Abstract
HIV-1 protease inhibitors (PIs) exhibit different protein binding affinities and achieve variable plasma and tissue concentrations. Degree of plasma protein binding may impact central nervous system penetration. This cross-sectional study assessed cerebrospinal fluid (CSF) unbound PI concentrations, HIV-1 RNA, and neopterin levels in subjects receiving either ritonavir-boosted darunavir (DRV), 95% plasma protein bound, or atazanavir (ATV), 86% bound. Unbound PI trough concentrations were measured using rapid equilibrium dialysis and liquid chromatography/tandem mass spectrometry. Plasma and CSF HIV-1 RNA and neopterin were measured by Ampliprep/COBAS® Taqman® 2.0 assay (Roche) and enzyme-linked immunosorbent assay (ALPCO), respectively. CSF/plasma unbound drug concentration ratio was higher for ATV, 0.09 [95% confidence interval (CI) 0.06–0.12] than DRV, 0.04 (95%CI 0.03–0.06). Unbound CSF concentrations were lower than protein adjusted wild-type inhibitory concentration-50 (IC50) in all ATV and 1 DRV-treated subjects (P < 0.001). CSF HIV-1 RNA was detected in 2/15 ATV and 4/15 DRV subjects (P = 0.65). CSF neopterin levels were low and similar between arms. ATV relative to DRV had higher CSF/plasma unbound drug ratio. Low CSF HIV-1 RNA and neopterin suggest that both regimens resulted in CSF virologic suppression and controlled inflammation.
Keywords: HIV/AIDS, central nervous system, clinical pharmacology
Despite highly active antiretroviral therapy (HAART), human immunodeficiency virus-1 (HIV-1) continues to elude eradication. Even in the setting of plasma virologic suppression, HIV-1 RNA can be found in peripheral blood mononuclear cells (PBMCs), genital secretions, gastrointestinal lymphoid tissue, and cerebrospinal fluid (CSF).1,2 Limited penetration of antiretrovirals (ARVs) into these sites may play a role in viral persistence.2,3
HIV-1 invades the central nervous system (CNS) early after infection via macrophages, monocytes, and dendritic cells.4 Over time, HIV-associated neurocognitive disorders (HAND) can develop,5 contributing significantly to morbidity6 and early mortality.7 While severity of HAND has decreased, prevalence remains unchanged since the pre-HAART era: 36.2–44.8%,6 prompting a concern for suboptimal CNS control of HIV. This is supported by reports of elevated neopterin, an inflammatory biomarker, and measurable HIV RNA in the CSF of patients on long term HAART,8,9 both predictive markers for development of HIV-associated dementia.10,11 Some studies suggest that limited CNS penetration by ARVs is associated with HAND.12,13
Molecular size, lipophilicity, affinity for efflux pump transporters, and degree of plasma protein binding are factors that impact drug CNS penetration.14 Lipophilic drugs, including HIV protease inhibitors (PIs), are highly bound to plasma protein, predominantly alpha1-acid glycoprotein (AAG). The plasma unbound (free) drug component is considered pharmacologically active and readily crosses the blood–brain and blood–CSF barriers15,16 therefore highly bound drugs such as PIs may have limited CNS penetration. Two PIs, atazanavir (ATV) and darunavir (DRV), are recommended by the Department of Health and Human Services (DHHS) as part of first-line once daily regimens for ARV-naïve patients when boosted with ritonavir (RTV) and given with a backbone of tenofovir disoproxil fumarate/emtricitabine (TDF/FTC).17 Both PIs have characteristics that could impede tissue and CNS penetration, including large molecular size and affinity for efflux pump transporters. However, they differ in their affinity for plasma AAG: ATV is 86% plasma protein bound in vitro whereas DRV is 95% bound.18,19 The degree to which they successfully penetrate the CNS remains unclear. Best et al showed low or undetectable CSF ATV levels in nearly 25% of study participants.20 but measured only total drug concentrations with a lower quantitation limit of 5 ng/mL. In contrast, 3 prior studies have shown that DRV can attain drug concentrations in the CSF exceeding IC50 21–23 ; however, the single study measuring unbound drug concentrations22 examined participants receiving twice daily DRV rather than the DHHS recommended once daily dosing schedule for patients without history of DRV resistance.17 Additionally, measuring random total drug concentrations rather than troughs limited all previous studies.
To address these limitations, we evaluated the CSF penetration of once daily DRV relative to ATV, both boosted with RTV and administered with the same background TDF/FTC regimen. We hypothesized that ATV, a PI with lower degree of plasma protein binding, would achieve a higher CSF/plasma unbound drug concentration ratio than DRV. In addition, we compared unbound CSF PI concentrations to their drug-specific IC50 and assessed relationships with CSF HIV-1 RNA and neopterin.
Methods
Study Design
A cross-sectional study of HIV-1-infected patients was conducted at the Grady Health System Infectious Diseases Program in Atlanta, Georgia from May 2012 through December 2012. The Emory University Institutional Review Board (IRB) and Grady Research Oversight Committee approved this study. Written informed consent was obtained from all study participants.
Eligibility Criteria
HIV-1 infected adults (≥18 years) receiving a regimen of once daily TDF/FTC (300/200 mg) plus RTV (100 mg)-boosted ATV (300 mg) or DRV (800 mg) were eligible to participate if they had documented virologic suppression (plasma HIV-1 RNA below the assay limit of detection) for ≥6 consecutive months leading up to the study (with ≥1 undetectable plasma viral load in the previous 90 days). Exclusion criteria included pregnancy, abnormal liver function testing (aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase >5 times upper limit of normal), creatinine clearance below 50 mL/min (Cockcroft-Gault equation), coagulopathy (International Standardized Ratio >1.4 or prothrombin time >14 seconds), recent neurologic abnormalities or CNS infections (≤6 months prior), or currently taking medications with known PI interactions. Subjects meeting these criteria were identified through clinic and pharmacy records.
Timing and Collection of Specimens
Participants’ demographic characteristics, HIV clinical history, concurrent medications, and comorbidities were collected by review of both electronic medical records and patient paper charts. Study visits were scheduled 24 hours from time of last patient-reported ARV dose (approximate plasma trough time) for paired plasma and CSF collection. Blood was collected from brachial venipuncture into 10 mL cell preparation tubes (CPT) with sodium citrate and spun at 2300 rpm for 20 minutes. The plasma fraction was collected and centrifuged again at 2300 rpm for 10 minutes to eliminate erythrocytes. CSF was collected via lumbar puncture using sterile technique. Plasma and CSF 1 mL aliquots were stored at −80°Celsius until analysis.
Plasma and CSF Drug Concentration Measurements
Unbound (free) PI values were achieved using rapid equilibrium dialysis (RED) to obtain unbound fractions24 and liquid chromatography/tandem mass spectrometry (LC-MS/MS) for quantitation (AB Sciex 6500 triple quadrupole mass spectrometer Foster City, CA, USA and Eksigent Micro HPLC, Dublin, CA, USA), as previously described.25 Plasma and CSF samples were dialyzed into phosphate buffered saline at 37°C with shaking for 5 hours in RED chambers (Thermo Scientific Pierce, Rockford, IL, USA). After incubation, aliquots of the resultant dialysates were spiked with internal standards: DRV for the ATV participants and ATV for the DRV samples. Drug concentrations were then quantified via LC-MS/MS by positive ion multiple reaction monitoring (MRM) as outlined in Supplemental Table S1, with a detectable range of 0.1–1000 ng/mL. Drugs were separated with a Halo C18 column (50 × 0.5 mm2, 2.7 μm particles) using a linear gradient from 50% to 99% acetonitrile with 0.1% formic acid over a 5 minute acquisition time (Supplemental Figure S1).
Plasma and CSF HIV-1 RNA Quantitation
Plasma and CSF HIV-1 RNA were measured using the COBAS® Ampliprep/COBAS® Taqman® version 2.0 HIV-1 assay (Roche Molecular Systems, Inc., Pleasanton, CA)26 which detects 20–10,000,000 copies/mL in plasma, with a sensitivity of 98.3% and specificity of 99.4%. Commercial reverse transcriptase PCR assays have been shown to successfully quantitate HIV-1 RNA in other biological compartments, including CSF.27 Because dilution of samples was performed to achieve proper volume necessary for HIV-1 RNA quantification, the lower limit of detection for CSF and plasma samples in this study was 40 copies/mL.
Plasma and CSF Neopterin Quantitation
Neopterin, a biomarker for CNS immune activation, is primarily released from monocytes and macrophages9 Plasma and CSF neopterin levels were determined using the enzyme immunoassay from ALPCO (Salem, NH, USA). All procedures were performed according to manufacturer's specifications. The quantitative range was 2.00–250.00 nM with intra-assay percent coefficients of variation of 2.13% at 7.98 nM and 10.02% at 83.97 nM. The inter-assay percent coefficients of variation were 5.80% at 8.29 nM and 3.79% at 80.55 nM.
Inhibitory Concentration-50 (IC50) Determination
The mean protein-unbound wild-type IC50 used for ATV and DRV was 1.7 and 0.4 ng/mL, respectively. This was previously derived from standardized in vitro phenotypic drug susceptibility measurements of wild-type clinical isolates tested between 2009 and 2010, compiled in the Monogram Biosciences database.28 The isolates were defined as wild type if previously described29 drug-selected mutations in protease and reverse transcriptase were not detected.
Sample Size Calculation
The primary outcome of interest was unbound CSF/plasma drug trough concentration ratios for ATV and DRV. As preliminary data on unbound CSF trough concentrations were unavailable, the study was powered to estimate the correlation coefficient between free drug CSF and plasma concentration separately for each group. A sample size of 15 participants for each arm achieves 88% statistical power to detect a difference of 0.70 between the null hypothesis correlation of 0 and the alternative hypothesis correlation of 0.70 using a two-sided hypothesis test with a statistical significance level of 0.05.
Statistical Analyses
Demographic and clinical characteristics were summarized by descriptive statistics. Differences between ATV and DRV arms were examined by Chi-square or Fisher's exact test for categorical variables or Wilcoxon rank sum test for continuous variables.
Plasma and CSF unbound drug concentrations were log transformed, and geometric means and 95% confidence intervals (CI) were reported for each PI in ng/mL. Geometric mean ratios for the CSF/plasma unbound drug concentrations were calculated and compared for ATV and DRV. If 95% confidence intervals of geometric means or geometric mean ratios did not overlap between arms, this was considered a statistically significant difference.
To assess the relationship between CSF unbound drug concentration and detection of CSF HIV-1 RNA, a point biserial correlation test was performed for the ATV and DRV arms. Additionally, Spearman rank correlation coefficients were used to separately evaluate associations between CSF unbound PI concentrations, plasma unbound PI concentrations, and CSF neopterin levels. A Chi-square test was performed to compare the proportions of participants in each arm achieving CSF unbound PI concentrations that exceeded the drug-specific IC50.
All analyses were performed using SAS® 9.3 (SAS Institute, Cary, NC, USA) software, with an alpha level of 0.05.
Results
Demographic and Clinical Characteristics
Three hundred thirty-six patients were identified as receiving ATV-based regimens; 300 did not meet eligibility criteria, 21 declined to participate, and 15 were enrolled. Two hundred sixty-five individuals were receiving DRV-based regimens: 242 did not meet eligibility criteria, 8 declined, and 15 enrolled. Most common reason for exclusion was having detectable plasma HIV-1 RNA within the last 6 months.
Table 1 summarizes demographic and clinical characteristics of the study population. Of the 30 patients enrolled, 23/30 (76.7%) were male, 26/30 (86.7%) were black, and median age was 46.9 years (interquartile range, IQR, 37.9–51.9). There were 5 females in the ATV group compared to 2 in the DRV group, however, this difference was not statistically significant, p = 0.4. Other characteristics, including age, racial composition, most recent CD4 count, and duration of undetectable plasma HIV-1 RNA, were similar between arms.
Table 1.
Participant Demographics
| Atazanavir (n = 15), n (%) or Median (IQR) | Darunavir (n = 15), n (%) or Median (IQR) | All (n=30), n (%) or Median (IQR) | |
|---|---|---|---|
| Male sex | 10 (66.7) | 13 (86.7) | 23 (76.7) |
| Age in years | 44.5 (31.8–49.8) | 49.3 (40.7–55.3) | 46.9 (37.9–51.9) |
| Race | |||
| Black | 13 (86.7) | 13 (86.7) | 26 (86.7) |
| White | 2 (13.3) | 2 (13.3) | 4 (13.3) |
| HIV risk factors | |||
| MSM | 5 (33.3) | 7 (46.7) | 12 (40) |
| Heterosexual sex | 8 (53.3) | 7 (46.7) | 15(50) |
| MSM and heterosexual sex | 1 (6.7) | 1 (6.7) | 2 (6.7) |
| Unknown | 1 (6.7) | 0 (0) | 1 (3.3) |
| Nadir CD4 count in cells/μL* | 117 (44–173) | 13 (6–108) | 62 (10–128) |
| CD4 within 90 days in cells/μL | 318 (222–484) | 288 (136–409) | 307 (218–450) |
| Months on current ARV regimen* | 34.0 (14.7–40.4) | 18.8 (13.8–23.4) | 21.8 (14.1–34.0) |
| Missed doses of ARVs in 30 days | 1 (0–1) | 0 (0–1) | 0 (0–1) |
| Months with undetectable plasma HIV-1 | 20.0 (9.2–29.6) | 19.1 (8.0–23.9) | 19.5 (9.2–26.1) |
| Creatinine clearance (mL/minute) | 101 (67–119) | 100 (90–122) | 101 (80–119) |
| Alkaline phosphatase (U/mL)* | 79 (68–99) | 61 (51–70) | 69 (54–85) |
| Total bilirubin (mg/dL)* | 1.2 (0.9–2.6) | 0.5 (0.4–0.6) | 0.7 (0.5–1.4) |
| Hepatitis B or C coinfection | 1 (6.7) | 1 (6.7) | 2 (6.7) |
| CNS infection >6 months prior | 2 (13.3) | 2 (13.3) | 4 (13.3) |
Variables compared between arms with Wilcoxon rank sum test (continuous) or Chi-square or Fisher's exact test (categorical).
IQR, interquartile range, 25th –75th quartile; MSM, men who have sex with men; ARV, anti-retroviral; CNS, central nervous system.
P < 0.05.
Plasma and CSF PI Concentrations
Timing of plasma and CSF sampling relative to last reported ARV dose approached plasma trough times (median 23.8 hours, IQR 23.5–24.3, Supplement Table S1) for both arms. Blood and CSF specimens were collected a median of 35 minutes apart (IQR 30– 45 minutes; Supplement Table S1).
There were 2 participants in the ATV arm and 1 participant in the DRV arm with nearly undetectable total plasma drug concentrations (<50 ng/mL), consistent with medication nonadherence, therefore these subjects were removed from drug trough concentration analyses. All other participants had total and unbound CSF and plasma PI concentrations above the assay's limit of quantitation. For ATV (n = 13), mean total plasma and CSF concen trations were 295.8 ng/mL (95%CI 204.2–428.3) and 8.7 ng/mL (95%CI 7.2–10.5) while mean unbound plasma and CSF concentrations were 7.1 ng/mL (95% CI 5.1–10.0) and 0.6 ng/mL (95%CI 0.4–0.9), respectively. For DRV (n = 14), mean total plasma and CSF concentrations were 1907.0 ng/mL (95%CI 1461.0– 2491.2) and 8.5 ng/mL (95%CI 6.0–12.1) while mean unbound plasma and CSF concentrations were 54.4 ng/ mL (95%CI 42.1–70.4) and 2.3 ng/mL (95%CI 1.4–3.7), respectively (Table 2). Plasma and CSF unbound drug concentrations were positively correlated for both ATV and DRV: Spearman rank correlation coefficient r = 0.77, p < 0.01 and r = 0.72, p < 0.01, respectively (Figure 1)..
Table 2.
Total and Unbound Atazanavir and Darunavir Concentrations in Plasma and CSF
| Atazanavir (n = 13)* |
Darunavir (n = 14)* |
|||
|---|---|---|---|---|
| Geometric Mean or Ratio | 95%CI | Geometric Mean or Ratio | 95%CI | |
| Total drug concentrations | ||||
| Plasma (ng/mL) | 295.8 | 204.2–428.3 | 1907.0 | 1461.0–2491.2 |
| CSF (ng/mL) | 8.7 | 7.2–10.5 | 8.5 | 6.0–12.1 |
| CSF/plasma ratio | 0.03 | 0.02–0.04 | 0.005 | 0.004–0.006 |
| Unbound drug concentrations | ||||
| Plasma (ng/mL) | 7.1 | 5.1–10.0 | 54.4 | 42.1–70.4 |
| CSF (ng/mL) | 0.6 | 0.4–0.9 | 2.3 | 1.4–3.7 |
| CSF/plasma ratio | 0.09 | 0.06–0.1 | 0.04 | 0.03–0.06 |
CSF, cerebrospinal fluid; CI, confidence interval.
Participants with total plasma drug concentrations <50 ng/mL were excluded from analyses due to medication nonadherence, resulting in n = 13 for ATV and n = 14 for DRV.
Figure 1.
Relationships between plasma and cerebrospinal fluid (CSF) unbound drug concentrations for atazanavir (ATV) and darunavir (DRV). (a) ATV (blue diamonds), Spearman rank correlation coefficient r = 0.77, P < 0.01, and (b) DRV (red diamonds), Spearman rank correlation coefficient r = 0.72, P < 0.01.
Unbound CSF/plasma geometric mean ratio was higher for ATV compared to DRV, 0.09 (95%CI 0.06–0.12) and 0.04 (95%CI 0.03–0.06), respectively (Table 2). Despite a higher CSF/plasma concentration ratio for ATV, no participant in the ATV arm achieved unbound CSF ATV levels that exceeded the wild-type IC50 compared to 14/15 participants (93.3%) in the DRV arm, p < 0.001. In fact, the mean ATV CSF concentration/IC50 ratio was 0.4 (standard error 0.1) whereas mean DRV CSF concentration/IC50 ratio was 8.0 (standard error 2.1), Figure 2.
Figure 2.
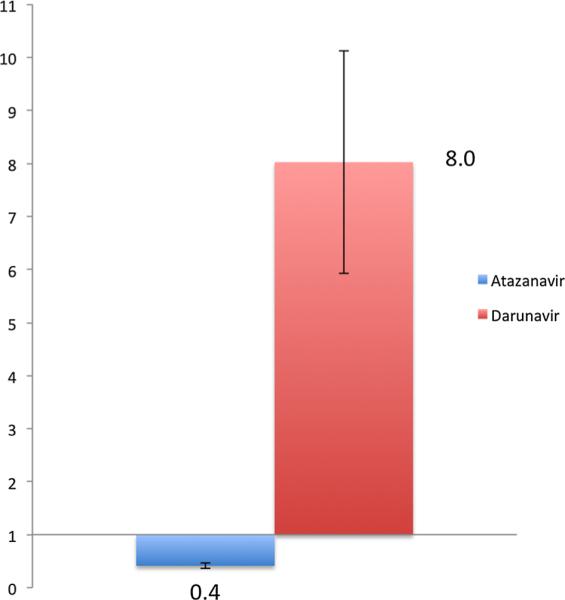
Mean cerebrospinal fluid (CSF) unbound trough concentration, standardized for each drug's wild-type protein-unbound IC50 (ATV = 1.7 ng/mL and DRV = 0.4 ng/mL) between ATV (n = 13) and DRV (n = 14). Mean unbound ATV CSF concentration was less than half of the IC50—ATV CSF concentration/IC50 ratio was 0.4 (standard error 0.1). In contrast, mean DRV CSF concentration was 8 times greater than IC50—mean DRV CSF concentration/IC50 ratio was 8.0 (standard error 2.1).
Plasma Protein Binding
Proteins, including AAG and albumin, were not directly quantified in either plasma or CSF. Proportion of drug bound to protein in plasma was calculated indirectly from the unbound fraction. Interestingly, proportion of protein-bound drug for ATV was a median (IQR) of 97.7% (96.7– 98.2%) in plasma, higher than the 86% reported in the literature.18 In contrast, median (IQR) proportion of DRV bound to protein in plasma was 96.5% (96.5–97.9%), close to the previously reported literature value.19
Plasma and CSF HIV-1 RNA
Despite documentation of undetectable plasma viral load for ≥6 months, 10 of 15 subjects in the ATV arm and 4 of 15 in the DRV arm had detectable plasma HIV-1 RNA on the day of their study visit, p = 0.03 (Table 3). In patients with detectable plasma viral load at study entry, median HIV-1 RNA was 225 copies/mL (IQR 140–310) for the ATV arm and 200 copies/mL (IQR 95–3520) for the DRV arm. Except for two outliers (920 copies/mL in the ATV arm and 6790 copies/mL in the DRV arm), all other plasma HIV-1 RNA viral loads were <400 copies/mL. Neither of these viremic outliers had low plasma PI concentrations: there was no association seen between plasma HIV-1 RNA and plasma unbound PI concentrations for either ATV or DRV (Spearman rank correlation r = 0.13, p = 0.6 and r = 0.13, p = 0.7, respectively).
Table 3.
HIV-1 RNA and Neopterin Levels in CSF and Plasma
| Atazanavir (n = 15) | Darunavir (n = 15) | P-Value Comparing Arms | |
|---|---|---|---|
| Detectable CSF HIV-1 RNAa, n (%) | 2 (13.3) | 4 (26.7) | 0.7 |
| Median copies/mL (IQR) | 220 (120–320) | 50 (45–55) | |
| Detectable plasma HIV-1 RNA, n (%) | 10 (66.7) | 4 (26.7) | 0.03 |
| Median copies/mL (IQR) | 225 (140–310) | 200 (95–3520) | |
| CSF neopterin in ng/mL, mean (SD) | 1.8 (0.7) | 1.7 (0.4) | 0.9 |
| Plasma neopterin in ng/mL, mean (SD) | 2.0 (0.9) | 1.7 (0.4) | 0.3 |
Continuous variables compared between arms using two-sided two-sample t-test.
Categorical variables compared between arms using Chi-square test or Fisher's exact test.
CSF, cerebrospinal fluid; SD, standard deviation.
Detectable HIV-1 RNA defined as ≥40 copies/mL
Of the 30 study participants, 6 (20%) had detectable CSF HIV-1 RNA (≥40 copies/mL): 2 in the ATV arm and 4 in the DRV arm (p = 0.7). Of the subjects with detectable CSF HIV RNA, those in the ATV arm had viral loads of 120 and 320 copies/mL whereas the DRV participants had lower viral load copies, ranging from 40 to 60 copies/mL. The 2 participants with detectable CSF HIV-1 RNA in the ATV arm also had detectable plasma HIV-1 RNA whereas only 1 of 4 subjects with detectable plasma CSF HIV-1 RNA in the DRV arm had detectable plasma HIV-1 RNA. Point biserial Pearson correlation tests showed no association between detection of CSF HIV-1 RNA and PI unbound CSF concentrations for ATV (r = 0.26, p = 0.3) and weak association for DRV (r = 0.54, p = 0.04).
Plasma and CSF Neopterin
Mean plasma neopterin was comparable between the ATV and DRV arms (2.0, SD 0.9 ng/mL and 1.7, SD 0.4 ng/mL), p = 0.3 (Table 3). CSF neopterin levels were also very similar between the ATV and DRV arms, 1.8 ng/mL (SD 0.7) and 1.7 ng/mL (SD 0.4), respectively. No association was seen between unbound CSF PI concentrations and CSF neopterin for either arm (Spearman rank correlation, ATV, r = 0.13, p = 0.6 and DRV, r = 0.29, p = 0.3).
Discussion
Both total (bound + unbound) and unbound CSF and plasma drug trough concentrations of ATV and DRV were determined in a group of HIV-1 infected adults receiving identical doses of TDF/FTC and RTV boosting. In contrast, prior literature has predominantly reported only random total PI concentrations in populations receiving different backbone ARVs, causing difficulty with interpretation of results.
While the mean total plasma trough concentration for ATV in this study was lower than previously reported,30 inter-patient variability of plasma ATV trough levels is known to be high.31 Our total ATV CSF concentrations were low and similar to previous findings by Best et al.20 With regard to unbound ATV plasma concentrations, one prior study found a wide range of random levels: 8.1– 291.7 ng/mL32; our findings show a mean closer to the lower end of that range, which is likely representative of samples drawn at plasma trough times. Unbound ATV CSF trough concentrations have not been previously reported in the literature to our knowledge but were found to be well below the wild-type IC50 of 1.7 ng/mL, even after removing two participants with suspected medication nonadherence. Total plasma and CSF DRV trough concentrations in this study were consistent with prior reports in the literature for once daily DRV dosing.21 However, both mean unbound plasma and CSF DRV levels measured were considerably lower than data reported by Croteau et al: the median unbound DRV concentration in Croteau's study was 538 ng/mL for plasma (IQR 369–968) and 50.2 ng/mL (IQR 35.0–72.6) for CSF.22 This is most likely explained by the difference in DRV dosing and post-dose sampling times between the two studies: all participants in the Croteau study were receiving DRV 600 mg twice daily compared to 800 mg daily dosing in our study. Furthermore, both CSF and plasma in the DRV arm of this study were sampled 24 hours (±1 hour) post-ARV dose compared to median of 6.7 hours (IQR 3.4–12.4) for plasma and 7.2 hours (IQR 3.8–12.9) for CSF in the Croteau study.22 As reported in prior studies for twice daily DRV, our results show that participants receiving once daily dosing also attain unbound CSF DRV concentrations that exceed the wild-type IC50.
For both PIs, unbound CSF concentrations were positively correlated to unbound plasma drug concentrations, consistent with prior findings in the literature20,22; this suggests that unbound plasma drug concentrations could be a useful and less invasive tool to predict CSF drug concentrations.
Proportion of ATV bound to plasma protein (97%) was higher than previously reported from in vitro studies (86%). There are several possible explanations for this finding. To begin, our study was cross-sectional and degree of plasma protein binding was calculated only at trough times, which may not accurately represent in vivo binding throughout the entire dosing interval. Boffito et al showed that lopinavir (LPV) protein binding in vivo changed significantly over the course of the 12 hour dosing interval: the unbound percentage of LPV was found to be statistically significantly higher after 2 hours than baseline (time 0 or trough).33 Therefore, concentration-dependent protein binding could explain our findings for ATV. Additionally, elevated AAG in the ATV arm may have played a role, as a prior study examining AAG concentrations in patients taking ATV showed considerable inter-patient variability.34 Elevated AAG decreased the apparent clearance of ATV in that study, suggesting that this could decrease the protein-unbound ATV fraction. To explore this in greater detail, longitudinal studies evaluating relationships between degree of plasma protein binding, AAG concentrations, and CSF unbound concentrations measured at multiple time points over full dosing intervals are necessary.
Our findings show that the unbound CSF/plasma drug trough concentration ratio was higher in patients receiving a regimen of once daily ATV than once daily DRV. However, given that participants on the ATV regimen attained much lower absolute CSF and plasma concentrations relative to IC50 than those on DRV, it is unlikely that the difference in CSF/plasma ratios indicates superior clinical effect but rather calls attention to the need for further exploration of plasma protein binding as a factor predicting relative penetration of a drug's unbound fraction into the CSF.
While unbound CSF ATV concentrations were lower than DRV concentrations relative to drug-specific IC50 in our study, detectable CSF viremia was uncommon in both arms. There are several possible explanations for this discrepancy. To start, the other backbone ARVs (TDF and FTC) may be partly responsible for viral control, compensating for ATV's low CSF levels. For example, FTC is measurable in the CSF and has several drug characteristics, including low molecular weight and low degree of plasma protein binding, that may facilitate blood–brain barrier and brain–CSF penetration.35 Also, CSF drug concentrations may not accurately represent brain parenchyma penetration, since drugs may not distribute homogenously over the brain due to metabolism, active transport mechanisms, and local changes in blood–brain barrier functionality secondary to HIV infection.36 Because detectable CSF HIV-1 RNA was such a rare finding, studies with larger sample sizes would be necessary to determine whether a true difference in frequency or severity of CSF HIV viremia exists between patients receiving ATV or DRV-based HAART.
No correlation was seen between CSF PI unbound concentrations and CSF neopterin in this study, consistent with findings from Hagberg et al.9 However, Hagberg et al reported mean CSF neopterin levels of 2.7 ng/mL (SD 2.6) for virologically suppressed HIV infected patients on HAART, slightly higher than our findings in both study arms. Regardless, our median CSF neopterin levels for both DRV and ATV-treated participants were still higher than what Hagberg et al previously described for HIV seronegative controls (mean 1.3 ng/mL, SD 0.6), indicating some persistent immune activation despite long-term therapy. It remains unclear whether this inflammation is driven by continued HIV replication in the CNS compartment or by chronic indolent injury.37
Limitations of this study include its small sample size and cross-sectional study design, which may result in unmeasured differences between the ATV and DRV groups. In addition, timing of last ARV dose and adherence to medications was subject to recall bias, as it was primarily determined by patient report. Although 14/30 study participants (10/15 in ATV arm and 4/15 in DRV arm) had detectable plasma HIV-1 RNA at their study visit despite having recent documented undetectable viral loads at clinic appointments, no correlation was seen between unbound drug plasma concentrations and plasma HIV-1 RNA. Because plasma HIV-1 RNA was measured in clinical specimens using Abbott RealTime HIV-1 Assay (Abbott RT) and in research specimens using Roche Diagnostics COBAS® Ampliprep/COBAS® Taqman® version 2.0 HIV-1 assay (Roche CAP/CTM-48 V2), varying sensitivity and specificity among these HIV assays is a more likely explanation for detectable plasma viremia than ARV non-adherence. Van Hensburg et al showed that the Abbott RT assay underestimated HIV viral loads by 0.26–0.30 log10 copies/mL while the Roche CAP/CTM-48 V2 overestimated by 0.17–0.31 log10 copies/mL.38 Finally, CSF measurements of drug concentrations were used as a proxy for the CNS and may not accurately represent penetration into the brain parenchyma; invasive techniques such as intracerebral micro-dialysis may give us a better picture of drug pharmacokinetics in the brain tissue36 but cannot be considered an ethical alternative in clinically stable patients.
In conclusion, results of this study prompt further investigation of plasma protein binding as a factor that could predict the degree to which PIs penetrate CSF from plasma, a potentially valuable tool in the development of future CNS drug therapeutics. In addition, prior studies have shown that plasma protein binding impacts drug accumulation in intracellular (PBMC),39 rectal, and seminal tissue,40 suggesting that this factor may be useful in the development of predictive models for compartmental drug penetration and the creation of novel pharmaceutical agents targeting elusive sites.
Supplementary Material
Acknowledgments
We are grateful to the faculty, staff, and research participants at the Ponce de Leon Center for their time and dedication to this project.
This work was supported by Bristol-Myers Squibb with an independent investigator initiated grant. I.O. and C.D. have received funding from Bristol-Myers Squibb. J.L. has received grants and administrative support from Gilead Sciences.
Funding
This study was supported by Bristol-Myers Squibb, the Emory CFAR (P30 AI050409), and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. Author contributions were as follows: C.D. and I.O. were lead study investigators, designed and wrote the study protocol, acquired/analyzed/interpreted data, and wrote and critically reviewed the article. S.P. developed and performed the assays for protease inhibitor quantification and wrote and critically reviewed the article. V.M., J.L., W.A., and A.S. were study investigators and wrote and critically reviewed the manuscript. K.E. conducted statistical analyses, interpreted data, and wrote and critically reviewed the article. A.V. contributed to data interpretation and wrote and critically reviewed the article. R.A and E.A. assisted in analyzing and interpreting the pharmacologic data and wrote and critically reviewed the manuscript.
Declaration of Conflicting Interests
S.P., V.M., W.A., R.A., A.S., K.E, E.A, and A.V. have no conflicts of interest to disclose.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- 1.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37(3):377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011;270(6):550–560. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 3.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS. 2013;8(3):190–195. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour VG, Shiramizu BT, Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol. 2010;87(4):621–626. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neuro-cognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis RJ, Deutsch R, Heaton RK, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- 8.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis. 1996;174(2):294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- 11.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175(4):963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 12.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liner KJ, II, Hall CD, Robertson KR. Effects of antiretroviral therapy on cognitive impairment. Curr HIV/AIDS Rep. 2008;5(2):64–71. doi: 10.1007/s11904-008-0011-7. [DOI] [PubMed] [Google Scholar]
- 14.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boffito M, Back DJ, Blaschke TF, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses. 2003;19(9):825–835. doi: 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- 16.Lindup WE, Orme MC. Clinical pharmacology: plasma protein binding of drugs. Br Med J (Clin Res Ed) 1981;282(6259):212–214. doi: 10.1136/bmj.282.6259.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panel on antiretroviral guidelines for adults and adolescents . Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; [January 10, 2014]. http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- 18.Le Tiec C, Barrail A, Goujard C, Taburet AM. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin Pharmacokinet. 2005;44(10):1035–1050. doi: 10.2165/00003088-200544100-00003. [DOI] [PubMed] [Google Scholar]
- 19.Robertson J, Feinberg J. Darunavir: a nonpeptidic protease inhibitor for antiretroviral-naive and treatment-experienced adults with HIV infection. Expert Opin Pharmacother. 2012;13(9):1363–1375. doi: 10.1517/14656566.2012.681776. [DOI] [PubMed] [Google Scholar]
- 20.Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–87. doi: 10.1097/QAD.0b013e328317a702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once-daily dosing and pharmacogenetics. AIDS. 2012;26(12):1529–1533. doi: 10.1097/QAD.0b013e3283553619. [DOI] [PubMed] [Google Scholar]
- 22.Croteau D, Rossi SS, Best BM, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90% inhibitory concentration. J Antimicrob Chemother. 2013;68(3):684–689. doi: 10.1093/jac/dks441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz A, Izadkhashti A, Price RW, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25(4):457–461. doi: 10.1089/aid.2008.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters NJ, Jones R, Williams G, Sohal B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J Pharm Sci. 2008;97(10):4586–4595. doi: 10.1002/jps.21317. [DOI] [PubMed] [Google Scholar]
- 25.Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B. 2010;878(19):1455–1465. doi: 10.1016/j.jchromb.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Damond F, Avettand-Fenoel V, Collin G, et al. Evaluation of an upgraded version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test for HIV-1 load quantification. J Clin Microbiol. 2010;48(4):1413–1416. doi: 10.1128/JCM.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamat A, Ravi V, Desai A, et al. Quantitation of HIV-1 RNA levels in plasma and CSF of asymptomatic HIV-1 infected patients from South India using a TaqMan real time PCR assay. J Clin Virol. 2007;39(1):9–15. doi: 10.1016/j.jcv.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother. 2012;56(11):5938–5945. doi: 10.1128/AAC.00691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48(2):437–443. doi: 10.1128/AAC.48.2.437-443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squibb B-M, editor. Atazanavir [package insert] Princeton, NJ: 2013. [January 10, 2014]. www.reyataz.com. [Google Scholar]
- 31.Crutchley RD, Ma Q, Sulaiman A, Hochreitter J, Morse GD. Within-patient atazanavir trough concentration monitoring in HIV-1-infected patients. J Pharm Pract. 2011;24(2):216–222. doi: 10.1177/0897190010380923. [DOI] [PubMed] [Google Scholar]
- 32.Fayet A, Beguin A, de Tejada BM, et al. Determination of unbound antiretroviral drug concentrations by a modified ultrafiltration method reveals high variability in the free fraction. Ther Drug Monit. 2008;30(4):511–522. doi: 10.1097/FTD.0b013e3181817318. [DOI] [PubMed] [Google Scholar]
- 33.Boffito M, Hoggard PG, Lindup WE, et al. Lopinavir protein binding in vivo through the 12-hour dosing interval. Ther Drug Monit. 2004;26(1):35–39. doi: 10.1097/00007691-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Barrail-Tran A, Mentre F, Cosson C, et al. Influence of alpha-1 glycoprotein acid concentrations and variants on atazanavir pharmacokinetics in HIV-infected patients included in the ANRS 107 trial. Antimicrob Agents Chemother. 2010;54(2):614–619. doi: 10.1128/AAC.00797-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calcagno A, Bonora S, Simiele M, et al. Tenofovir and emtricitabine cerebrospinal fluid-to-plasma ratios correlate to the extent of blood-brainbarrier damage. AIDS. 2011;25(11):1437–1439. doi: 10.1097/QAD.0b013e3283489cb1. [DOI] [PubMed] [Google Scholar]
- 36.de Lange EC, Danhof M. Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41(10):691–703. doi: 10.2165/00003088-200241100-00001. [DOI] [PubMed] [Google Scholar]
- 37.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well-controlled plasma viral load. AIDS. 2012 doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rensburg EJ, Tait K, Watt A, Schall R. Comparative evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 version 2 test using the TaqMan 48 analyzer and the Abbott RealTime HIV-1 assay. J Clin Microbiol. 2011;49(1):377–379. doi: 10.1128/JCM.01285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones K, Hoggard PG, Khoo S, Maher B, Back DJ. Effect of alpha1-acid glycoprotein on the intracellular accumulation of the HIV protease inhibitors saquinavir, ritonavir and indinavir in vitro. Br J Clin Pharmacol. 2001;51(1):99–102. doi: 10.1046/j.0306-5251.2001.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown KC, Patterson KB, Jennings SH, et al. Single- and multiple-dose pharmacokinetics of darunavir plus ritonavir and etravirine in semen and rectal tissue of HIV-negative men. J Acquir Immune Defic Syndr. 2012;61(2):138–144. doi: 10.1097/QAI.0b013e31825cb645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



