Abstract
Amyotrophic Lateral Sclerosis (ALS) is a rapidly progressive neurodegenerative disorder with fatal prognosis. Cellular therapy has been studied for ALS in various animal models and these advances have highlighted its potential to be a treatment modality. This is a retrospective controlled cohort study of total 57 patients. Out of these, 37 patients underwent autologous bone marrow mononuclear cell transplantation in addition to standard rehabilitation and Riluzole. Control group consisted of 20 patients who did not receive cell transplantation. The survival duration since the onset of the disease for both the groups was computed using a Kaplan-Meier Survival analysis and compared using log-rank test. Effect of age at onset, type of onset and lithium on survival duration in the intervention group was analyzed. Mean survival duration of patients in intervention group was 87.76 months which was higher than the control group mean survival duration of 57.38 months. Survival duration was significantly (p = 0.039) higher in people with the onset of the disease below 50 years of age. Limb onset and lithium also showed positive influence on the survival duration. Mean survival duration of the intervention group was also higher than the survival duration of ALS patients in previous epidemiological studies. In addition to the standard treatment with Riluzole, early intervention with combination of BMMNCs transplantation and Lithium may have a positive effect on the survival duration in ALS. Prospective randomized controlled studies with a larger sample size and rigorous methodology are required for conclusive findings.
Keywords: Motor Neuron Disease, Amyotrophic Lateral Sclerosis, stem cells, cellular therapy, stem cell therapy, autologous bone marrow mononuclear cells
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a rapidly progressive neurodegenerative disorder with a fatal prognosis. Incidence of the disease is variable in different parts of the world with higher incidence of 2.08 per 100000 population is seen in Europe where as a lower incidence of 0.46 per 100000 population is seen in Japan and China [1]. The disease is rapidly progressive and affects both the quantity and quality of life [2]. One of the most commonly used diagnostic criteria for ALS is revised El-Escorial criteria [3] which categories the ALS patients as Definite, probable and possible ALS groups. These criteria include patients with both upper motor neuron (UMN) and lower motor neuron (LMN) signs.
Rapid progression and unknown etiology has contributed to inability to provide an effective treatment for ALS. The current management strategies are palliative. Treatment with Riluzole and assistive technologies like mechanical ventilation and percutaneous endoscopic gastrotomy (PEG) have a limited influence on the survival of these patients [4,5] and the prognosis remains grim. Advances in cellular therapy have highlighted its potential to be a treatment modality in neurodegenerative disorders like ALS. Due to the unique capacity of self-renewal and differentiation into different types of mature cells, Stem cells may aid in modifying or arresting the deterioration. Cellular therapy has been shown to be effective and safe for various neurological diseases [6-9]. Its potential in the treatment of ALS has been explored in various animal studies [10-12]. Preliminary trials with autologous bone marrow derived mononuclear cells have shown beneficial effects in ALS [13,14]. The evidence is still inconclusive about the effectiveness of cellular therapy in influencing the survival of individuals suffering from ALS due to inadequate sample size and heterogeneous methodologies. We conducted a retrospective analysis of the patients diagnosed with ALS treated with autologous bone marrow mononuclear cells (BMMNCs) intrathecal transplantation; to identify the effect of cellular therapy on survival duration. Our secondary objective was to analyze the effect of age at onset, bulbar onset and lithium on thesurvival durationof the patients post cellular therapy.
Materials and methods
Study design, participants eligibility criteria and recruitment
Primary objective was to identify the effect of cellular therapy on the survival duration since the onset of the disease. Secondary objective was to analyze the effects of age at onset, Lithium and bulbar onset on the survival duration of the patients.
This is a retrospective control cohort study to compare the survival duration of the patients that underwent cellular transplantation and control group that did not receive cell transplantation. We also compared the survival duration calculated in the study population with historical epidemiological populations.
We analyzed the information of the patients from December 2008 till August 2013. For primary screening patients with the diagnoses of Motor Neuron Disease (MND) and/or ALS were selected. These patients were then categorized into ALS, Primary Lateral Sclerosis (PLS), Progressive Bulbar Palsy (PBP), Progressive Spinal Muscular Atrophy (PSMA) and PMA according to the international statistical classification of the diseases and related health problems, 10th revision (ICD 10) [15]. Patients with ALS were then further subdivided into definite, probable and suspected ALS according to the ‘World federation of Neurology El-escorial research criteria’. The patients with the diagnosis of definite ALS who underwent cellular therapy were included in the intervention group. Detailed information of these patients was compiled for further analysis. For the control group we selectedpatients with the diagnosis of definite ALS from our out-patient department, who did not undergo cellular therapy.
The intervention group was further divided into two groups of patients with the age of onset below and above 50 years. The survival duration of these two groups was then compared using the Kaplan-Meier survival analysis to analyze the effect of age at onset. The entire intervention group was then divided into the groups of patients who received lithium and those who did not receive lithium. The comparison of survival duration was repeated for these two groups as well. Lastly the intervention group was divided into two groups of patients with limb onset and bulbar onset and the survival duration was compared.
Inclusion criteria: Patients with the diagnoses of definite ALS; clear information about date of onset of symptoms, documented contact information and follow up information were included for the analysis.
Exclusion criteria: Patients with diagnoses of PLS, PBP or PSMA, PMA, monomelicamyotrophy, madras motor neuron disease; patients with co-morbidities like presence of acute infections such as HIV/HBV/HCV, malignancies, bleeding tendencies, renal failure, severe liver dysfunction and other acute medical conditions such as respiratory infection and pyrexia were excluded.
Intervention
All the patients in the intervention group underwent a standardized intervention protocol of autologous BMMNCs intrathecal transplantation followed by standard rehabilitation. The inclusion was based on the Revised World Medical Association Helsinki Declaration for Ethical Principles for medical research involving human subjects [16]. The ethical approval for the intervention was sought from Institutional Committee for Stem Cell Research and Therapy (IC-SCRT) as per Indian Council of Medical Research (ICMR) guidelines. All the patients were explained about the unproven nature of the therapy and potential use of the clinical data for research analysis and publication without disclosing any identifiable personal information. This was followed by an informed documented consent from the patient.
Pre intervention procedures
Pre intervention surgical fitness was ascertained by standard investigations. Patients were administered Granulocyte Colony Stimulating Factor (GCSF), 48 hours and 24 hours before the harvest and transplantation of BMMNCs to enhance the mobilization of the BMMNCs [17].
All the patients underwent a thorough clinical evaluation. An experienced physiotherapist assessed and documented the muscle strength as per Modified- Medical Research Councils Manual Muscle Testing to identify the weakest muscles. Motor points of these muscles were marked for intramuscular transplantation of BMMNCs.
Procurement of autologous BMMNCs
Procedure was performed in the operation theatre with aseptic conditions maintained. The patient assumed supine position. Approximately, 100 ml to 120 ml of bone marrow was aspirated under local anesthesia from the region of anterior superior iliac spine using the bone marrow aspiration needle and collected in the heparinized tubes.
Isolation of autologous BMMNCs
This was performed in the stem cell laboratory under aseptic conditions. The sample was then centrifuged and using the density gradient method mononuclear cells were separated. The layer of mononuclear cells was transferred to a sterile centrifuge tube using a sterile autopipette dispenser. The separated mononuclear cell pellet was analyzed under microscope using Trypan blue to check for viability of the mononuclear cells. Cell viability and cell counting was done manually and confirmed using Tally cell counter. The average cell count was 80.5 x 106 with 96.7 % average cell viability. Sample was sent for characterization of cell surface marker CD34+. During the FACS analysis CD34 PE antibody was used for the identification.
Mode of transplantation
The separated autologous BMMNCs were injected on the same day. Two third amount was injected, intrathecally using an 18G Touhy needle at the level between fourth and fifth lumbar vertebrae. One third amount wasdiluted in the patient’s own CSF accumulated during intrathecal transplantation and injected intramuscularly at the motor-points of the specific muscles. The average numbers of cells injected was calculated. Simultaneously 20 mg/kg body weight methyl prednisolone in 500 ml Ringer Lactate was given intravenously to subside the immediate local inflammation. Patientswere monitored for any immediate adverse events and throughout the duration of the follow up.
Post transplantation advice
Patients underwent a standard multidisciplinary rehabilitation in the subsequent four days before discharge and were advised to continue the same at home post discharge [18]. The standard Riluzole medication was continued. In addition Lithium at 300 mg twice a day was prescribedto patients who could tolerate it (no allergy, normal liver and kidney function, able to swallow tablets and availability of serum lithium level monitoring). Regular monitoring of lithium levels was mandatory (levels were maintained between 0.5-0.8 mmol/lit). Lithium regimen was continued for six weeks post intervention.
Patient follow up and outcome measures
Survival duration was used as an outcome measure. According to the clinical protocol patients were advised to come for a follow up examination every three months post transplantation. Documented information regarding the symptomatic improvement at these follow ups was analyzed. All the patients were also contacted telephonically or through email to gather the most recent ALS-FRS scores and survival information, at the time of analysis.
Statistical analysis
Demographic data analysis
Baseline demographic data of all the patients was gathered. Mean age at onset and at intervention was calculated. Distribution of both the intervention and control group according to age and gender was computed. Descriptive statistics for the demographic data was calculated as mean (Standard Deviation) for continuous variables and as percentage for categorical variables. Both the control and intervention group were tested for the baseline differences. Unpaired students T-test was performed to find out if there was significant difference between mean age at onset of the disease for the two groups.
Survival analysis
Normality of the distribution was calculated using Shapiro-wilk test. Further analysis of survival was conducted using Kaplan-Meier survival analysis to compare the cumulative probabilities of survival for both the intervention and control groups. The Intervention group was further divided into subgroups to analyze the effect of various factors on the survival of patients. The intervention group was divided according to age, type of onset and lithium. Two separate Kaplan-Meier survival curves were plotted for patients in ALS group with age less than andmore than 50 years at onset of the disease. These two curves were then compared using the log rank test. The same was repeated for patients with bulbar onset and limb onset and the survival duration was compared. It was also repeated for the patients who received Lithium and who did not receive Lithium (as they could not tolerate). Survival duration of the patients in the intervention group was also compared to the survival duration of patients in previous epidemiological studies.
Statistical analysis for both the groups was conducted using SPSS 20.0.
Results
Sample size and demographic distribution (Table 1)
Table 1.
Demographic distribution of intervention and control group
| Characteristic | Intervention group | Control group |
|---|---|---|
| Gender | ||
| Females | 15 (40.54%) | 8 (40.00%) |
| Males | 22 (59.44%) | 12 (60.00%) |
| Lithium | ||
| Number of patients who were prescribed lithium | 15 (40.54%) | 0 (0.00%) |
| Number of patients who were not prescribed lithium | 22 (59.45%) | 20 (100.00%) |
| Type of onset | ||
| Percentage of patients with bulbar onset | 27.02% | 6.25% |
| Percentage of patients with limb onset | 72.97% | 93.75% |
| Average age in years at onset of symptoms (SD) | 48.89 (10.79) | 53.11 (11.49) |
Intervention group
A total of 37 patients diagnosed with definite ALS and who had received cell transplantation were included in the analysis. There were 15 (40.54%) females and 22 (59.46%) males. Mean age at onset was 48.89 (10.79). Bulbar onset was noted in 20 (66.66%) patients; 15 (40.54%) patients received lithium after intervention. The survival status was known for 33 patients, 4 patients could not be contacted telephonically during the latest follow up and therefore were considered lost to follow up for the Kaplan-Meier survival analysis. Their survival duration was considered till the last follow up. All the 37 patients were contacted telephonically or by email and the survival status was found out, however the information was insufficient to score ALS-FRS. The follow up ALS-FRS scales were recorded telephonically for 6 patients post stem cell therapy.
Control group
From the out-patient department a total of 20 patients diagnosed with ALS and without any cell transplantation were included in the control group. There were 8 (40.00%) females and 12 (60.00%) males. Mean age at onset was 53.11 (11.49).
Survival analysis (Table 2)
Table 2.
Survival analysis
| Survival analysis | Intervention group | Control group |
|---|---|---|
| Total mortality | 48.64% | 50.00% |
| Range of survival duration (months) | 13-158 | 26-84 |
| Mean survival duration (months) | 87.76 (10.45) | 57.38 (5.31) |
The baseline comparison with an unpaired student’s T-test showed no significant difference (p = 0.216) between the age at onset for the patients in intervention and control group. Percentage distribution of males and females in both the groups was also similar. Shapiro–Wilk test suggested that the survival of the patients since the onset of the disease was normally distributed for both control and intervention group (p > 0.05).
Intervention group
Total mortality in the intervention group was 17 out of 37 (45.94%) patients. Status of survival was not known for 4 patients and number of patients alive was 16 (48.48%) (Table 2). There were 15 patients who survived for more than 5 years since the onset of the disease and 6 patients survived for more than 8 years since the onset of the disease. The highest survival duration was 158 months since the onset of the diseaseand the patient was surviving. Mean survival duration since the onset of the disease was 87.76 (10.45) months (Range: 13 months to 158 months).
Control group
Total mortality in the control group was 10 out of 20 (50.00%) patients. Status of survival was known for all the patients. 10 (50.00%) of the control group patients were alive (Table 2). There were 3 patients who survived more than 5 years since the onset of the disease and none survived more than 8 years since the onset of the disease. The highest survival in the control group was 84 months since the onset of the disease and patient was surviving. Duration of the survival since the onset of the disease ranged from 26 months to 84 months. Mean survival duration was 57.38 (5.31) months since the onset of the disease.
Comparison of survival duration
Mean survival since the onset of the disease, estimated as 50% cumulative probability of survival by Kaplan-Meier survival analysis (Figure 1) was 87.76 (10.45) months in the intervention group and 57.38 (5.31) months for the control group. The intervention group showed higher survival duration by 30.38 months as compared to the control group. Statistical significance calculated with Log rank test was p = 0.133. The survival duration in the intervention group was also found to be significantly higher than the survival duration of patients with definite ALS in previous epidemiological studies (Table 3).
Figure 1.
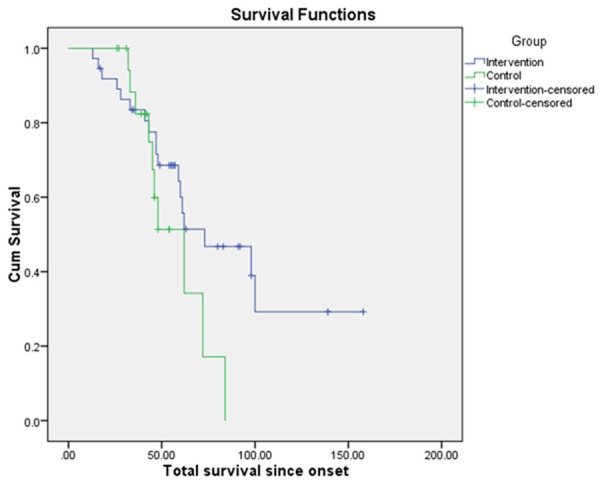
Kaplan Meier survival analysis comparing the mean survival duration of the intervention and control group.
Table 3.
Mean and median survival duration as observed in previous epidemiological studies and its comparison with the current findings
| Author | Year | Country | Sample size | Mean age of the population in years (SD) | Mean or median Survival since onset | Comparison with the results of our study |
|---|---|---|---|---|---|---|
| Haverkamp et al. [23] | 1995 | USA | 1200 | 55.7 | 37.5 months | 87.76 months |
| Forbes et al. [24] | 2004 | Scotland | 1226 | Men - 65.2 | 25 months | 87.76 months |
| Women - 67.7 | 5 year survival probability - 11% | 5 year survival probability - 62.3% | ||||
| Milul et al. [25] | 2005 | Italy | 79 | 64.4 | 39.2 months | 87.76 months |
| Osuntukun et al. [26] | 1974 | Nigeria | 92 | 39.2 (1.6) | Dead - 73 months | mean survival 87.76 months |
| Alive - 79 months | mean survival 87.76 months | |||||
| Radhakrishnan K [27] | 1986 | Libya | 23 | 51 | 42 | 87.76 months |
| Norris et al [28] | 1993 | USA | 708 | Range - 25 -74 | 24-44 years - 71.5 months | 24-44 years - 138.7 months |
| 45-54 years - 35 months | 45-54 years - 76.80 months | |||||
| 55-74 years - 32.5 months | 55-74 years - 57.19 months | |||||
| Alcaz S [29] | 1997 | Serbia | 58 | Men - 56.2 | 2 years survival probability - 62% | 2 years survival probability - 89% |
| Women - 56.6 | 5 years survival probability - 27% | 5 years survival probability - 62.3% | ||||
| 7 year survival probability - 27% | 7 year survival probability - 43.2% | |||||
| Eisen et al. [30] | 1993 | USA | 246 | Not available | < 40 years - 98.4 months | 77 months - |
| 61-70 years - 31.2 months | 53 months - | |||||
| Traynor et al. [31] | 2000 | Ireland | 388 | Men - 63.3 | Definite ALS - 27 months | Definite ALS - 88.86 months |
| Women - 64.4 | Probable - 30 months | Probable ALS - not included | ||||
| Sorenson et al. [32] | 2002 | USA | 77 | 63 | 23 months | 87.76 months |
| Tysnes et al. [33] | 1991 | Norway | 60.9 | 28 months | 87.76 months | |
| Range - 34 to 82 | ||||||
| Turner et al. [34] | 2003 | England | 769 | Long survivors - 43, short survivors - 57, others - 62 | 43 months | 87.76 months |
Analysis of various factors on survival duration (Table 4) (Figures 2, 3 and 4)
Table 4.
Effect of prognostic factors on the median survival since the onset of the disease
| Prognostic factor | Median survival since the onset of the diseases as calculated by Kaplan-Meier survival analysis | P-Value as calculated by comparing the Kaplan-meier survival curves using log-rank test |
|---|---|---|
| Lithium | ||
| Patients who were given lithium | 106.73 (15.69) | 0.121 |
| Patients who were not given lithium | 66.83 (7.52) | |
| Age at onset | ||
| Patients with the age below 50 years at onset | 113.34 (15.45) | 0.039* |
| Patients with the age above 50 years at onset | 63.02 (7.7) | |
| Onset | ||
| Bulbar | 78.01 (14.23) | 0.902 |
| Limb | 90.24 (13.27) | |
Statistically significant (p < 0.05).
Figure 2.
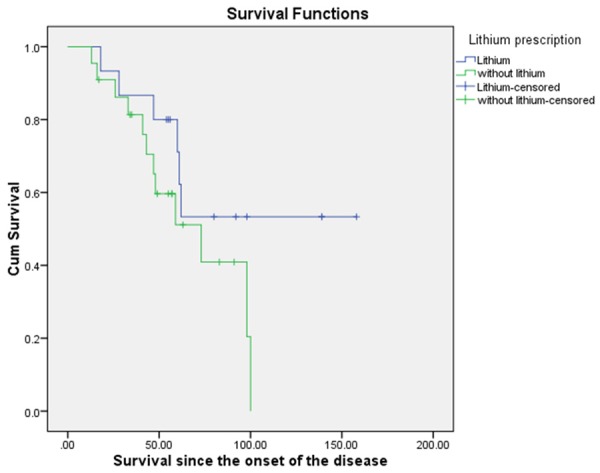
Kaplan-Meier Survival comparison for the patients in intervention group with or without Lithium prescription.
Figure 3.
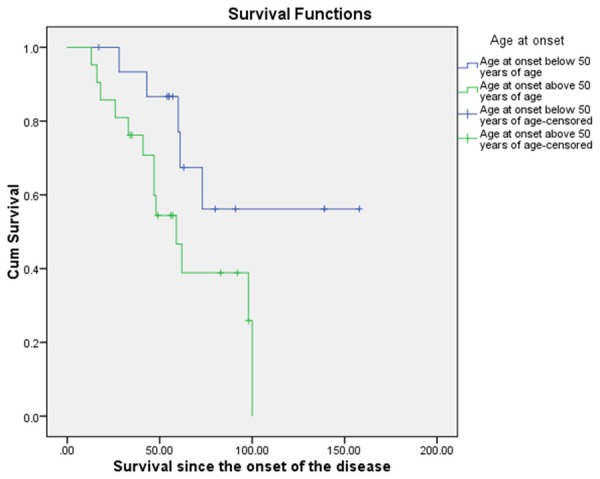
Kaplan-Meier Survival comparison for the patients in intervention group below and above 50 years of age at the onset.
Figure 4.
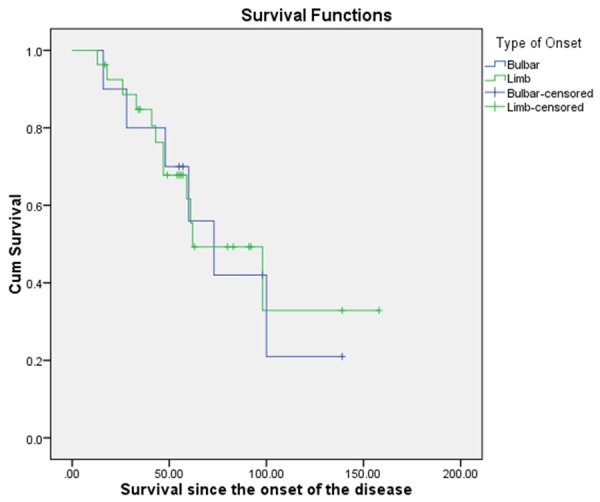
Kaplan-Meier Survival comparison for the patients in intervention group with bulbar onset and limb onset.
Different factors were analyzed to find out their effect on the total survival post intervention. There was a statistically significant increased survival duration, 114.59 (14.50) noted in patients below the age of 50 years as compared to patients above the age of 50 years, 62.74 (7.4). There was a clinically significant difference in the survival duration of patients who received lithium and the patients that did not receive lithium. The patients who received lithium showed higher mean survival of 106.73 (15.69) months as compared to the once that did not receive lithium, 66.83 (7.52) (p = 0.121). Patients who presented with limb onset showed mean survival duration of 90.24 (12.27) months which was higher than compared to those with bulbar onset 78.01 (14.23) (p = 0.902).
Adverse events (Table 5)
Table 5.
Adverse events observed in the immediate post transplantation period
| Adverse Events | Present during the period of follow up | Absent during the period of follow up | ||
|---|---|---|---|---|
|
| ||||
| Procedure related | Cellular transplantation related | Procedure related | Cellular transplantation related | |
| Minor | • Spinal Headache (30.43%) | None | • Bleeding at the site of injection | None |
| • Nausea (4.34%) | • Bleeding at the site of aspiration | |||
| • Vomiting (4.34%) | ||||
| • Pain at the site of injection (4.34%) | ||||
| • Pain at the site of aspiration (8.67%) | ||||
| • Fatigue (4.34 %) | ||||
| Major | None | • Neurological deficits | • Allergic reaction | |
| • Nerve root damage | ||||
| • Parasthesia in Lower Limb | ||||
| • Loss of sensation in lower limb | ||||
| • Loss of motor function in the lower limbs | ||||
| • Hematoma at the site of injection | ||||
| • Hematoma at the site of aspiration | ||||
| • Local infection at the site of injection or aspiration | ||||
| • Meningismus or meningitis | ||||
| • Systemic or brain infection | ||||
| • Bowel or bladder incontinence | ||||
| • Respiratory distress | ||||
| • Cardiac failure | ||||
| • Sudden onset of respiratory discomfort | ||||
| • Breathlessness | ||||
Procedure related
In this study we noted only minor side effects like nausea, spinal headache, vomiting, pain at the site of injection or aspiration, and parasthesia in lower limbs which were associated with the technique of intrathecal transplantation. These side effects did not interfere with the outcome of the treatment.
Cellular transplant related
There were no major or minor cellular transplant related side effects noted.
Discussion
ALS is a rapidly progressing neurodegenerative disease. The current management strategies show very limited effect on the survival of the patients. In the current analysis we computed the survival probabilities for the patients of ALS treated with autologous BMMNCs intrathecal and intramuscular transplantation and compared these survival probabilities with a control group. Mononuclear cells have been used earlier for the treatment of ALS. Some of the studies have isolated these cells from peripheral blood whereas the others have isolated it from the bone marrow [13,14,19-21]. Various other types of stem cells like autologous mesenchymal stem cells (MSCs), Neural stem cells (NSCs) and allogenic umbilical cord stem cells (UCSc) have also been used in the clinical studies of ALS. All these cell types show beneficial effects however due to lack of comparative studies and heterogeneous methodologies the evidence still remains inconclusive [22].
Predicted survival of the patients in the study population was analyzed using Kaplan-Meier survival analysis. The mean survival duration estimated for the intervention group was found to be 87.76 (10.45) months as compared to 57.38 (5.31) months in the control group. The comparison of survival duration shows a clinically significant difference of 30.38 months (2.53 years). The estimated survival duration in the intervention group is significantly more than the mean survival observed in previous epidemiological studies (Table 3) [23-34]. There is one epidemiological study estimating the mean survival duration of ALS patients in Indian population. But 89.5% of patients were censored in the Kaplan-Meier analysis as their survival status was unknown. This study may have overestimated the results and thus we did not comparethe results [35]. Age at onset has been found to be inversely proportional to the average survival of the ALS patients [9,31]. Four of the studies had the average age comparable to our population [27,29,31,34]; the mean survival duration as observed in our population was significantly higher. In spite of lower mean age at onset in two previous studies [26,28] they had lower survival duration (79 months and 71.5 months) as compared to the current study (87.76 months). The male and female distribution was varied in all of the above studies. The incidence of ALS is found to be higher in males as compared to females however the evidence is inconclusive about the difference in the survival. Some epidemiological articles suggest that there is no statistically significant difference between the survival of males and females with ALS [36,37]. The mean survival duration in this study was significantly longer as compared to the enhanced survival duration in patients treated with Riluzole [38-40]. Two articles present retrospective analyses identifying the prognostic factors for ALS. Rooney et al. 2013 [38] showed the mean survival for the patients taking Riluzole in an Irish population to be 17.52 months; which was significantly (p ≤ 0.001) higher as compared to the ones that did not take Riluzole (Median survival-10 months). Lee et al. 2013 [39] studied the population in Taiwan and found median survival of patients with Riluzole to be 67.75 months, with an increase of 18.17 months as compared to the control population. A systematic review by Miller et al. 2009, [40] of 974 patients treated with Riluzole and 503 patients treated with placebo showed that the survival was higher in the group treated with Riluzole with mean survival of 17.7 months. There was an increase in mean survival of the patients by 2.3 months at the dose of 100 mg. The mean survival observed in this study population (87.76 months) was significantly higher than the previous reports of extended survival with Riluzole. There was no beneficial effect of Riluzole was observedon muscle strength and bulbar symptoms in patients who were prescribed Riluzole. There was only minimal beneficial effect observed in the limb function. There were various side effects associated with Riluzole but none of these were shown to have an effect on the treatment outcome [40]. In the current analysis improvements were noted in various bulbar functions like speech, swallowing and respiratory capacity. There was also improvement noted in fine motor activities of the hand and ability to maintain sitting and standing balance. There were a few minor side effects noted in some patients and some major side effects noted in only one patient that did not interfere in the outcome of cellular therapy.
Prognostic factors for the survival of ALS patients post cellular therapy
Prescription of Lithium post cellular transplantation
The Kaplan-Meier survival curves for patients with and without lithium showed a clinically significant difference with patients who received lithium showed higher survival since the onset of the disease, 106.73 (15.69) months, as compared to the patients who did not receive lithium 66.83 (7.52) months (Figure 2). The difference between the mean survivalsince onset (39.9 months) of these two groups was therefore clinically significant. When compared using Log-rank test it did not achieve statistical significance (p = 0.121). A systematic review of literature including a total of 1100 patients treated with Lithium showed no statistically significant differences in the rates of functional decline, deterioration of respiratory functionor survival time since onset of the disease as compared to patients treated with Riluzole or placebo [41]. The rationale for prescribing Lithium to our patients was however to enhance the survival and potency of the transplanted cells [42]. It is interesting to note that although there is a clinically significant difference the small sample size may have underestimated the statistical significance. The effect of lithium in conjunction with cellular therapy is therefore recommended to be tested with a larger sample size.
Age at onset
Age at onset has been shown to be a strong prognostic factor in ALS. It is inversely proportional to survival time. Lower age of onset correlates with better survival [24-37,43]. In our population patients below the age of 50 years showed a significantly (P = 0.039) higher survival, 113.34 (15.45) months as compared to the patients with the age of onset more than 50 years of age, 68.32 (10.74) months (Figure 3). Younger patients had significantly higher survival duration. Age at onset influences survival duration in ALS and older patients may have poor prognosis.
Onset of the disease
The onset of the disease is identified based on the region of the body in which the symptoms first manifest. Manifestation of the disease first in the bulbar region is associated with poor prognosis and significantly shorter survival duration [44]. The survival duration is not significantly different when the age of onset is above 70 years [45]. In younger population bulbar onset leads to poor prognosis. In our population there was no statistically significant difference (p = 0.902) between the survival duration of patients with bulbar onset and limb onset. Mean survival duration for patients with bulbar onset was 78.01 (14.23) months and for limb onset it was 90.24 (13.27) months (Figure 4). Bulbar onset may be associated with poor prognosis in ALS.
Duration between onset to intervention
Early diagnosis and early intervention has been shown to have a positive prognostic effect on the survival duration in ALS [23]. However survival prognosis is influenced significantly with rate of progression of the disease. Therefore duration between onset of the diseaseand intervention cannot be assessed singularly. The functional decline and rate of progression must be taken into account. In our analysis the data available was not sufficient to carry out the analysis using existing statistical methods.
ALS-FRS
In addition to the clinically significant higher survival than compared to the control group, other clinical outcome measures like ALS-FRS scale also showed beneficial effect. ALS-FRS is a simple outcome measure that can be administered telephonically [46]. The summary of change in the ALS-FRS scores of our patients is given in Table 6. Previous studies have reported decline of ALS-FRS scale scores to be a prognostic indicator of the disease. Various epidemiological studies have reported various rates of decline of ALS-FRS scale scores. De Carvahelo et al 2005 [47] report a 17% decline of ALS-FRS scores every 6 months. Whereas Magnus et al 2002 [48] report a decline of 12 points over the period of 36 months. In our population two patients with ALS-FRS scale scores of 7 and 10 before transplantation were found to have maintained the scores 43 months after transplantation. Two patients showed less than 17% decline of ALS-FRS scores for every six months. One patient showed a decline of 17 points, the scores reduced from 34 to 17 in 44 months. The second patient showed a decline of 2 points of the scale, from 24 to 22 in 18 months. There were two patients who were on an artificial ventilatory support at the time of follow up and the rate of reduction in the ALS-FRS scores was not influenced by intervention (Table 6). These findings may suggest a beneficial effect of cell transplantation on rate of progression of the disease.
Table 6.
Patient characteristics and follow up ALS-FRS scores
| Age at onset | Gender | Survival in months since the onset of the disease | Survival in months after the intervention | Duration in months between onset and intervention | ALS-FRS score before SCT | ALS-FRS score after SCT at the most recent telephonic follow up | |
|---|---|---|---|---|---|---|---|
| Patient I | 50 | M | 49 | 45 | 4 | 37 | 1 |
| Patient II | 56 | M | 92 | 44 | 48 | 34 | 17 |
| Patient III | 57 | M | 56 | 44 | 12 | 17 | 2 |
| Patient IV | 40 | F | 139 | 43 | 96 | 7 | 7 |
| Patient V | 28 | M | 139 | 43 | 96 | 10 | 10 |
| Patient VI | 35 | F | 54 | 18 | 36 | 24 | 22 |
Clinical observations
Survival analysis of the patients that underwent cellular therapy showed higher survival as compared to the control group. It is also important however to know if there was any improvement in the physical limitations and bulbar symptoms experienced by these patients. In the retrospective analysis that we conducted the information was not sufficient to analyze and comment about the improvements noted in the limb and bulbar function however there were several reports of such improvements. Majority of the patients reported clearer and louder speech, improved tongue movements and reduced speech fatigability. Some of the patients showed improvement in various bulbar symptoms like reduced chocking, improved swallowing, reduced saliva drooling, increase respiratory capacity. Few showed better neck control. The limb function was better in few patients. Majority of patients improved in fine motor activities, static and dynamic standing and sitting balance. Some patients showed improvement in the lower extremity function and ambulation. All these changes were noted in the early post transplantation period (till 3 months). The sustainability of these improvements could not be studied in this population due to insufficient data. Future trials should be designed to study the clinical symptomatology in more detail. We observed that intervention in the earlier stage of the disease was associated with more likelihood of response. One can hypothesize that since the neural damage is limited in the early stage of the disease, the microcellular environment is more conducive to the cell transplantation. Time to intervention needs to be studied in depth.
Neuropathophysiology of Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis (ALS) is characterized by progressive axonal degeneration of motor neurons in the spinal cord and motor cortex. The disease process selectively affects only motor neurons sparing the sensory system. Various mechanisms have been presumed to contribute to the neuropathophysiology of ALS [49]. Recent advances have suggested involvement of non neuronal cells like glial cells [50]. Up regulation of superoxide dismutase leads to unchecked intracellular peroxidation reactions and subsequent oxidative stress. Astrocytes also play a role in development of ALS; astrocytic glutamate is up regulated.Excitoxicity, autoimmunity and a widespread neuroinflammatory response are also implicated. Recently various genes have also been found to be involved [51]. Pathophysiology of ALS is heterogeneous and therefore makes it difficult to manage the disease. So far the only drug that has shown some efficacy in altering the disease process is the N Methyl B aspartate (NMDA) receptor antagonist riluzole, which helps to reduce the over excitation of the neurons in presence of elevated extracellular glutamate levels. However this only increases the life span of patients suffering from ALS by 3-5 months [39,40] and the disease still continues to be fatal.
Mechanism of action of bone marrow mononuclear cells
In recent years cellular therapy has shown some promise in altering various pathophysiological processes in ALS. Although replacement of degenerated motor neurons is the goal of transplantation therapy, various factors could influence the outcome of the transplanted cells. Some of these factors are survival of transplanted cells, generation of functional motor units and appropriate projection of neuronal fibers for long distances to reach to the muscle. Currently the transplantation of these cells aims to support and protect the existing motor neurons from undergoing degeneration.The mechanism of action of bone marrow mononuclear cells in ALS is twofold; to protect the existing motor neurons and to replace the degenerated motor neurons. BMMNCs have shown neurogenic potential however the functionality of the regenerated neurons is debatable in the highly toxic cellular microenvironment observed in patients with ALS [52]. Neuroprotective mechanisms are therefore considered to be more potent in bringing about the changes seen in ALS post transplantation. BMMNCs have shown a neurotrophic effect in various neurodegenerative diseases [14,53,54]. These cells are capable of homing onto the injured sites, as guided by various chemo attractant pathways [55]. Modification of theexaggerated microglial response by immunomodulatory effects is also observed. Various secreted neurotrophic factors like connective tissue growth factor, fibroblast growth factor 2 and 7 andvarious interleukins are responsible for cell proliferation and cytoprotection [56]. Reduced levels of TNF-α, IL-1β, IL-1α, IL-6 and increased levels of IL-10 lead to an anti-inflammatory effect on the neural microenvironment [56-58]; therefore, enhancing the neuronal repair. Further secretion of growth factors like vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), brain fibroblast growth factor (bFGF) leads to neoangiogenesis and up regulation of hormones like erythropoietin [56]. The cascade of events triggered due to these leads to formation of new vessels as well as increased bold flow. Improved blood circulation helps retrieving the lost tissue functions. Therefore BMMNCs may be instrumental in arresting the disease progression through above mentioned mechanisms.
Synthesis of evidence for mononuclear cell therapy in ALS
Mononuclear cells have been previously explored to see their effect in ALS. Some of these trials have used peripheral blood mononuclear cells [59,60]. Various routes of transplantation were used. Transplantation in the frontal motor cortex resulted in significantly higher survival duration. These studies suggest the potential of MNCs for the treatment of ALS.Several other researchers have isolated the MNCs from the bone marrow and have studies their effects in patients of ALS. Deda et al. 2009 [13], treated 13 patients with bulbar symptoms and loss of functional movement. The cells were injected in the anterior part of the spinal cord at the level of C1-C2 after performing a laminectomy. Out of the 13 patients treated 3 patients died within the first 9 months of the transplantation due to lung infection and myocardial infarction. The other 10 patients showed improvement in muscle strength and bulbar symptoms like speech, swallowing, and breathing. One of the patients who was on the mechanical ventilator showed spontaneous breathing at some occasions after cellular therapy. Prabhakar et al. 2012 [61] transplanted the autologous BMMNCs intrathecally in ten patients with confirmed ALS. These patients were followed up for one year and time to 4 points drop in the ALS-Functional rating scale revised (ALSFRS-R) was calculated. The time calculated was 16.7 months which was more than shown in previous studies. Blanquer M et al. 2010 [62] treated cases of confirmed ALS with intraspinal autologous BMMNCs. These were followed up for 3 years. These cases showed arrested progression of the drop in forced vital capacity and various neurological scales measured. Autologous BMMNCs have therefore shown some beneficial effects in previous trials. A single arm open label study by Blanquer et al 2012 [14] suggests that intraspinal administration of BMMNCs leads to neurotrophic effect and preserves more number of motor neurons in the treated spinal segments as compared to the non-treated spinal segments. Our results are consistent with the previous findings of the safe use and beneficial effects of BMMNCs.
Limitations of the study
The analysis was of a retrospective cohort, with a relatively small sample size for a survival analysis which is a limitation for conclusive findings. There are various other factors that may potentially influence the outcome of the cellular therapy, like the number of transplantations and the duration of the time between subsequent transplantation; these could not be analyzed with the available information. Time to intervention is an important determinant of the treatment outcome. In our analysis the data was not sufficient to analyze this and future should investigate this factor in depth.
Research implications
Further prospective trails with rigorous methodology making use of randomization, blinding and larger sample size need to be carried out for conclusive evidence. There are different cell types that are being studied for the treatment of ALS, it is key to compare the effects of different cell types. Combination of cellular transplantation with various other neuroprotective regimens should also be studied to find the treatment option that gives best possible results in ALS. Effect of lithium in combination with the cellular therapy and the frequency of transplantations need to be analyzed. While designing the trials randomization of the patients should be done taking into consideration multiple factors that may influence the outcome of the intervention. We observed improvement in the clinical outcomes immediately post transplantation. The duration for which the improvements are maintained needs to be analyzed with a rigorous methodology. Such findings will help identify the time at which the transplantation may be repeated.
Conclusion
This study shows increased survival duration by 30.38 months in patients undergoing autologous BMMNCs transplantation as compared to the control population. This was also higher as compared to the previous epidemiological reports from different regions of the world. Various factors may also influence the survival duration especially age at the onset of the disease and type of onset. Although statistical significance was not reached, Lithium shows a beneficial effect on survival duration in combination with BMMNCs transplantation. In addition to the standard treatment with Riluzole, there is a possibility that early intervention with combination of BMMNCs transplantation and Lithium may have a positive effect on the duration of survival in ALS. Further prospective studies with a larger sample size and a rigorous methodology including randomization and blinding are required for conclusive findings.
Disclosure of conflict of interest
The authors do not have any conflicts of interests to declare.
References
- 1.Chiò A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, White LA. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41:118–30. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravits J, Appel S, Baloh RH, Barohn R, Brooks BR, Elman L, Floeter MK, Henderson C, Lomen-Hoerth C, Macklis JD, McCluskey L, Mitsumoto H, Przedborski S, Rothstein J, Trojanowski JQ, van den Berg LH, Ringel S. Deciphering amyotrophiclateral sclerosis: what phenotype, neuropathology and genetics are telling usabout pathogenesis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(Suppl 1):5–18. doi: 10.3109/21678421.2013.778548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 4.Lee CT, Chiu YW, Wang KC, Hwang CS, Lin KH, Lee IT, Tsai CP. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. J Epidemiol. 2013;23:35–40. doi: 10.2188/jea.JE20120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2013;3:CD004427. doi: 10.1002/14651858.CD004427.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, Jacob VC. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21(Suppl 1):S79–90. doi: 10.3727/096368912X633798. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, Shetty A, Mishra P, Kali M, Biju H, Badhe P. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013:623875. doi: 10.1155/2013/623875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Gokulchandran N, Sane H, Badhe P, Kulkarni P, Lohia M, Nagrajan A, Thomas N. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. Journal of Neurorestoratology. 2013;1:13–22. [Google Scholar]
- 10.Rizvanov AA, Guseva DS, Salafutdinov II, Kudryashova NV, Bashirov FV, Kiyasov AP, Yalvaç ME, Gazizov IM, Kaligin MS, Sahin F, Mukhamedyarov MA, Palotás A, Islamov RR. Genetically modified human umbilical cord blood cells expressing vascular endothelial growth factor and fibroblast growth factor 2 differentiate into glial cells after transplantation into amyotrophic lateral sclerosis transgenic mice. Exp Biol Med (Maywood) 2011;236:91–8. doi: 10.1258/ebm.2010.010172. [DOI] [PubMed] [Google Scholar]
- 11.Garbuzova-Davis S, Rodrigues MC, Mirtyl S, Turner S, Mitha S, Sodhi J, Suthakaran S, Eve DJ, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS. PLoS One. 2012;7:e31254. doi: 10.1371/journal.pone.0031254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution,migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–70. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- 13.Deda H, Inci MC, Kürekçi AE, Sav A, Kayihan K, Ozgün E, Ustünsoy GE, Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11:18–25. doi: 10.1080/14653240802549470. [DOI] [PubMed] [Google Scholar]
- 14.Blanquer M, Moraleda JM, Iniesta F, Gómez-Espuch J, Meca-Lallana J, Villaverde R, Pérez-Espejo MÁ, Ruíz-López FJ, García Santos JM, Bleda P, Izura V, Sáez M, De Mingo P, Vivancos L, Carles R, Jiménez J, Hernández J, Guardiola J, Del Rio ST, Antúnez C, De la Rosa P, Majado MJ, Sánchez-Salinas A, López J, Martínez-Lage JF, Martínez S. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. 2012;30:1277–85. doi: 10.1002/stem.1080. [DOI] [PubMed] [Google Scholar]
- 15. http://apps.who.int/classifications/icd10/browse/2010/en#/G12.2.
- 16.Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: Past, present and future. Br J Clin Pharmacol. 2004;57:695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 18.Ng L, Khan F, Mathers S. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2009:CD007425. doi: 10.1002/14651858.CD007425.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, Gutierrez-Jimenez E, Zazueta-Fierro OE, Meza JA, Couret-Alcaraz P, Hernandez-Torre M. Stem cell transplantation in amyotrophic lateral sclerosis patients: methodological approach, safety, and feasibility. Cell Transplant. 2012;21:1899–907. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 20.Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas JE, Caro E, Gutierrez-Jimenez E, Segura JJ. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26–34. doi: 10.1080/14653240802644651. [DOI] [PubMed] [Google Scholar]
- 21.Blanquer M, Pérez Espejo MA, Iniesta F, Gómez Espuch J, Meca J, Villaverde R, Izura V, de Mingo P, Martínez-Lage J, Martínez S, Moraleda JM. Bone marrow stem cell transplantation in amyotrophic lateral sclerosis: technical aspects andpreliminary results from a clinical trial. Methods Find Exp Clin Pharmacol. 2010;32(Suppl A):31–7. [PubMed] [Google Scholar]
- 22.Meamar R, Nasr-Esfahani MH, Mousavi SA, Basiri K. Stem cell therapy in amyotrophic lateral sclerosis. J Clin Neurosci. 2013;20:1659–63. doi: 10.1016/j.jocn.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 24.Forbes RB, Colville S, Cran GW, Swingler RJ Scottish Motor Neurone Disease Register. Unexpected decline in survival from amyotrophic lateral sclerosis/motor neurone disease. J NeurolNeurosurg Psychiatry. 2004;75:1753–5. doi: 10.1136/jnnp.2003.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millul A, Beghi E, Logroscino G, Micheli A, Vitelli E, Zardi A. Survival of patients with amyotrophic lateral sclerosis in a population-based registry. Neuroepidemiology. 2005;25:114–9. doi: 10.1159/000086353. [DOI] [PubMed] [Google Scholar]
- 26.Osuntokun BO, Adeuja AO, Bademosi O. The prognosis of motor neuron disease in Nigerian africans. A prospective study of 92 patients. Brain. 1974;97:385–94. doi: 10.1093/brain/97.1.385. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan K, Ashok PP, Sridharan R, Mousa ME. Descriptive epidemiology of motor neuron disease in Benghazi, Libya. Neuroepidemiology. 1986;5:47–54. doi: 10.1159/000110812. [DOI] [PubMed] [Google Scholar]
- 28.Norris F, Shepherd R, Denys E, U K, Mukai E, Elias L, Holden D, Norris H. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. 1993;118:48–55. doi: 10.1016/0022-510x(93)90245-t. [DOI] [PubMed] [Google Scholar]
- 29.Alcaz S, Jarebinski M, Pekmezović T, Marinković Z, Apostolski S. Survival in amyotrophic lateral sclerosis. Srp Arh Celok Lek. 1997;125:19–23. [PubMed] [Google Scholar]
- 30.Eisen A, Schulzer M, MacNeil M, Pant B, Mak E. Duration of amyotrophic lateral sclerosis is age dependent. Muscle Nerve. 1993;16:27–32. doi: 10.1002/mus.880160107. [DOI] [PubMed] [Google Scholar]
- 31.Traynor BJ, Codd MB, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria. Arch Neurol. 2000;57:1171–6. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- 32.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59:280–2. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 33.Tysnes OB, Vollset SE, Aarli JA. Epidemiology of amyotrophic lateral sclerosis in Hordaland county, western Norway. Acta Neurol Scand. 1991;83:280–5. doi: 10.1111/j.1600-0404.1991.tb04701.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A. Prolonged survival in motor neuron disease: a descriptive study of the King’s data base 1990–2002. J Neurol Neurosurg Psychiatry. 2003;74:995–7. doi: 10.1136/jnnp.74.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalini A, Thennarasu K, Gourie-Devi M, Shenoy S, Kulshreshtha D. Clinical characteristics and survival pattern of 1,153 patients with amyotrophic lateral sclerosis: experience over 30 years from India. J Neurol Sci. 2008;272:60–70. doi: 10.1016/j.jns.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Talman P, Forbes A, Mathers S. Clinical phenotypes and natural progression for motor neuron disease: Analysis from an Australian database. Amyotroph Lateral Scler. 2009;10:79–84. doi: 10.1080/17482960802195871. [DOI] [PubMed] [Google Scholar]
- 37.Murphy M, Quinn S, Young J, Parkin P, Taylor B. Increasing incidence of ALS in Canterbury, New Zealand: A 22-year study. Neurology. 2008;71:1889–1895. doi: 10.1212/01.wnl.0000336653.65605.ac. [DOI] [PubMed] [Google Scholar]
- 38.Rooney J, Byrne S, Heverin M, Corr B, Elamin M, Staines A, Goldacre B, Hardiman O. Survival Analysis of Irish Amyotrophic Lateral Sclerosis Patients Diagnosed from 1995-2010. PLoS One. 2013;8:e74733. doi: 10.1371/journal.pone.0074733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CT, Chiu YW, Wang KC, Hwang CS, Lin KH, Lee IT, Tsai CP. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. J Epidemiol. 2013;23:35–40. doi: 10.2188/jea.JE20120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamez J, Salvado M, Martínez de la Ossa A, Badia M. Lithium for treatment of amyotrophic lateral sclerosis: much ado about nothing. Neurologia. 2013 doi: 10.1016/j.nrl.2013.02.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Richman CM, Kinnealey A, Hoffman PC. Granulopoietic effects of lithium on human bone marrow in vitro. Exp Hematol. 1981;9:449–55. Gallicchio VS, Chen MG. Influence of lithium on proliferation of hematopoietic stem cells. Exp Hematol 1981; 9: 804-10. [PubMed] [Google Scholar]
- 43.Atsuta N, Watanabe H, Ito M, Tanaka F, Tamakoshi A, Nakano I, Aoki M, Tsuji S, Yuasa T, Takano H, Hayashi H, Kuzuhara S, Sobue G Research Committee on the Neurodegenerative Diseases of Japan. Age at onset influences on wide-ranged clinical features of sporadic amyotrophic lateral sclerosis. J Neurol Sci. 2009;276:163–9. doi: 10.1016/j.jns.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Rooney J, Byrne S, Heverin M, Corr B, Elamin M, Staines A, Goldacre B, Hardiman O. Survival Analysis of Irish Amyotrophic Lateral Sclerosis Patients Diagnosed from 1995-2010. PLoS One. 2013;8:e74733. doi: 10.1371/journal.pone.0074733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kataoka H, Kiriyama T, Kobayashi Y, Horikawa H, Ueno S. Clinical outcomes and serum uric acid levels in elderly patients with amyotrophic lateral sclerosis aged ≥ 70 years. Am J Neurodegener Dis. 2013;2:140–4. [PMC free article] [PubMed] [Google Scholar]
- 46.Mannino M, Cellura E, Grimaldi G, Volanti P, Piccoli F, La Bella V. Telephone follow-up for patients with amyotrophic lateral sclerosis. Eur J Neurol. 2007;14:79–84. doi: 10.1111/j.1468-1331.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 47.De Carvalho M, Costa J, Swash M. Clinical trials in ALS: a review of the role of clinical and neurophysiological measurements. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:202–12. doi: 10.1080/14660820510011997. [DOI] [PubMed] [Google Scholar]
- 48.Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25:709–14. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 49.Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 2012;124:339–52. doi: 10.1007/s00401-012-1022-4. [DOI] [PubMed] [Google Scholar]
- 50.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 51.Walczak P, Chen N, Hudson JE, Willing AE, Garbuzova-Davis SN, Song S, Sanberg PR, Sanchez-Ramos J, Bickford PC, Zigova T. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J Neurosci Res. 2004;76:244–54. doi: 10.1002/jnr.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover LE, Tajiri N, Weinbren NL, Ishikawa H, Shinozuka K, Kaneko Y, Watterson DM, Borlongan CV. A Step-up Approach for Cell Therapy in Stroke: Translational Hurdles of Bone Marrow-Derived StemCells. Translational Stroke Research. 2012;3:90–98. doi: 10.1007/s12975-011-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo X, Bu X, Li Z, Yan Z, Jiang J, Zhou Z. Comparison of autologous bone marrow mononuclear cells transplantation and mobilization by granulocyte colony-stimulating factor in experimental spinal injury. Int J Neurosci. 2012;122:723–33. doi: 10.3109/00207454.2012.716109. [DOI] [PubMed] [Google Scholar]
- 54.Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X, Wang J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222–9. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, Aronowski J, Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869–76. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenneman M, Sharma S, Harting M, Strong R, Cox CS Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–9. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, Gutierrez-Jimenez E, Zazueta-Fierro OE, Meza JA, Couret-Alcaraz P, Hernandez-Torre M. Stem cell transplantation in amyotrophic lateral sclerosis patients: methodological approach, safety, and feasibility. Cell Transplant. 2012;21:1899–907. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 60.Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas JE, Caro E, Gutierrez-Jimenez E, Segura JJ. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26–34. doi: 10.1080/14653240802644651. [DOI] [PubMed] [Google Scholar]
- 61.Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study. Neurol India. 2012;60:465–9. doi: 10.4103/0028-3886.103185. [DOI] [PubMed] [Google Scholar]
- 62.Blanquer M, Pérez Espejo MA, Iniesta F, Gómez Espuch J, Meca J, Villaverde R, Izura V, de Mingo P, Martínez-Lage J, Martínez S, Moraleda JM. [Bone marrow stem cell transplantation in amyotrophic lateral sclerosis: technical aspects and preliminary results from a clinical trial] . Methods Find Exp Clin Pharmacol. 2010;32(Suppl A):31–7. [PubMed] [Google Scholar]


