Abstract
We speculate that cortical reactions evoked by swallowing activity may be abnormal in patients with central infarction with dysphagia. The present study aimed to detect functional imaging features of cerebral cortex in central dysphagia patients by using blood oxygen level-dependent functional magnetic resonance imaging techniques. The results showed that when normal controls swallowed, primary motor cortex (BA4), insula (BA13), premotor cortex (BA6/8), supramarginal gyrus (BA40), and anterior cingulate cortex (BA24/32) were activated, and that the size of the activated areas were larger in the left hemisphere compared with the right. In recurrent cerebral infarction patients with central dysphagia, BA4, BA13, BA40 and BA6/8 areas were activated, while the degree of activation in BA24/32 was decreased. Additionally, more areas were activated, including posterior cingulate cortex (BA23/31), visual association cortex (BA18/19), primary auditory cortex (BA41) and parahippocampal cortex (BA36). Somatosensory association cortex (BA7) and left cerebellum in patients with recurrent cerebral infarction with central dysphagia were also activated. Experimental findings suggest that the cerebral cortex has obvious hemisphere lateralization in response to swallowing, and patients with recurrent cerebral infarction with central dysphagia show compensatory recombination phenomena of neurological functions. In rehabilitative treatment, using the favorite food of patients can stimulate swallowing through visual, auditory, and other nerve conduction pathways, thus promoting compensatory recombination of the central cortex functions.
Keywords: nerve regeneration, blood oxygen level-dependent functional magnetic resonance imaging, cerebral ischemia, dysphagia, function restructuring, cerebral cortex, neural regeneration
Introduction
Dysphagia is a common sequela of stroke (Daniels et al., 1998; Martino et al., 2005) estimated to occur in 37–78% of stroke cases (Wiles, 1991; Black-Schaffer et al., 1999; Martino et al., 2000; Meng et al., 2000; Han et al., 2001; Rogus-Pulia and Robbins, 2013). It can lead to several complications, such as aspiration pneumonia, malnutrition, and dehydration (Ney et al., 2009; Taylor et al., 2013), and the mortality rate in patients with swallowing dysfunction after stroke was significantly higher than in those with no swallowing dysfunction (Steinhagen et al., 2009). Therefore, identifying characteristics of central dysfunction and possible mechanisms of functional reorganization, and formulating effective programs for rehabilitating swallowing should help improve the effectiveness of dysphagia therapy. A noninvasive technique such as blood oxygen level-dependent functional magnetic resonance imaging (MRI) could provide information regarding the location, intensity, and dynamics of neural activity in swallowing-related brain regions (Hamdy et al., 1999; Martin et al., 2001, 2004; Ertekin and Aydogdu, 2003; Logemann, 2007). Here, we analyzed swallowing-related activity and compensatory reorganization in the brains of dysphagia patients with cerebral infarctions in a broad attempt to provide a theoretical basis for effective swallowing rehabilitation.
Subjects and Methods
Subjects
Ten right-handed patients with cerebral infarction were hospitalized in the Department of Neurology, Kailuan General Hospital, China from January 2013 to December 2013.
Patients first presented with dysphagia and both sides of the brain or pons had multiple cerebral infarctions that were confirmed by MRI (T1- and T2-weighted images). In 10 cases (seven male, three female), one of these infarctions was confirmed using diffusion weighted imaging as the new acute infarct and met the inclusion criteria (see below). Five patients had new lesions on the right (right recurrent cerebral infarction group) and five had new lesions on the left (left recurrent cerebral infarction group). The present study was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and was reviewed and approved by the Medical Ethics Committee from Affiliated Kailuan General Hospital of Hebei United University in China. All patients gave their signed informed consent.
Inclusion criteria
No patients had difficulty in swallowing, choking coughs, hoarseness, or other abnormalities before suffering from cerebral infarction. All exhibited swallowing dysfunction for the first time within 1 week after cerebral infarction. Clinical examination found no signs of disease or peripheral nerve damage in the mouth, tongue, throat, or other swallowing-motion organs, and patients were ultimately diagnosed as having central nervous system swallowing dysfunction (Li et al., 2009). Results from the Kubota water test (Martino et al., 2005) were between levels 3–5, life signs were stable, consciousness, spirit, intelligence and verbal expression were normal, and patients cooperated with the physical examination. Scores on the anxiety depression scale (Murakami et al., 2013) were < 7 points. The damage to the nervous system was evaluated by NIHSS score for every patient (Mann and Hankey, 2001).
Exclusion criteria
Patients were excluded if their consciousness, spirit, or intelligence were disordered, if they did not cooperate with the physical examination, if they had a history of head or neck cancer, local diseases of the throat or esophagus, or esophagus-related dysphagia. Additionally, those with failure in visceral function or who were in a critical condition were also excluded. Further, because of the use of MRI, those who had metal objects, such as pacemakers or coronary stents were excluded, as were those with atrial fibrillation.
We also included 10 individuals (six male and four female; aged 53.2 ± 14.9 years) in a normal control group. These participants were recruited from healthy adults who participated in a physical examination at Kailuan General Hospital. All were right-handed and in good health, without any organic disease. Scores on the anxiety depression scale were < 7 points, and they had not imbibed any alcoholic beverages within 12 hours of the scan. Patients exhibited no uncomfortable symptoms or positive neurological signs before testing.
Blood oxygen level-dependent functional MRI data acquisition
We used a GE Signa Twin Speed 1.5T MRI system (GE Healthcare, Wauwatosa, WI, USA) to determine brain regions active during swallowing. After positioning, a T1WI FSPGR-3D scan that employed a single-shot EPI-temporal plane gradient echo sequence was used to image the basic anatomy. Activation of motor-related regions was scanned with blood oxygen level-dependent functional MRI using a T2WI gradient echo-pulse sequence. For single-temporal excitation echo-planar imaging, the position was defined parallel to the joint line (AC-PC), and the scanning covered 18 layers with the following parameters: single acquisition, time of repetition = 3,000 ms, time of echo = 60 ms, flip angle = 90°, field of view = 24 cm × 24 cm, thickness = 5 mm, gap = 1.5 mm, frequency encoding direction = R/L.
Using a block design, blood oxygen level-dependent functional MRI scanning lasted 8 minutes. Subjects first lay on the examination bed quietly and received instruction and brief training in the simulation process, and then their heads were fixed in a birdcage head-coil to minimize the movement of lips, tongue, and head. Subjects were asked to bite a plastic 2-mm tube with the middle part of their lips. A 50-mL syringe was connected to the other end of the tube, and swallowing blocks began. Each block lasted 30 seconds, with 2 mL of pure room-temperature water being injected into the tube every 5 seconds. Subjects immediately swallowed each time water was injected, yielding six swallows per block. Rest (control) blocks lasting 30 seconds followed each swallowing block. During the rest block, water was not injected into the tube, and participants did not engage in any swallowing action. Each experiment consisted of 16 blocks (eight swallowing, eight rest) for a total of 48 swallows (Figure 1).
Figure 1.
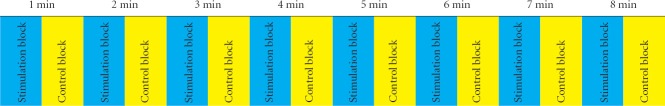
Experimental block design.
Swallowing blocks and control blocks alternated with each lasting 30 seconds. One experiment included 16 blocks. Each of the eight swallowing blocks resulted in six swallows, while no swallows were recorded in any control block. min: Minute.
Functional MRI data processing
SPM5 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK) software was used to model brain activity and determine brain regions associated with swallowing in the two groups of participants. Convolution of the swallowing time, interval, and hemodynamic function were used to analyze the whole-brain voxel signal correlation. The activation volume (represented by the number of active voxels) and intensity (represented by T value—higher T value indicated the stronger intensity) were calculated, MNI coordinate position generated by SPM5 was converted into standard Talairach coordinates with Brain-Map GingerALE 2.1.1 software (Research Imaging Institute of the University of Texas Health Science Center San Antonio, San Antonio, TX, USA). Talairach Client 2.4 software (Research Imaging Institute of the University of Texas Health Science Center San Antonio) was used to view specific anatomical locations. Data from subjects with three-dimensional translation of the head greater than 0.5 mm and three-dimensional rotation greater than 0.5° were discarded. Two-sample t-tests were used to determine regions of activation. Statistical probability threshold was set at P < 0.05. The extent threshold was 10 pixels. Excel 2003 software (Microsoft Corporation, Redmond, WA, USA) was used to set up a database and SPSS 13.0 software (SPSS, Chicago, IL, USA) was used to analyze the activated region. Activation volume and intensity of brain areas calculated by SUN workstations and SPM are expressed as the mean ± SD. A non-parametric rank sum test was used for statistical analysis. Significance was set at P < 0.05.
Laterality index (LI) was defined as: (left hemisphere activation volume – right hemisphere activation volume)/(left hemisphere activation volume + right hemisphere activation volume) × 100% (Malandraki et al., 2011b). An LI ≥ 10% was considered left lateralization and an LI ≤ –10% was considered right lateralization (Ogura et al., 2012).
Results
Regions activated by independent swallowing
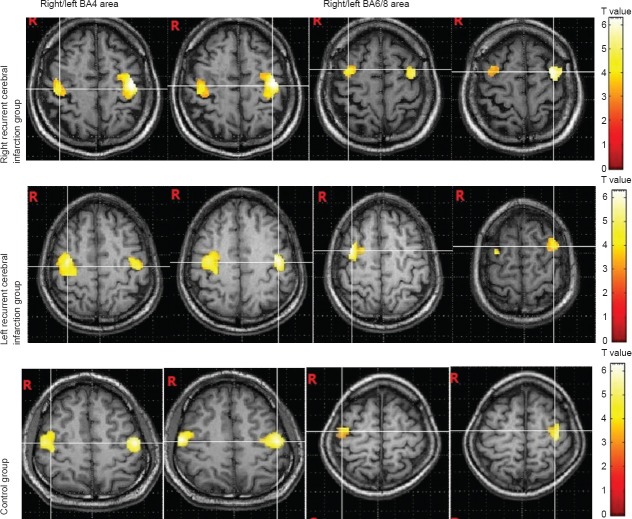
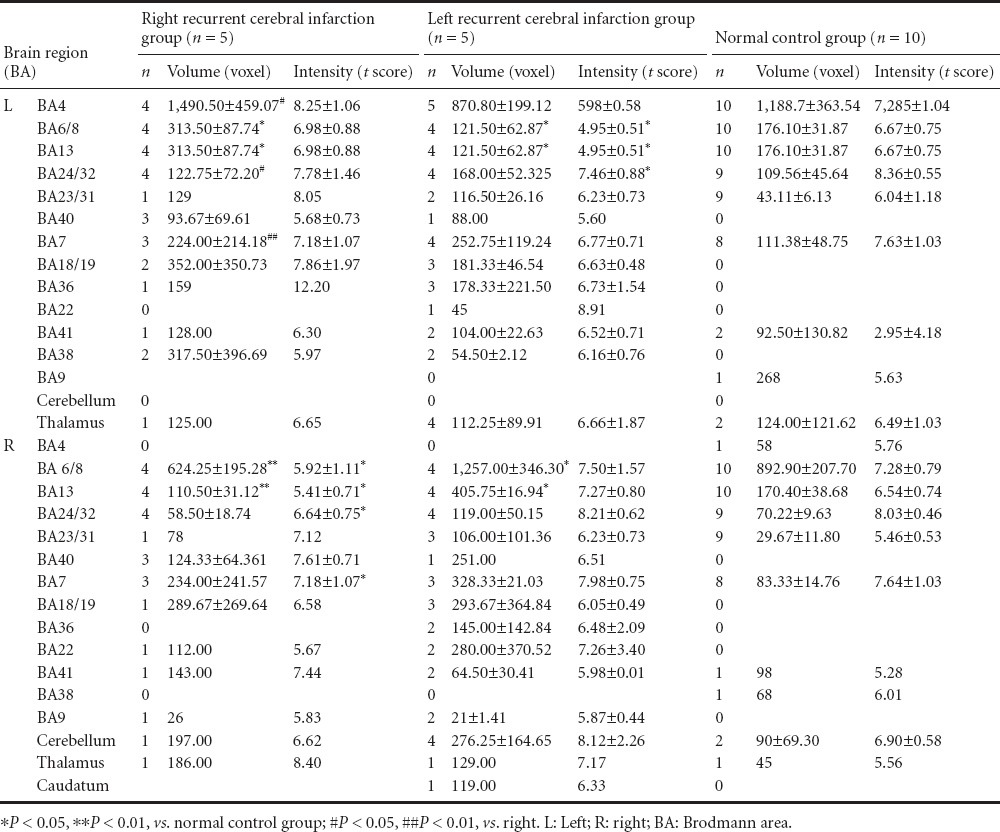
Mean NIHSS scores in the patient group were 4.0 ± 2.5 points (range: 3–10 points) based on the functional MRI examination. All patients completed all tests successfully. Both sides of Brodmann's area (BA) 4, BA40, BA13, BA22 around the sylvian fissure and BA6/8, BA24, BA32, and the BA38/22/41 region of the superior temporal gyrus and other regions such as the hypothalamus and parts of the brain center were activated when control participants engaged in swallowing tests. Compared with the control group, areas more active in patients with right recurrence of cerebral infarction were BA4, BA13, BA40, and BA6/8. Areas with significantly reduced activity included bilateral BA24/32. Other areas with increased activity included bilateral BA23/31, BA36, right BA9 and BA18/19, and left BA41. Activation of bilateral BA23/31 in patients with left recurrent cerebral infarction was not as obvious as that in patients with recurrent acute cerebral infarction, however bilateral BA7, BA18/19, BA36, left BA41, right BA9, and cerebellum were significantly activated. Blood oxygen level-dependent functional MRI scanning results are shown in Table 1.
Table 1.
Blood oxygen level-dependent functional magnetic resonance imaging results during swallowing for dysphagia patients with acute recurrent cerebral infarction
The laterality of brain regions activated by swallowing
When the control group performed swallowing, the overall extent of activation was greater in the left hemisphere than in the right hemisphere (LI value: 15.22%, P < 0.05). A more detailed analysis revealed that activation volumes in left medial frontal gyrus (BA4) and left insula (BA13) were larger than corresponding regions in the right hemisphere (LI values: BA4 = 13.49%, BA13 = 19.02%; P < 0.05). Further, left hemisphere activation volumes in BA40 and BA32 were obviously larger than those in the right hemisphere (LI values: BA40 = 10.89%, BA32 = 61.29%; P < 0.01). In contrast, activation volumes in left BA6/8 and left BA24 were not different from those in the right hemisphere (LI values: BA6/8 = 2.02%, BA24 = 0.66%; P > 0.05).
Brain related hubs activated by swallowing
In the right cerebral infarction group, activation volumes of right BA4 and BA6/8 were smaller than those of the control group (P < 0.01), while those for BA13 and BA40 were not (P > 0.05). Further, while activation volume for left BA6/8 was greater than that of the control group (P < 0.05), those for left BA4, BA13, and BA40 were not (P > 0.05).
In the left cerebral infarction group, activation volumes of left BA4 and BA6/8 were smaller than those of the control group (P < 0.05), while those for BA13 and BA40 were not (P > 0.05). Additionally, while activation volumes for right BA4 and BA6/8 were larger than those of the control group (P < 0.05), those for right BA13 and BA40 were not (P > 0.05; Figure 2).
Figure 2.
Activation during swallowing of primary motor cortex (BA4) and premotor cortex (BA6/8) in the right/left cerebral infarction group and the control group.
Patients with right cerebral infarctions and swallowing dysfunction exhibited smaller activation volume in right BA4 and right BA6/8 and larger activation volume in left BA6/8 compared with controls. Activation volume in left BA4 did not differ between these patients and controls. Similarly, patients with left cerebral infarction and swallowing dysfunction exhibited smaller activation volume in left BA4 and BA6/8 and larger activation volume in right BA4 and BA6/8. R: Right.
Activation intensity in swallowing
During swallowing, left BA4, BA6/8, BA13, BA40, BA24, and BA32 of the control group were not more active than corresponding locations in the right hemisphere (P > 0.05; Table 1).
For the right cerebral infarction group, right BA4, BA6/8, BA13, and BA40 were less active than the corresponding areas in the control group (P < 0.05). This was not true for left BA4, BA6/8, BA13, and BA40 (P > 0.05; Table 1).
For the left recurrent cerebral infarction group, left BA4, BA6/8, and BA13 were less active than the corresponding areas in the control group (P < 0.05), but left BA40 was not (P > 0.05). Additionally, activity levels in right BA4, BA6/8, BA13, and BA40 did not differ between groups (P > 0.05; Table 1).
Discussion
Studies have shown that the most prominent swallow-related activation foci correspond to the lateral pericentral and perisylvian cortices, anterior cingulate cortex, premotor cortex, insula/operculum, and supramarginal gyrus (D’Esposito et al., 2003; Lowell et al., 2008; Malandraki et al., 2010, 2011a; Peck et al., 2010). However, the compensatory mechanisms and functional reorganization that occur in dysphagia remain unclear clear. Further studies are needed to develop effective individualized rehabilitation programs for dysphagia. We found that there was no obvious or opharyngeal sensory cortex activation during normal swallowing, but premotor, frontal eye fields (BA6/8), and anterior cingulate (BA24/32) were activated, along with the lateral fissure oropharyngeal area surrounding primary motor cortex (BA4), insula (BA13), supramarginal gyrus (BA40), and temporal cortex BA38/22. This evidence indicated that motor planning and execution (beginning in primary motor cortex) were most important for autonomous swallowing. These results are consistent with previously reports (Kern et al., 2001; Gazzaniga et al., 2002; Ettlin et al., 2004; Fukunaga et al., 2005; Humbert et al., 2011; Babaei et al., 2012; Galovic et al., 2013). They also suggest that oropharyngeal tactile stimulation was not the main factor that induced swallowing. Physiological needs, smell, and language are all appetite-related stimuli that might be major factors in initiating independent swallowing. However, in-depth study on the cortical activity related to these stimuli must be done to draw definite conclusions. The cortical motor areas and supramarginal gyrus were important for planning swallowing, and oropharyngeal motor region was the central region responsible for initiating swallowing (Forman et al., 1995; Lotze et al., 1999; Komisaruk et al., 2002; Satow et al., 2004; Lacourse et al., 2005; Saad et al., 2006; Szameitat et al., 2007).
However, here we examined patients with central dysphagia when it resulted from unilateral recurrent cerebral infarction at acute stage. We found that regardless of the side of the infarction, BA4, BA13, BA40, and BA6/8 were still the main regions of active cortex. In contrast, activation in bilateral cingulate cortex (BA24/32) was significantly reduced, suggesting that regions necessary for planning and execution of swallowing were still activated in patients with dysphagia, but that the region needed for initiating swallowing was significantly reduced. In patients with acute recurrent right non-dominant cerebral infarction, we found that bilateral posterior cingulate cortex (BA23/31) was obviously activated, and that right parahippocampal cortex (BA36), prefrontal cortex (BA9), left occipital cortex (BA18/19), and primary auditory cortex (BA41) were all activated. This evidence indicates that brain damage leading to swallowing disorders resulted in compensatory functional reorganization involving brain regions that control the initiation of swallowing, such as cingulate cortex, prefrontal cortex, visual cortex, and audio cortex. Stimulating patients with their favorite food through visual and auditory channels might promote functional recovery of swallow initiation. We will conduct further studies on this phenomenon (Uğurbil et al., 2000; Power et al., 2007; Babaei et al., 2010; Cola et al., 2010).
In patients with acute recurrent left dominant cerebral infarction, we found that the activation of bilateral posterior cingulate cortex (BA23/31) was not as obvious as in those with acute cerebral infarction in the right hemisphere, but somatosensory cortex (BA7), occipital cortex (BA18/19), and the cerebellum were significantly activated. We also found that bilateral parahippocampal cortex (BA36), left primary auditory cortex (BA41), and right lateral prefrontal cortex (BA9) were activated, indicating that BA7, BA18/19, and the cerebellum played an important role in restructuring compensatory function in the motor initiation of swallowing in patients with dysphagia. Patients with central swallowing disorders could do passive swallowing masonic-coordination training through visual stimulation of food and feeling, and it is possible to promote the reorganization of cortical function, thereby accelerating the recovery of swallowing behavior. It is worth observing in our clinical work (Kumar et al., 2011; Michou et al., 2012; Yang et al., 2012).
Our results showed that in the process of normal swallowing, the extent of cortical activation in the left or right hemisphere was quite different (depending on the side of the injury), but that the intensity was not. The extent of activation in primary oropharyngeal cortex (BA4) was significantly larger than other cortical regions, and was greater in the left hemisphere than in the right. Consistent with previous reports, this suggested that the size of the activated area was normal, and that especially in the dominant hemisphere, it was an important factor for implementing normal autonomous swallowing (Regard and Landis, 1997; Jubault et al., 2007; Price, 2010). Similarly, the size of supramarginal gyrus (BA40) activation in the dominant (left) hemisphere was also significantly greater than that in the non-dominant one, while that of the supplementary motor area was did not differ across hemispheres, although it was smaller than what we observed for BA4. However, the activation intensity of these regions was not significantly different. These results further illustrated that BA40 in both the supramarginal gyrus and premotor cortex/supplementary motor area were important centers for planning the movements needed for swallowing. Volume of activation of the apply center of dominant hemisphere in a normal state is an important security condition for autonomic swallowing completed accurately and efficiently.
Meanwhile, we also observed that both swallowing coordination centers in the posterior lobe of cerebellar cortex were activated in some adults, resulting in smoother and more coordinated swallowing. When the normal independent swallowing began, the size of primary motor cortex activation in the left hemisphere was greater than in the right, the total LI value and activation in the central gyrus, anterior cingulate cortex, and insula were all greater than 10%. The LI value in BA6/8 was significantly less than 10% and that in BA40 was approximately 10%. Therefore, initiation and execution of independent swallowing was obviously lateralized (Daniels et al., 2006; Martin et al., 2007; Teismann et al., 2007; Babaei et al., 2012; Li et al., 2014). Thus, while independent swallowing was regulated by the central gyrus, anterior cingulate cortex, insula, and supramarginal gyrus in the dominant hemisphere, it was planned bilaterally.
However, in patients with right recurrent cerebral infarction, the extent of activation in BA4 and BA6/8 on the same side of the lesion decreased, while that of BA40 and BA13 areas was significantly different. Additionally, activation volume in BA6/8 contralateral to the lesion was greater, and that in BA4, BA40, and BA13 showed no significant differences. Similarly, in those with left recurrent cerebral infarction, activation volume in BA4 and BA6/8 ipsilateral to the lesion were smaller than on the right, and those of BA40 and BA13 showed no significant differences. Further, activation volume of BA4 and BA6/8 contralateral to the lesion were larger, and those of BA4, BA40, and BA13 showed no significant differences. Therefore, when the non-dominant hemisphere infarction occurred, activation volume of the regions needed to initiate swallowing (such as BA13) did not differ across hemispheres, but those of regions needed for planning the action (such as BA6/8)were reduced on the side of the lesion and increased on the side opposite the lesion. Activation volume in BA40 showed no significant difference between hemispheres, a smaller region of activation in primary motor cortex (BA4), but no compensatory contralateral increase. In those with a dominant hemispheric infarction, the volume of activation for regions needed to initiate swallowing did not differ across hemispheres, a reduced extent of activation in BA6/8 on the side of the lesion side, and a compensatory increase in activation volume contralaterally. However, activation volume in BA40 was not significantly different activation volume in the primary motor cortex (BA4) was reduced on the side of the lesion, and no compensatory contralateral increase was observed.
Our findings showed that the size of cortical activity was lateralized when normal swallowing occurred, but that the activation intensity of both regions related to swallowing was not. However, activation intensity of the central gyrus, anterior cingulate cortex, insula, and supramarginal gyrus has been shown to be an obvious change in dysphagia patients (Ertelt et al., 2007; Steinhagen et al., 2009; Babaei et al., 2013). We showed that activation intensity in ipsilateral BA4, BA6/8, BA40, and BA13 was reduced in patients with right recurrent cerebral infarction of the non-dominant hemisphere, but the intensity was not obviously different when contralateral central cortex was activated. The activation intensity of BA4, BA6/8, and BA13 were reduced, while that of BA40 was not obviously different when ipsilateral central cortex was activated. Activation intensity of BA4, BA6/8, BA13, and BA40 also had no obvious differences when contralateral central cortex was activated in patients with left recurrent infarction of the non-dominant hemisphere. Therefore activation intensity was not obviously different in ipsilateral swallowing related regions in patients with dysphagia after acute cerebral infarction, but it decreased in ipsilateral swallowing centers and was not generally affected in BA40, a dominant swallowing center.
Pleasant stimulation related to appetite could activate the swallowing centers, such as the anterior insula and the anterior cingulate cortex, and then activate the motor cortex related to planning and execution of swallowing, such as the supramarginal gyrus and premotor area. The activation volume of central cortex is the basis for regular swallowing motor activity. The central regulation of swallowing has obvious advantages in dominant hemisphere laterality. In patients with dysphagia resulting from recurrent acute cerebral infarction, while regions dedicated to planning and execution of swallowing were active, the size of activity was reduced in regions involved in the initiation of movement, while the intensity in the contralateral swallowing regions had no obvious change, nor did that in BA40 area of the dominant side. In our study, non-response bias and investigation bias might have occurred, and we patiently explained this to patients to improve compliance with the examination. Therefore, making individualized rehabilitation programs for dysphagia patients based on the results of this study will promote compensatory reorganization of cortical centers and further improve the effect of rehabilitation treatment. Meanwhile, to fully understand the impact and role of each nerve and biological function of the body, we need to further research the characteristics and compensatory reorganization mechanisms of brain function in a variety of sensory dysfunctions and cognitive impairments. We will expand the sample size in order to make conclusions that can be used for clinical rehabilitation treatment.
Acknowledgments:
We would like to thank M.D. Wen-jiang Zhang from Tangshan Vocational Technical College in China for software applications and M.D. Ling-min Meng from Kailuan General Hospital in China for the help in medical statistics. This work gained supports and technical assistance of teachers from Hebei United University in China.
Footnotes
Conflicts of interest: None declared.
Copyedited by Phillips A, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Babaei A, Kern M, Antonik S, Mepani R, Ward BD, Li SJ, Hyde J, Shaker R. Enhancing effects of flavored nutritive stimuli on cortical swallowing network activity. Am J Physiol Gastrointest Liver Physiol. 2010;299:G422–429. doi: 10.1152/ajpgi.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei A, Siwiec RM, Kern M, Douglas Ward B, Li SJ, Shaker R. Intrinsic functional connectivity of the brain swallowing network during subliminal esophageal acid stimulation. Neurogastroenterol Motil. 2013;25:992–e779. doi: 10.1111/nmo.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei A, Ward BD, Ahmad S, Patel A, Nencka A, Li SJ, Hyde J, Shaker R. Reproducibility of swallow-induced cortical BOLD positive and negative fMRI activity. Am J Physiol Gastrointest Liver Physiol. 2012;303:G600–609. doi: 10.1152/ajpgi.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black-Schaffer RM, Kirsteins AE, Harvey RL. Stroke rehabilitation. 2. Co-morbidities and complications. Arch Phys Med Rehabil. 1999;80:S8–16. doi: 10.1016/s0003-9993(99)90096-5. [DOI] [PubMed] [Google Scholar]
- Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–486. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Brailey K, Priestly DH, Herrington LR, Weisberg LA, Foundas AL. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Corey DM, Fraychinaud A, DePolo A, Foundas AL. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–27. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, Buccino G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36(Suppl 2):T164–173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Ettlin DA, Zhang H, Lutz K, Järmann T, Meier D, Gallo LM, Jäncke L, Palla S. Cortical activation resulting from painless vibrotactile dental stimulation measured by functional magnetic resonance imaging (FMRI) J Dent Res. 2004;83:757–761. doi: 10.1177/154405910408301004. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Uematsu H, Sugimoto K. Influences of aging on taste perception and oral somatic sensation. J Gerontol A Biol Sci Med Sci. 2005;60:109–113. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- Galovic M, Leisi N, Müller M, Weber J, Abela E, Kägi G, Weder B. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44:2760–2767. doi: 10.1161/STROKEAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. New York: W.W.Norton & Company; 2002. Cognitive Neuroscience: the Biology of the Mind. [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: A functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82:677–682. doi: 10.1053/apmr.2001.21939. [DOI] [PubMed] [Google Scholar]
- Humbert IA, McLaren DG, Malandraki G, Johnson SC, Robbins J. Swallowing intentional off-state in aging and Alzheimer's disease: preliminary study. J Alzheimers Dis. 2011;26:347–354. doi: 10.3233/JAD-2011-110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubault T, Ody C, Koechlin E. Serial organization of human behavior in the inferior parietal cortex. J Neurosci. 2007;27:11028–11036. doi: 10.1523/JNEUROSCI.1986-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G531–538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Mosier KM, Liu WC, Criminale C, Zaborszky L, Whipple B, Kalnin A. Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. Am J Neuroradiol. 2002;23:609–617. [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, Schlaug G. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42:1035–1040. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse MG, Orr EL, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage. 2005;27:505–519. doi: 10.1016/j.neuroimage.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry. 2009;80:1320–1329. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- Li S, Ma Z, Tu S, Zhou M, Chen S, Guo Z, Gong Q, He L, Huang X, Yao D, Lui S, Yu B, Wang X, Zhou D, He C. Altered resting-state functional and white matter tract connectivity in stroke patients with dysphagia. Neurorehabil Neural Repair. 2014;28:260–272. doi: 10.1177/1545968313508227. [DOI] [PubMed] [Google Scholar]
- Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol. 2007;21:563–573. doi: 10.1016/j.bpg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–295. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Johnson S, Robbins J. Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head Neck. 2011a;33(Suppl 1):S14–S20. doi: 10.1002/hed.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC. Age-related differences in laterality of cortical activations in swallowing. Dysphagia. 2010;25:238–249. doi: 10.1007/s00455-009-9250-z. [DOI] [PubMed] [Google Scholar]
- Malandraki GA, Perlman AL, Karampinos DC, Sutton BP. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp. 2011b;32:730–743. doi: 10.1002/hbm.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G, Hankey GJ. Initial clinical and demographic predictors of swallowing impairment following acute stroke. Dysphagia. 2001;16:208–215. doi: 10.1007/s00455-001-0069-5. [DOI] [PubMed] [Google Scholar]
- Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. Cerebral cortical processing of swallowing in older adults. Exp Brain Res. 2007;176:12–22. doi: 10.1007/s00221-006-0592-6. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Martino R, Pron G, Diamant N. Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15:19–30. doi: 10.1007/s004559910006. [DOI] [PubMed] [Google Scholar]
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil. 2000;79:170–175. doi: 10.1097/00002060-200003000-00010. [DOI] [PubMed] [Google Scholar]
- Michou E, Mistry S, Jefferson S, Singh S, Rothwell J, Hamdy S. Targeting unlesioned pharyngeal motor cortex improves swallowing in healthy individuals and after dysphagic stroke. Gastroenterology. 2012;142:29–38. doi: 10.1053/j.gastro.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Hama S, Yamashita H, Onoda K, Kobayashi M, Kanazawa J, Yamawaki S, Kurisu K. Neuroanatomic pathways associated with poststroke affective and apathetic depression. Am J Geriatr Psychiatry. 2013;21:840–847. doi: 10.1016/j.jagp.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutr Clin Pract. 2009;24:395–413. doi: 10.1177/0884533609332005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura E, Matsuyama M, Goto TK, Nakamura Y, Koyano K. Brain activation during oral exercises used for dysphagia rehabilitation in healthy human subjects: a functional magnetic resonance imaging study. Dysphagia. 2012;27:353–360. doi: 10.1007/s00455-011-9374-9. [DOI] [PubMed] [Google Scholar]
- Peck KK, Branski RC, Lazarus C, Cody V, Kraus D, Haupage S, Ganz C, Holodny AI, Kraus DH. Cortical activation during swallowing rehabilitation maneuvers: a functional MRI study of healthy controls. Laryngoscope. 2010;120:2153–2159. doi: 10.1002/lary.21125. [DOI] [PubMed] [Google Scholar]
- Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. J Neurol Neurosurg Psychiatry. 2007;78:141–146. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Regard M, Landis T. “Gourmand syndrome”: eating passion associated with right anterior lesions. Neurology. 1997;48:1185–1190. doi: 10.1212/wnl.48.5.1185. [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia N, Robbins J. Approaches to the rehabilitation of dysphagia in acute poststroke patients. Semin Speech Lang. 2013;34:154–169. doi: 10.1055/s-0033-1358368. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Chen G, Reynolds RC, Christidis PP, Hammett KR, Bellgowan PS, Cox RW. Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Hum Brain Mapp. 2006;27:417–424. doi: 10.1002/hbm.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow T, Ikeda A, Yamamoto J, Begum T, Thuy DHD, Matsuhashi M, Mima T, Nagamine T, Baba K, Mihara T, Inoue Y, Miyamoto S, Hashimoto N, Shibasaki H. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: a movement-related cortical potential study. Am J Physiol Gastrointest Liver Physiol. 2004;287:G459–G470. doi: 10.1152/ajpgi.00323.2003. [DOI] [PubMed] [Google Scholar]
- Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40:1903–1906. doi: 10.1161/STROKEAHA.108.535468. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Shen S, Sterr A. Motor imagery of complex everyday movements. An fMRI study. Neuroimage. 2007;34:702–713. doi: 10.1016/j.neuroimage.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Taylor JK, Fleming GB, Singanayagam A, Hill AT, Chalmers JD. Risk factors for aspiration in community-acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126:995–1001. doi: 10.1016/j.amjmed.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007;8:62. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğurbil K, Adriany G, Andersen P, Chen W, Gruetter R, Hu X, Merkle H, Kim DS, Kim SG, Strupp J, Zhu XH, Ogawa S. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng. 2000;2:633–660. doi: 10.1146/annurev.bioeng.2.1.633. [DOI] [PubMed] [Google Scholar]
- Wiles CM. Neurogenic dysphagia. J Neurol Neurosurg Psychiatry. 1991;54:1037–1039. doi: 10.1136/jnnp.54.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Baek SR, Shin J, Lim JY, Jang HJ, Kim YK, Paik NJ. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30:303–311. doi: 10.3233/RNN-2012-110213. [DOI] [PubMed] [Google Scholar]