes at room temperature. The cells were then washed in PBS, mounted on slides with mounting medium, and examined by fluorescence microscopy (Olympus BX51, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SEM of three independent experiments. Where indicated, statistical analyses used one-way analysis of variance and the Student-Newman-Keuls tests with SPSS 13.0 software (SPSS, Chicago, IL, USA). A P < 0.05 level was considered statistically significant.
Results
Curcumin protected neurons against OGD-induced injury
To determine whether pretreatment or post-treatment with curcumin protects against death of neurons induced by transient OGD, we exposed primary cultured rat cortical neurons to 10 μM curcumin for 24 hours prior to the OGD insult, or 5 μM curcumin for 24 hours after a 1 hour period of OGD, as described in the Methods section.
As shown in Figure 1A, the number of injured cells observed after exposure to 10 μM curcumin was significantly decreased compared with the OGD/R group (39.21 ± 2.63% vs. 64.78 ± 3.17%, P < 0.05), which indicated that with 24 hours of reoxygenation, pretreatment with curcumin conferred significant protection against delayed cell injury. As shown in Figure 1B, 5 μM curcumin post-treatment reduced the cell injury to 43.58 ± 3.14% of the level in the control group. The vehicle-treated cells experienced 64.78 ± 3.17% cell injury (P < 0.05).
Figure 1.
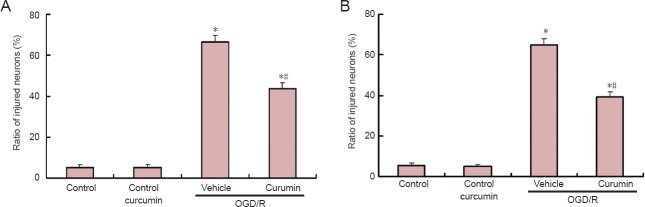
Curcumin protects neurons against oxygen-glucose deprivation-induced injury.
Curcumin pretreatment protects neurons against cell injury. (A) Rat cortical neuron cultures pretreated with curcumin (10 μM) or vehicle (dimethyl sulfoxide 0.01%) for 1 hour and reoxygenation for 24 hours. Control cells were incubated for 24 hours with either curcumin or vehicle in neurobasal medium without serum. (B) Rat cortical neuron cultures were exposed to oxygen-glucose deprivation in a deoxygenated, glucose-free ionic shift solution for 1 hour. Curcumin (5 μM) was added to the culture at the beginning of reoxygenation and cultures were maintained for 24 hours. After 24 hours of reoxygenation, the injury ratio of cortical neurons was determined by propidium iodide/Hoechst staining. Data are expressed as the mean ± SEM (n = 4). Data were analyzed using one-way analysis of variance and the Student Newman-Keuls post-hoc test. *P < 0.01, vs. control group; #P < 0.05, vs. OGD/R + vehicle group. OGD/R: Oxygen-glucose deprivation/reoxygenation.
Curcumin post-treatment inhibited early DNA/RNA oxidation after transient OGD
To determine whether post-treatment with curcumin protects against oxidative stress induced by transient OGD, we evaluated the ability of post-treatment with curcumin to inhibit DNA/RNA oxidation early during reoxygenation, which precedes delayed cell injury. As shown in Figure 2, the relative fluorescence of 8-hydroxydeoxyguanosine following 24 hours of reoxygenation after a 1-hour period of OGD was significantly lower for curcumin-treated cells than for vehicle-treated cells (1,105 ± 46 U vs. 1,634 ± 93 U; P < 0.05). However, it was not significantly greater than for cells not exposed to OGD. This suggests that oxidative modifications to DNA/RNA must have occurred early during reoxygenation in this model, and that these modifications were inhibited by post-treatment with curcumin.
Figure 2.
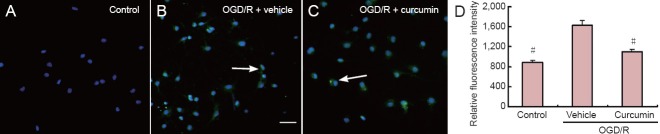
Immunoreactivity of 8-hydroxydeoxyguanosine (8-OHdG) in cortical neurons after 24 hours of reoxygenation (immunocytochemical staining).
Curcumin post-treatment inhibits early DNA/RNA oxidation after transient oxygen-glucose deprivation. (A) DNA/RNA oxidation was detected by immunocytochemical staining for 8-OHdG, a common marker for the evaluation of oxidative DNA damage. 8-OHdG is shown in green and nuclei were counterstained blue with 4,6-diamidino-2-phenylindole. Arrows show 8-OHdG and Hoechst double-positive cells. (B) Relative fluorescence intensities were obtained as described in the Methods and are expressed as the mean ± SEM (n = 4). Data were analyzed using one-way analysis of variance and the Student Newman-Keuls post-hoc test. #P < 0.05, vs. OGD/R + vehicle group. Scale bar: 100 μm. OGD/R: Oxygen-glucose deprivation/ reoxygenation.
Curcumin increased Nrf2 protein expression and induced nuclear accumulation of Nrf2
To determine whether the observed protection offered by curcumin pretreatment and post-treatment was accompanied by activation of the Nrf2 pathway of gene expression, neuronal cultures were exposed to curcumin or vehicle and the Nrf2 protein levels were analyzed after 24 hours of reoxygenation. As shown in Figure 3A, in the curcumin-pretreated group, Nrf2 immunoreactivity in nuclear fractions showed a double band around 90 kDa. A discrepancy between the predicted molecular weight (66.9 kDa) and the observed molecular weight has been reported previously, and is likely due to the abundance of acidic residues in Nrf2 which lead to abnormal migration in electrophoresis gels (Chan et al., 1993; Moi et al., 1994). The phosphorylated form of Nrf2 was almost undetectable under untreated conditions (control) or in vehicle-treated cells. As shown in Figure 3A, curcumin pretreatment increased the phosphorylated form of Nrf2 by 3.23-fold compared with 1.22-fold in vehicle-treated cells (P < 0.05). As shown in Figure 3B, in the curcumin-post-treatment group, the protein level of the phosphorylated form of Nrf2 detected in nuclear fractions was 1.90-fold higher in curcumin-treated cells than in vehicle-treated cells (P < 0.05), as indicated by western blot analysis. These results indicate that curcumin can increase Nrf2 protein expression and induce nuclear accumulation of Nrf2 protein regardless of whether the curcumin is administered before or after OGD insult.
Figure 3.
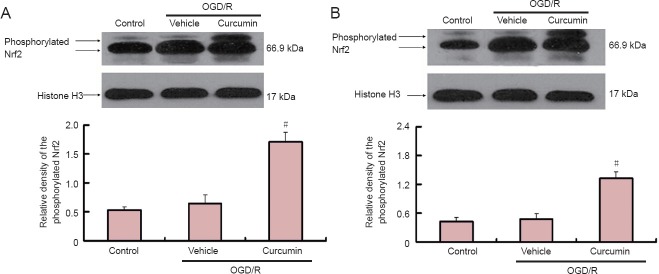
Curcumin increases Nrf2 protein expression in rat cortical neurons (western blot analysis).
(A) Cortical neurons were pretreated for 24 hours with curcumin or vehicle followed by OGD/R, or maintained in neurobasal medium without serum (control). (B) Cortical neurons were post-treated for 24 hours with 5 μM curcumin after a 1 hour period of OGD. After 24 hours, the nuclear fractions were isolated as described in the Methods. The immunoblots were probed with anti-Nrf2 antibody then stripped and re-probed for histone H3 as loading control (n = 4). The values were square transformed and the data were analyzed using one-way analysis of variance and the Student-Newman-Keuls post-hoc test. #P < 0.05, vs. OGD/R + vehicle group. Data were expressed as the mean ± SEM. OGD/R: Oxygen-glucose deprivation/reoxygenation; Nrf2: nuclear factor erythroid 2-related factor 2.
Curcumin increased the nuclear translocation of Nrf2
To determine whether Nrf2 undergoes nuclear translocation in neurons exposed to curcumin after OGD insult, we used immunocytochemical analysis and fluorescence microscopy to evaluate the nuclear translocation of Nrf2. Immunocytochemical analysis showed Nrf2 to be mostly in the cytoplasm in untreated neurons (Figure 4). In neurons post-treated with curcumin for 24 hours after a 1 hour period of OGD, Cy3 signaling for Nrf2 was observed in the nucleus.
Figure 4.
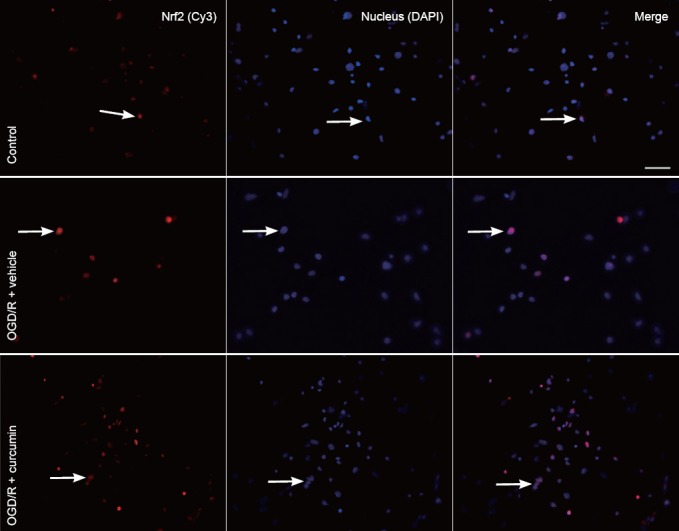
Curcumin increases the nuclear translocation of Nrf2 in cortical neurons (immunocytochemical staining, fluorescence microscope).
Cortical neurons cultured on chamber slides were exposed to 5 μM curcumin after a 1 hour period of OGD. After 24 hours of reoxygenation, neurons were fixed in 4% paraformaldehyde, permeabilized, and immunostained for active Nrf2 [cyanine-3 (Cy3); red]. The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; blue). A merge of Cy3 and DAPI is shown in the third panel. The images presented here are representative of multiple fields from three independent experiments. Arrows show the curcumin-induced translocation of Nrf2 to the nucleus, cells expressing Nrf2 in the cytoplasm, but not in the nucleus. After the neurons were treated with curcumin for 24 hours, a 1 hour exposure to OGD caused increased Cy3 signaling for Nrf2 in the nucleus. This overlapped with DAPI staining. Scale bar: 100 μm. OGD/R: Oxygen-glucose deprivation/reoxygenation; Nrf2: nuclear factor erythroid 2-related factor 2.
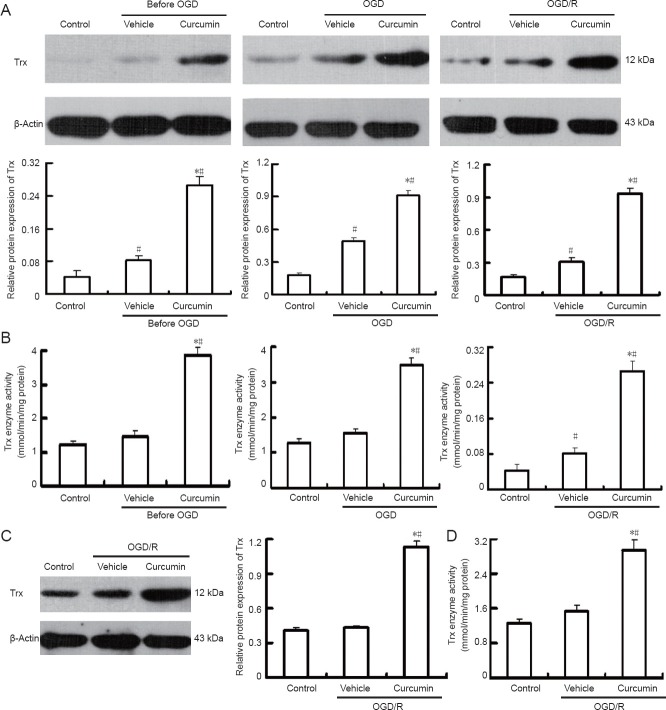
Curcumin induced up-regulation of thioredoxin protein and increased thioredoxin enzyme activity
The effect of curcumin on thioredoxin protein levels was measured before OGD, after OGD and after OGD/reoxygenation. As shown in the representative immunoblots in Figure 5A (upper panel) and by the densitometric analysis in Figure 5A (lower panel), curcumin pretreatment induced a statistically significant increase (0.27 ± 0.02) in thioredoxin total protein level over that observed 0.081 ± 0.011 in vehicle-treated cells (P < 0.01). At the end of OGD, the fold change in thioredoxin protein level was 0.93 ± 0.05 in curcumin-treated cells and 0.304 ± 0.033 in vehicle-treated cells (P < 0.01).
Figure 5.
Curcumin increases Trx expression and Trx enzyme activity in rat cortical neurons.
(A) Rat cortical neurons were pretreated for 24 hours with curcumin, then exposed to OGD (1 hour) and reoxygenation (24 hours). The immunoblots were probed with anti-Trx antibody, and then stripped and re-probed for β-actin as a loading control (n = 4). Controls represent the following: control-before OGD, neurons maintained in neurobasal medium without serum; control-end of OGD, neurons maintained 1 hour in serum-free neurobasal medium; control-24 hours reoxygenation, neurons maintained 24 hours in serum-free neurobasal medium. The ratio of densitometry values for Trx and β-actin was analyzed. *P < 0.05, vs. control group; #P < 0.01, vs. vehicle group. (B) The Trx enzyme activity in total cell lysates of neurons pretreated with curcumin was determined at the same time points as those used for measuring the Trx total protein level. The results are expressed as the mean ± SEM (n = 4). #P < 0.01, vs. control group; *P < 0.01, vs. vehicle group. (C) Curcumin post-treatment increases the protein espression level of Trx after 24 hours of REOX. The ratio of densitometry values of Trx and β-actin was analyzed (n = 4). #P < 0.01, vs. control group; *P < 0.01, vs. vehicle group. (D) Curcumin post-treatment increases Trx enzyme activity after 24 hours of REOX. The results are expressed as the mean ± SEM (n = 4). The values were square transformed and the data were analyzed using one-way analysis of variance and the Student-Newman-Keuls test. #P < 0.01, vs. control group; *P < 0.01, vs. vehicle group. OGD: Oxygen-glucose deprivation; OGD/R: oxygen-glucose deprivation/reoxygenation; Trx: thioredoxin; min: minute.
After 24 hours of reoxygenation, thioredoxin expression was still higher in curcumin-treated cells (0.92 ± 0.04) than in the vehicle-treated cells (0.49 ± 0.03; P < 0.01) relative to the level in control cells. Consistent with the increase in thioredoxin protein level, curcumin pretreatment also induced a 2.2-fold increase in thioredoxin enzyme activity over that observed in vehicle-treated cells (P < 0.01), as shown in Figure 5B. At the end of OGD, thioredoxin activity was 2.3 times higher in curcumin-treated cells than in vehicle-treated cells (P < 0.01). At 24 hours after reoxygenation, it was 2.7 times higher (P < 0.01).
Discussion
In the present study, we demonstrated that oxidative stress contributes to delayed cell injury in neurons after OGD/reoxygenation, and that protection against cell injury can be conferred by activating the Nrf2-mediated antioxidant response element pathway through the use of curcumin.
We provide experimental evidence that post-treatment and post-treatment with curcumin can significantly decrease delayed cell injury in neuron cultures subjected to OGD. Our pilot dose-response experiments in post-treated neurons showed that 5 μM of curcumin was less toxic than 10 μM (the dose used for the pretreatment studies). The higher sensitivity of neurons to curcumin under post-treatment versus pretreatment conditions might be explained by the fact that for the post-treatment experiments, the cells were maintained in serum-free medium, while pretreatment was performed in medium supplemented with 10% serum. Serum deprivation and the pro-oxidant activity of curcumin might both contribute to the higher toxicity observed at 10 μM.
A primary factor in initiation of the pathological response to the ischemia/reoxygenation injury is the generation of reactive oxygen species. The increase in the levels of reactive oxygen species produced upon reoxygenation appears to be essential to the development of delayed neuron death (Kubota et al., 2010). In our in vitro model, a significant increase in oxidative DNA/RNA damage was noted after OGD/reoxygenation, as indicated by the increased 8-hydroxydeoxyguanosine immunoreactivity. However, curcumin could inhibit this damage. Consistent with the ability of curcumin to reduce oxidative damage, levels of cell injury assessed at 24 hours of reoxygenation showed a reduction in delayed death after curcumin post-treatment, as indicated by propidium iodide/Hoechst staining.
Activation of Nrf2 by curcumin involves post-translational modifications that result in its release from Keap 1, which normally targets Nrf2 for degradation, Nrf2 protein accumulation, and translocation to the nucleus. In our model, activation of the Nrf2/antioxidant response element pathway by curcumin was indicated by the finding that pretreatment and post-treatment with curcumin, but not vehicle pretreatment, induced a significant increase in the level of phosphorylated Nrf2 protein in the nuclear fraction of neurons. There was also a significant increase in Nrf2 levels in neurons exposed to curcumin, probably because of enhanced stabilization of this transcription factor. This is why no decrease in cytosolic Nrf2 was observed in treated cells. Curcumin post-treatment promoted the nuclear translocation of Nrf2 detected by immunocytochemical analysis. In this way, our findings are consistent with reports indicating that several cytosolic kinases, such as Akt, PKC, and p38 are involved in transducing signals from antioxidants to the antioxidant response element through Nrf2 phosphorylation (Huang et al., 2002; Kang et al., 2002).
We further investigated the involvement of the Nrf2/antioxidant response element pathway in the activity of curcumin in neurons by analyzing the expression of endogenous antioxidant response element-regulated genes. Thioredoxin is constitutively present as a surface-associated sulfhydryl protein in the plasma membranes of a wide range of cells (Hirota et al., 2002). Recently, it was reviewed that thioredoxin is a ubiquitous protein with many roles, including acting as a regulator of mitochondrial metabolism (Yoshioka and Lee, 2013). It is a redox-sensitive molecule with a cytokine-like and chemokine-like ability to prevent cell injury due to oxidative stress (Arnér and Holmgren, 2000). Thioredoxin can act as an antioxidant or reactive oxygen species scavenger because of its ability to eliminate singlet oxygen, hydroxyl radicals, and hydrogen peroxide (Das and Das, 2000). Thioredoxin is highly inducible by many physicochemical stimuli, including UV irradiation, hypoxia, and hydrogen peroxide (Hirota et al., 2002). Its induction is considered to be transcriptionally regulated by antioxidant response element (Kim et al., 2003; Tanito et al., 2005). In this study, we demonstrated that both pretreatment and post-treatment with curcumin can increase both protein expression and enzyme activity of thioredoxin in whole-cell lysates at 24 hours after reoxygenation. These results also suggest that the mechanism by which exposure to curcumin protects neurons against OGD-induced delayed cell injury is likely to involve activation of the Nrf2/antioxidant response element pathway.
Although increases in levels of thioredoxin protein have been used as an indicator of curcumin action in rat cortical neurons, it does not imply that the protective effect can be mediated by a single enzyme. Increasing evidence (Balogun et al., 2003; Ye et al., 2007; Yang et al., 2009) has shown that curcumin can activate multiple Nrf2 response genes, including nicotinamide adenine dinucleotide phosphate:quinine oxidoreductase 1, heme oxygenase-1, and glutathione S-transferase, whose protein products participate in cellular defense.
In summary, our results support the concept of pretreatment and post-treatment against oxidative stress and delayed death by the use of agents that activate the Nrf2/antioxidant response element pathway. Curcumin was found to increase thioredoxin protein expression and enzyme activity and to protect neurons against cell injury regardless of whether it was administered before or after OGD. This shows that pharmacologic stimulation of antioxidant gene expression may be a promising approach to neuroprotection after cerebral ischemia.
Footnotes
Funding: This study was supported by grants from the National Natural Science Foundation of China, No. 81171090; Natural Science Foundation of Chongqing Education Committee of China, No. KJ110313; Foundation of Key State Laboratory of Neurobiology of Fudan University in China, No. 10-08; and Foundation of Key Laboratory of Ministry of Education of the Third Medical Military University in China.
Conflicts of interest: None declared.
Copyedited by McGowan D, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci U S A. 1993;90:11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Danilov CA, Fiskum G. Hyperoxia promotes astrocyte cell injury after oxygen and glucose deprivation. Glia. 2008;56:801–808. doi: 10.1002/glia.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KC, Das CK. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem Biophys Res Commun. 2000;277:443–447. doi: 10.1006/bbrc.2000.3689. [DOI] [PubMed] [Google Scholar]
- Eghwrudjakpor PO, Allison AB. Oxidative stress following traumatic brain injury: enhancement of endogenous antioxidant defense systems and the promise of improved outcome. Niger J Med. 2010;19:14–21. doi: 10.4314/njm.v19i1.52466. [DOI] [PubMed] [Google Scholar]
- Ge QF, Hu X, Ma ZQ, Liu JR, Zhang WP, Chen Z, Wei EQ. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacol Res. 2007;55:148–157. doi: 10.1016/j.phrs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Hirota K, Nakamura H, Masutani H, Yodoi J. Thioredoxin superfamily and thioredoxin-inducing agents. Ann N Y Acad Sci. 2002;957:189–199. doi: 10.1111/j.1749-6632.2002.tb02916.x. [DOI] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Kang ES, Kim GH, Kim HJ, Woo IS, Ham SA, Jin H, Kim MY, Lee JH, Chang KC, Seo HG, Hwang JY. Nrf2 regulates curcumin-induced aldose reductase expression indirectly via nuclear factor-kappaB. Pharmacol Res. 2008;58:15–21. doi: 10.1016/j.phrs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- Katsori AM, Chatzopoulou M, Dimas K, Kontogiorgis C, Patsilinakos A, Trangas T, Hadjipavlou-Litina D. Curcumin analogues as possible anti-proliferative & anti-inflammatory agents. Eur J Med Chem. 2011;46:2722–2735. doi: 10.1016/j.ejmech.2011.03.060. [DOI] [PubMed] [Google Scholar]
- Kim YC, Yamaguchi Y, Kondo N, Masutani H, Yodoi J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene. 2003;22:1860–1865. doi: 10.1038/sj.onc.1206369. [DOI] [PubMed] [Google Scholar]
- Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, Takeuchi T. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem. 2010;285:667–674. doi: 10.1074/jbc.M109.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Whitman SA, Jaramillo MC, Zhang DD. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol. 2013;27:99–105. doi: 10.1002/jbt.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ma X, Yu W, Lou Z, Mu D, Wang Y, Shen B, Qi S. Reperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in rats. PLoS One. 2012;7:e46498. doi: 10.1371/journal.pone.0046498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, Ha I, Han JS. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82:831–838. doi: 10.1002/jnr.20692. [DOI] [PubMed] [Google Scholar]
- Linares M, Marín-García P, Martínez-Chacón G, Pérez-Benavente S, Puyet A, Diez A, Bautista JM. Glutathione peroxidase contributes with heme oxygenase-1 to redox balance in mouse brain during the course of cerebral malaria. Biochim Biophys Acta. 2013;1832:2009–2018. doi: 10.1016/j.bbadis.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H: quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Léveillé F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1, 2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007;13:BR286–292. [PubMed] [Google Scholar]
- Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005;46:979–987. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang L, Chen ZB, Wu JY, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur J Pharmacol. 2009;609:40–44. doi: 10.1016/j.ejphar.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Xiang J, Tang YP, Zhou ZY, Wu P, Wang Z, Mori M, Cai DF. Apocynum venetum leaf extract protects rat cortical neurons from injury induced by oxygen and glucose deprivation in vitro. Can J Physiol Pharmacol. 2010;88:907–917. doi: 10.1139/y10-069. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Ye SF, Hou ZQ, Zhong LM, Zhang QQ. Effect of curcumin on the induction of glutathione S-transferases and NADP(H):quinone oxidoreductase and its possible mechanism of action. Yao Xue Xue Bao. 2007;42:376–380. [PubMed] [Google Scholar]
- Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]
- Yoshioka J, Lee RT. Thioredoxin-interacting protein and myocardial mitochondrial function in ischemia-reperfusion injury. Trends Cardiovasc Med. 2013;24:75–80. doi: 10.1016/j.tcm.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]