Abstract
Combined teriparatide and denosumab increases spine and hip bone mineral density more than either drug alone. The effect of this combination on skeletal microstructure and microarchitecture, however, is unknown. Because skeletal microstructure and microarchitecture are important components of skeletal integrity, we performed high-resolution peripheral QCT assessments at the distal tibia and radius in postmenopausal osteoporotic women randomized to receive teriparatide 20-µg daily (n=31), denosumab 60-mg every 6 months (n=33), or both (n=30) for 12 months. In the teriparatide group, total volumetric BMD (vBMD) did not change at either anatomic site but increased in both other groups at both sites. The increase in vBMD at the tibia was greater in the combination group (3.1±2.2%) than both the denosumab (2.2±1.9%) and teriparatide groups (−0.3±1.9%) (p<0.02 for both comparisons). Cortical vBMD decreased by 1.6±1.9% at the tibia and by 0.9±2.8% at the radius in the teriparatide group whereas it increased in both other groups at both sites. Tibia cortical vBMD increased more in the combination group (1.5±1.5%) than both monotherapy groups (p<0.04 for both comparisons). Cortical thickness did not change in the teriparatide group, but increased in both other groups. The increase in cortical thickness at the tibia was greater in the combination group (5.4±3.9%) than both monotherapy groups (p<0.01 for both comparisons). In the teriparatide group, radial cortical porosity increased by 20.9±37.6% and by 5.6±9.9% at the tibia but did not change in the other two groups. Bone stiffness and failure load, as estimated by finite element analysis, did not change in the teriparatide group but increased in the other two groups at both sites. Together, these findings suggest that the use of denosumab and teriparatide in combination improves HR-pQCT measures of bone quality more than either drug alone and may be of significant clinical benefit in the treatment of postmenopausal osteoporosis.
Keywords: osteoporosis, high-resolution peripheral quantitative computed tomography, microarchitecture, denosumab, teriparatide
Introduction
While therapeutic options for osteoporosis have expanded greatly over the past few decades, no single agent is able to restore skeletal integrity in most patients with advanced disease. Despite initial enthusiasm for an approach that would target both bone resorption and formation, studies investigating the efficacy of combined bisphosphonates and parathyroid hormone or teriparatide generally demonstrated minimal or no greater increase in bone mass compared to monotherapy.(1–4) In the Denosumab and Teriparatide Administration (DATA) study, we demonstrated that 12-months of combined denosumab and teriparatide therapy increases areal bone mineral density at the spine and hip as measured by dual X-ray absorptiometry (DXA) more than either treatment and more than has been reported with any currently approved treatment for postmenopausal osteoporosis.(5) The effects of combined denosumab and teriparatide on compartment-specific changes in bone density, changes in skeletal microarchitecture, or changes in bone strength, however, are unknown.
Until recently, treatment-induced bone microarchitectural changes could only be assessed by histomorphometric analyses of iliac crest bone biopsies. The development of high-resolution peripheral quantitative computed tomography (HR-pQCT), however, enables non-invasive assessments of cortical and trabecular bone volumetric density, geometry, and microarchitecture at peripheral anatomic sites in vivo. Only a few studies have investigated the effects of osteoporosis treatment on bone microarchitecture assessed by HR-pQCT (6–14) and no published studies have assessed the microarchitectural and compartmental structural changes in response to the combination of antiresorptive and anabolic therapy in humans. Furthermore, recent technical advances now allow for the HR-pQCT measurement of intra-cortical porosity, a parameter that is independently associated with prevalent hip fracture and is known to independently contribute to the age-related decrease in bone strength.(15–17) HR-pQCT images can also be used to estimate bone strength by micro-finite element analysis (µFEA), a technique that utilizes accepted engineering principles to estimate fracture resistance at these peripheral sites.(18–21) To define the effects of combination teriparatide and denosumab on cortical and trabecular bone density, microarchitecture, and predicted bone strength, we obtained HR-pQCT measurements of the distal tibia and radius in postmenopausal women randomized to receive 12 months of teriparatide, denosumab, or both medications in the DATA study. Specifically, we aimed to test the hypotheses that 12 months of combined teriparatide and denosumab would increase cortical and trabecular volumetric BMD, improve trabecular and cortical microstructure and geometry, and increase predicted bone strength more than either drug alone.
Methods
Subjects
Details of the DATA study subjects have been reported previously.(5) Briefly, we recruited postmenopausal women over age 45 at high risk of fracture from a single clinical site (Massachusetts General Hospital, Boston, MA). We defined high risk of fracture as T score −2.5 or lower at the spine, total hip, or femoral neck; a T score −2.0 or less with at least one BMD-independent risk factor (fracture after age 50 years, parental hip fracture after age 50 years, previous hyperthyroidism, inability to get up from a chair with arms raised, or current smoking);(22) or a T score −1.0 or less with history of fragility fracture. Women were excluded for use of glucocorticoids or oral bisphosphonates within 6 months of enrollment; use of estrogen, selective estrogen-receptor modulators, or calcitonin within 3 months of enrollment; or any prior use of intravenous bisphosphonates, parathyroid hormone, or strontium ranelate.
Protocol
Prior to randomization, women were stratified by age (younger than 65 years vs 65 years or older) and by previous bisphosphonate use. Subjects were then randomly assigned to open-label treatment of teriparatide 20 mcg subcutaneously once daily, denosumab 60 mg subcutaneously every six months, or both medications. Calcium and vitamin D were prescribed to achieve total daily intakes of 1,200 mg calcium and 25-hydroxyvitamin D concentrations greater than 20 ng/mL. The study was approved by the Partners Healthcare Institutional Review Board and registered on ClinicalTrials.gov, number NCT00926380.
HR-pQCT
Trabecular and cortical density and microarchitecture of the distal radius and tibia were assessed at 0, 3, 6, and 12 months using HR-pQCT (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland). Scans consisting of 110 CT slices over a 9.02 mm region of interest were acquired with an isotropic voxel size of 82 microns. The non-dominant arm and corresponding leg were scanned unless there was a prior fracture at that region, whereupon the dominant side was scanned. The volume of interest (VOI) shared by a participant’s 0, 3, 6, and 12 month scans was identified using the manufacturer’s 2D region-matching software which relied on the periosteal contours. In this shared VOI, we used semi-automated software to segment cortical and trabecular regions based on a threshold-based algorithm. Total, trabecular, and cortical bone density (Tot.vBMD, Tb.vBMD, Ct.vBMD, mg HA/cm3) were determined in addition to microarchitecture parameters of cortical thickness (Ct.Th, mm), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, mm−1), and trabecular separation (Tb. Sp, mm).
The cortical bone compartment in this shared VOI was then further segmented via an autocontouring process that generated periosteal and endosteal contours.(23) All automatically generated contours were inspected, and if the contour deviated from the visually apparent periosteal or endosteal margin, semi-manual adjustment was made. Using this extended cortical analysis, cortical tissue mineral density (Ct.TMD, mgHA/cm3) and cortical porosity (Ct.Po, %) were assessed at months 0 and 12. Ct.TMD is the density of the cortical bone excluding the resolved Haversian canals and pores. Cortical porosity is the volume of the resolved Haversian canals and intracortical pore space normalized by the sum of their volume plus the cortical solid tissue volume.
Micro-FEA was performed to estimate the biomechanical properties of the bone, as previously described.(18–20) Following image segmentation (described above), each bone voxel of the HR-pQCT distal radius and tibia images was converted to hexahedral finite elements having linear-elastic and isotropic material behavior, with a Young’s modulus and Poisson’s ratio of 10 GPa and 0.3, respectively. The µFEA model was subjected to uniaxial compression, and stiffness (kN/mm) and failure load (kN) were estimated at months 0 and 12. Failure load was estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000 µstrain.(24)
Two operators performed the endosteal contouring (assigning the same operator for all scans for each subject) and a third operator reviewed all periosteal and endosteal contouring for quality assurance. Images were graded as 1 (excellent), 2 (good), or 3 (poor) for motion artifact and scans with a quality score of 3 were excluded from analysis. Scans with a fracture in the region of interest were excluded from analysis. Same-day reproducibility for repeated measurements is 0.2 to 1.4% for density values, 0.3 to 8.6% for trabecular microarchitecture parameters, 0.6 to 2.4% for cortical microarchitecture parameters, 7.3 to 20.2% for cortical porosity measurements, and 2.1 to 3.0% for µFEA measures. These ranges are consistent with prior reports.(18, 25)
Statistical analysis
We performed a modified intention-to-treat analysis, which includes all data from women completing at least one post-baseline study visit. To compare the between-group changes in each parameter, we used repeated measures analysis of variance (ANOVA). Within group changes were assessed by paired t-test. The 0–12 month changes in each variable in subjects with previous bisphosphonate use, no previous use, and the entire cohort were compared by one-way ANOVA. Statistical analyses were performed with SAS for Windows (version 9.3). Data are presented as mean (SD) unless otherwise specified. Two sided p-values of 0.05 or less are considered significant.
Results
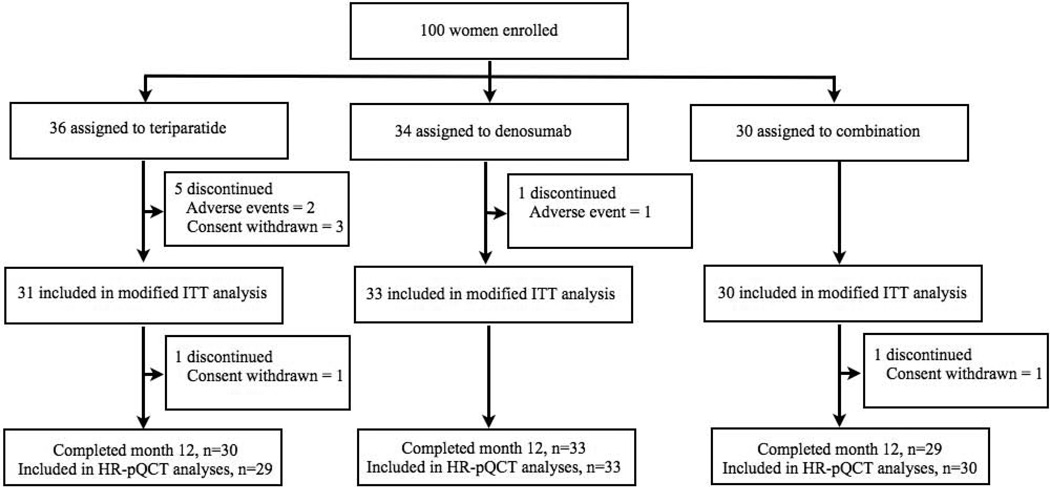
Of the 100 women enrolled, 94 women completed at least one post-baseline visit and are included in the modified intention-to-treat analysis (figure one). Six percent of radius scans and 3% of tibia scans were excluded due to motion artifact. When comparing the baseline and 12-month visit only, the shared VOI averaged 93% at the radius and 95% at the tibia. When comparing all four visits, the shared VOI averaged 86% at the radius and 91% at the tibia. Baseline clinical characteristics were similar among the three groups (table 1). Baseline HR-pQCT variables were similar among the three groups with exception of tibial cortical thickness, which was greater in the combination therapy group than in the denosumab group (table 2). It should be noted that there was no difference in overall treatment response in any HR-pQCT variable among subjects with or without a history of bisphosphonate exposure and the entire cohort (Supplemental Table).
Figure 1.
Subject disposition.
Table 1.
Baseline clinical characteristics of subjects included in the modified intention-to-treat analysis.
| Characteristic | Teriparatide group (N=31) |
Denosumab group (N=33) |
Combination group (N=30) |
|---|---|---|---|
| Age (year) | 66 ± 8 | 66 ± 8 | 66 ± 9 |
| Body mass index (kg/m2) | 25.5 ± 3.8 | 24.1 ± 3.9 | 25.4 ± 4.9 |
| Percent White, non-Hispanic | 31 (100%) | 30 (91%) | 27 (90%) |
| History of fragility fracture (no, %) | 16 (52%) | 12 (36%) | 10 (33%) |
| Previous oral bisphosphonate (BP) use (no, %) | 13 (42%) | 12 (36%) | 10 (33%) |
| Duration of oral BP use (months) | 40 ± 25 | 43 ± 27 | 28 ± 21 |
| Time since discontinuation of BP use (months) | 27 ± 20 | 36 ± 23 | 42 ± 17 |
| Serum 25-hydroxyvitamin D level (ng/mL) | 31 ± 9 | 35 ± 11 | 34 ± 12 |
| Serum alkaline phosphatase level (U/L) | 76 ± 17 | 79 ± 17 | 84 ± 21 |
| OC (ng/mL) | 49.0 ± 28.8 | 42.9 ± 19.4 | 52.2 ± 29.9 |
| P1NP (µg/L) | 46.0 ± 19.5 | 45.7 ± 16.7 | 49.3 ± 20.9 |
| CTX (ng/mL) | 0.36 ± 0.15 | 0.39 ± 0.21 | 0.43 ± 0.17 |
| Posterior-anterior spine BMD (g/cm2) | 0.823 ± 0.111 | 0.866 ± 0.088 | 0.856 ± 0.131 |
| Femoral neck BMD (g/cm2) | 0.643 ± 0.061 | 0.641 ± 0.086 | 0.642 ± 0.067 |
| Total hip BMD (g/cm2) | 0.757 ± 0.068 | 0.766 ± 0.100 | 0.759 ± 0.073 |
| One third radius BMD (g/cm2) | 0.612 ± 0.069 | 0.602 ± 0.082 | 0.613 ± 0.070 |
Table 2.
Baseline HR-pQCT characteristics of subjects included in the modified intention-to-treat analysis.
| Tibia | Radius | |||||||
|---|---|---|---|---|---|---|---|---|
| TPTD | DMAB | Both | P- value |
TPTD | DMAB | Both | P-value | |
| Tot.vBMD, mg HA /cm3 | 236.4 (44.3) | 224.7 (53.4) | 249.2 (43.8) | ns | 268.9 (55.4) | 257.1 (60.3) | 267.7 (56.8) | ns |
| Tb.vBMD, mg HA /cm3 | 133.3 (31.1) | 139.0 (37.4) | 142.3 (34.8) | ns | 122.9 (26.1) | 122.4 (33.9) | 122.5 (25.7) | ns |
| Ct.vBMD, mg HA /cm3 | 774.8 (59.4) | 756.6 (69.4) | 780.2 (65.4) | ns | 807.2 (80.4) | 802.6 (74.3) | 814.6 (69.4) | ns |
| Ct.Th, mm | 0.88 (0.25) | 0.77 (0.23) | 0.93 (0.20) | 0.02 | 0.63 (0.20) | 0.61 (0.18) | 0.63 (0.17) | ns |
| Tb.N, mm−1 | 1.59 (0.35) | 1.64 (0.47) | 1.60 (0.38) | ns | 1.65 (0.32) | 1.61 (0.42) | 1.57 (0.29) | ns |
| Tb.Sp, mm | 0.59 (0.18) | 0.61 (0.29) | 0.59 (0.17) | ns | 0.56 (0.14) | 0.62 (0.26) | 0.60 (0.13) | ns |
| Tb.Th, mm | 0.071 (0.013) | 0.072 (0.013) | 0.075 (0.016) | ns | 0.062 (0.007) | 0.064 (0.008) | 0.066 (0.012) | ns |
| Ct.Po, % | 8.7 (3.2) | 8.5 (2.7) | 9.1 (3.7) | ns | 2.8 (1.5) | 2.7 (1.2) | 2.6 (1.4) | ns |
| Stiffness, kN/mm | 166.3 (29.1) | 167.0 (31.9) | 167.4 (25.6) | ns | 64.4 (15.2) | 66.6 (15.7) | 61.8 (16.6) | ns |
| Failure Load, kN | 7.68 (1.04) | 7.74 (1.31) | 7.77 (1.05) | ns | 2.76 (5.32) | 2.83 (5.41) | 2.66 (4.48) | ns |
Values are mean (SD). Tot.vBMD = total vBMD; Tb.vBMD = trabecular vBMD; Ct.vBMD = cortical vBMD; Ct.Th = cortical thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; Ct.Po = cortical porosity.
Volumetric Density
Tibia
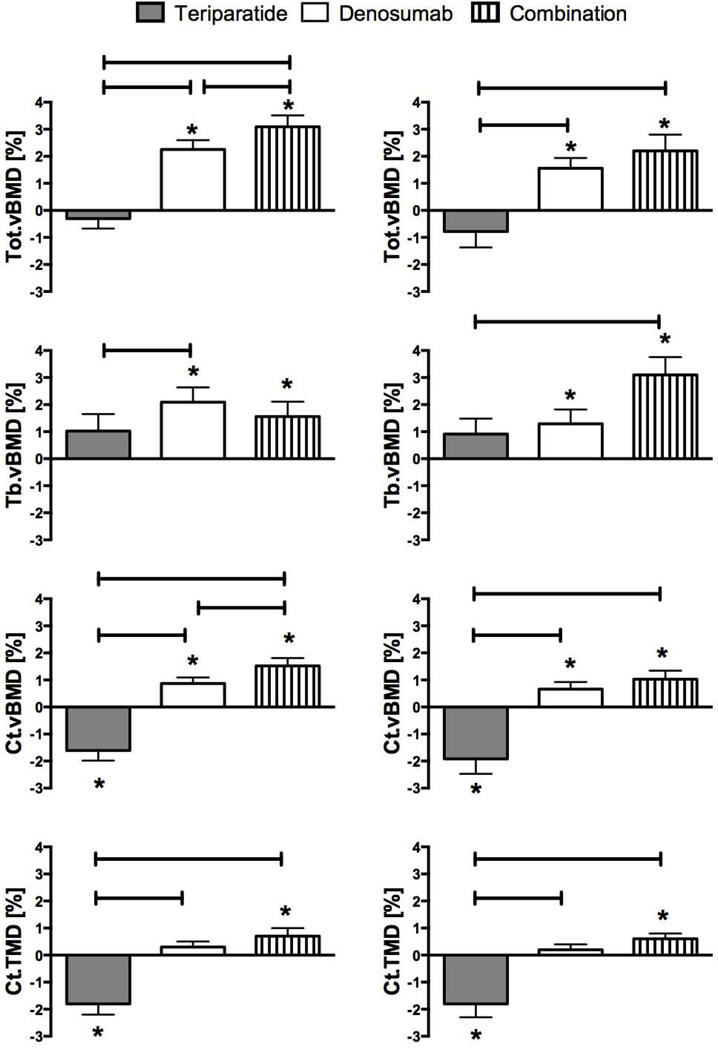
The mean changes in HR-pQCT volumetric density are shown in Figure 2. At the tibia, 12 months of teriparatide treatment did not change total vBMD, whereas denosumab increased total volumetric BMD by 2.2 ± 1.9% (p<0.001 versus baseline) and combination therapy increased total vBMD by 3.1 ± 2.2% (p<0.001 versus baseline). The combination of both drugs increased total volumetric BMD more than teriparatide or denosumab (p<0.001, combination versus teriparatide and p=0.014, combination versus denosumab). Similarly, trabecular vBMD did not change in the teriparatide group and increased in both the denosumab and combination groups, although no significant between-group differences were observed. Cortical vBMD decreased by 1.6 ± 1.9% in the teriparatide group, increased by 0.9 ± 1.2% in the denosumab group, and increased by 1.5 ± 1.5% in the combination group (p<0.001 versus baseline for all comparisons). Combination therapy increased cortical vBMD more than teriparatide and denosumab (p<0.001, combination versus teriparatide and p=0.035, combination versus denosumab). Cortical tissue mineral density decreased with teriparatide, remained stable with denosumab, and increased with combination therapy and the change in the combination therapy group differed significantly from the teriparatide group (p<0.001, combination versus teriparatide).
Figure 2.
Mean percent change (SEM) from baseline at distal tibia (left column) and distal radius (right column) in bone densities at 12 months. *p value <0.05 versus baseline, bars represent p value <0.05 between groups.
Radius
At the radius, total vBMD did not change in the teriparatide group but increased in both the denosumab (1.6 ± 2.1%) and combination therapy groups (2.2 ± 3.0%, p<0.01 for both within group comparisons). The increase in total vBMD was greater in the combination therapy group than in the teriparatide group only (p<0.001). Similarly, trabecular vBMD did not change in the teriparatide group but increased in the denosumab (1.3 ± 2.8%) and combination therapy (3.1 ± 3.3%) groups (p=0.05 for both within group comparisons). The increase in total vBMD was greater in the combination therapy group than the teriparatide group only (p=0.012). Cortical vBMD decreased by 1.9 ± 2.8% in the teriparatide group, increased by 0.7 ±1.4% in the denosumab group, and increased by 1.0 ± 1.6% in the combination groups (p<0.02 for all within group comparisons). Combination therapy increased cortical vBMD more than teriparatide (p<0.001, combination versus teriparatide). Cortical tissue mineral density decreased with teriparatide, remained stable with denosumab, and increased with combination (p<0.001, combination versus teriparatide).
Microarchitecture: geometry and structure
Tibia
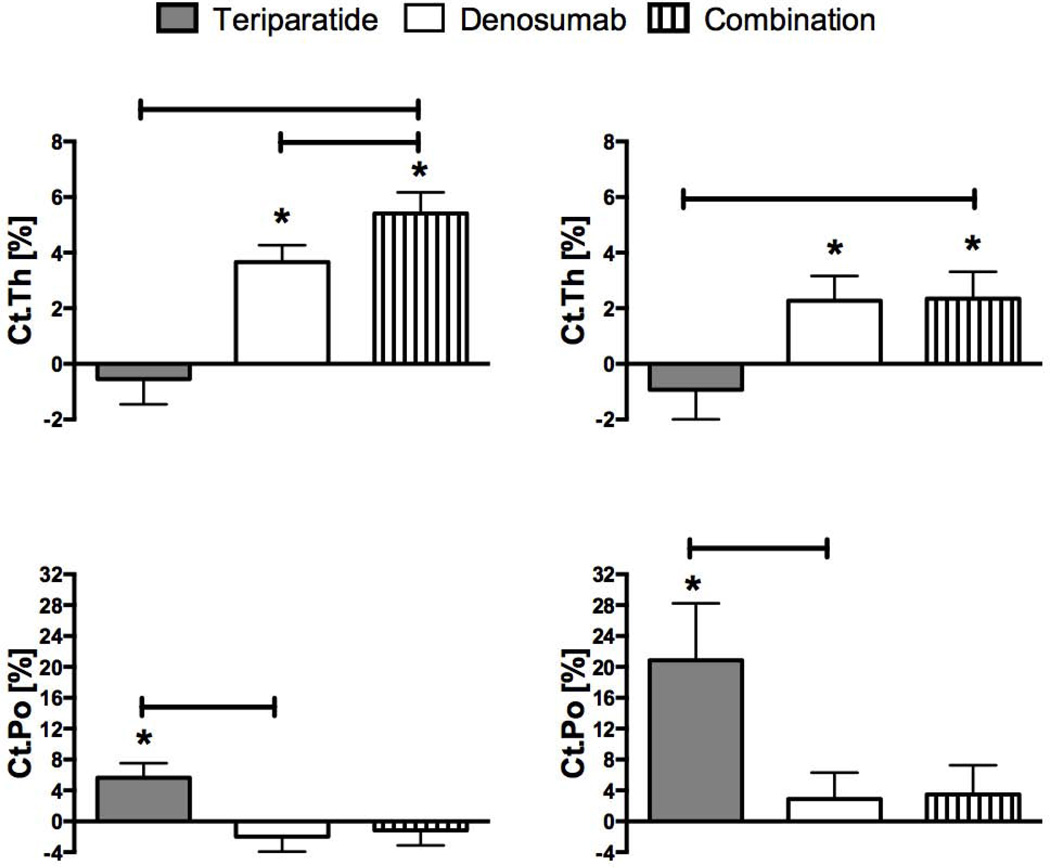
At the tibia, 12 months of teriparatide did not change cortical thickness whereas cortical thickness increased by 3.7 ± 3.3% in the denosumab group and by 5.4 ± 3.9% in the combination therapy group (p<0.001 versus baseline for the latter 2 groups) (figure 3). Combination therapy increased cortical thickness more than both teriparatide (p<0.001) and denosumab (p=0.009). Also at the tibia, cortical porosity increased by 5.6 ± 9.9% in the teriparatide group (p=0.006 versus baseline) but did not change in the other two groups.
Figure 3.
Mean percent change (SEM) from baseline at distal tibia (left) and distal radius (right) in cortical thickness and cortical porosity at 12 months. *p value <0.05 versus baseline, bars represent p value <0.05 between groups.
Radius
At the radius, cortical thickness did not change in the teriparatide group whereas it increased by 2.3 ± 4.8% in the denosumab group (p=0.016 versus baseline) and by 2.3 ± 4.8% in the combination therapy group (p=0.022 versus baseline). The increase in cortical thickness was greater in the combination group than the teriparatide group (p=0.014). Also at the radius, teriparatide increased cortical porosity by 20.9 ± 37.6% (p=0.009 versus baseline) whereas cortical porosity did not change in the other two groups. Note that while the percent changes in porosity appear to be large, the absolute changes are relatively modest. For example, while cortical porosity at the radius increased by 20.9% in the teriparatide group, this represents a change from 2.7% at baseline to 3.1% after 1 year of therapy.
At both the tibia and radius, changes in trabecular architecture were generally small and did not reach statistical significance (table 3).
Table 3.
Mean percent change (SD) from baseline in trabecular microarchitecture at 12 months.
| Tibia: % change (SD) | Radius: % change (SD) | |||||
|---|---|---|---|---|---|---|
| Teriparatide | Denosumab | Combination | Teriparatide | Denosumab | Combination | |
| Tb.Th | 1.8 (7.9) | 2.2 (7.0) | 3.8 (8.5) * | 2.5 (11.3) | 4.4 (10.7) * | 3.1 (11.5) |
| Tb.Sp | 0.9 (8.5) | 0.8 (7.7) | 0.4 (8.2) | 1.6 (12.0) | 2.2 (11.0) | 1.6 (12.6) |
| Tb.N | −0.3 (8.9) | −0.3 (7.3) | −0.1 (8.3) | −0.5 (11.7) | −1.3 (9.5) | −0.2 (13.1) |
Tb.Th = trabecular thickness; Tb.Sp = trabecular separation; Tb.N = trabecular number.
p value <0.05 versus baseline
Estimated bone strength
Tibia
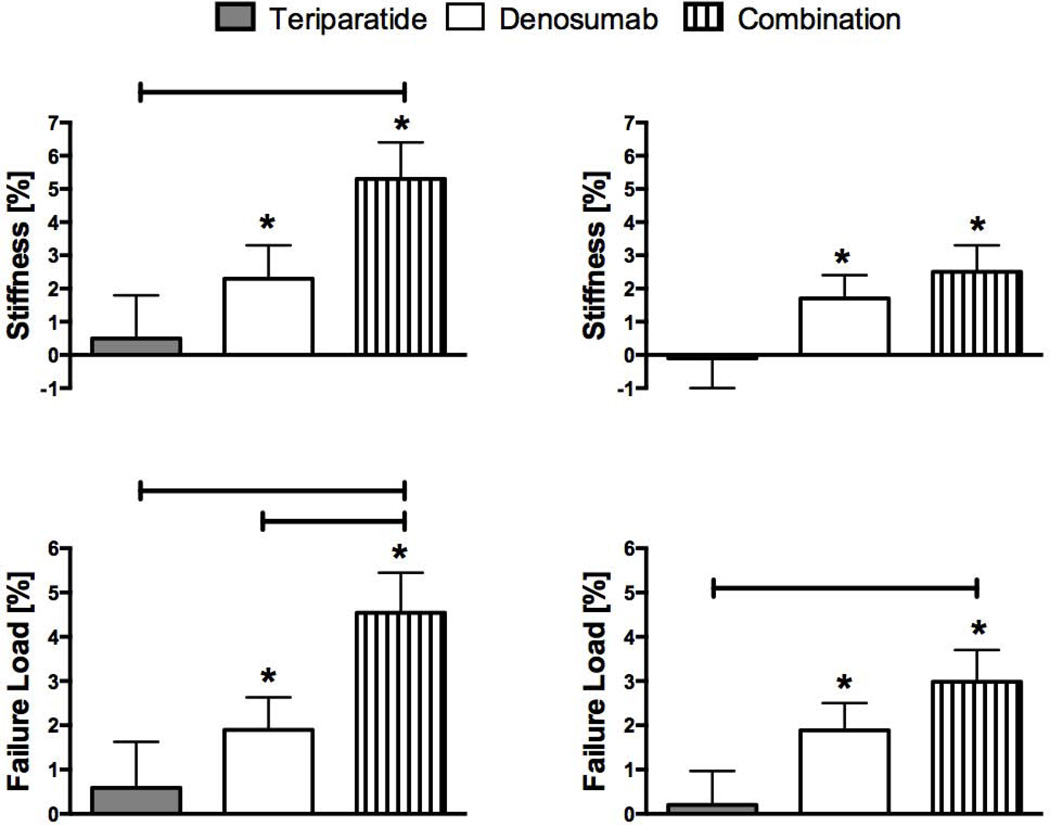
At the tibia, FEA-estimates of bone stiffness did not change in the teriparatide group but increased by 2.3 ± 5.3% in the denosumab group (p=0.027 versus baseline) and by 5.3 ± 5.5% in the combination therapy group (p<0.001 versus baseline) (figure 4). The increase in stiffness was greater in the combination therapy group than in the teriparatide group (p=0.005). Similarly, failure load did not change in the teriparatide group but increased by 1.9 ± 4.0% in the denosumab group (p=0.015 versus baseline) and by 4.5 ± 4.6% in the combination group (p<0.001 versus baseline). The increase in tibial failure load was greater in the combination group than in both other groups (p=0.005, combination versus teriparatide and p=0.041, combination versus denosumab).
Figure 4.
Mean percent change (SEM) from baseline at distal tibia (left) and distal radius (right) in stiffness and failure load at 12 months. *p value <0.05 versus baseline, bars represent p value <0.05 between groups.
Radius
Similar patterns in estimated bone strength were observed at the radius, though not all between-group comparisons were statistically significant.
Discussion
The results of this study demonstrate that 12 months of combined denosumab and teriparatide therapy improves peripheral cortical and total bone density, cortical microarchitecture, and estimated bone strength more than either drug alone. While superior efficacy is noted at both the tibia and the radius in the combination group, the advantage of the combined treatment is numerically greater at the tibia than at the radius. The mechanism underlying the differential effects at these two peripheral anatomic sites is uncertain but might reflect an ability of weight-bearing to amplify the skeletal benefits of TPTD in humans, as previously documented in experimental animals.(26) There was also greater effect with alendronate in humans as measured with HR-pQCT in the distal tibia compared with the distal radius.(6)
The significant increase in cortical thickness and cortical area along with the concomitant decrease in trabecular area (data not shown) at the radius and tibia in the denosumab and combination therapy groups suggests that resorptive cavities at the endocortical surface are potentially being filled in response to therapy. Alternatively, the apparent increase in cortical thickness may be secondary to increases in the mineralization of cortical bone, which can influence edge detection by threshold-based segmentation algorithms. The relative contributions of these mechanisms to the observed increase in cortical thickness cannot be determined with current methods.
The observed decrease in cortical density and increase in cortical porosity among teriparatide treated women are consistent with HR-pQCT changes observed in prior studies of postmenopausal osteoporosis in which teriparatide monotherapy was shown to either decrease peripheral cortical BMD, increase cortical porosity, or both.(9, 12) Moreover, numerous rodent and primate animal models show increases in cortical porosity with parathyroid hormone treatment.(27–29) Importantly, however, teriparatide-induced increases in cortical porosity do not appear to have deleterious effect on µFEA estimated failure load or stiffness as measured by HR-pQCT.(12) The explanation for the maintained skeletal integrity may relate to beneficial changes in trabecular microarchitecture not detected in a sample of this size. It is possible that these relatively small absolute changes in cortical porosity do not substantially impact µFEA strength predictions, as previously reported.(23) Additionally, assessment of cortical porosity that utilize a threshold-based analysis to differentiate cortical and trabecular bone may overestimate if there is substantial undermineralized bone. Methods of assessing cortical porosity that do not rely on a threshold-based analysis, such as a recently reported automated method of segmenting bone into its compact-appearing cortex, transitional zone, and trabecular compartment provide an alternate approach for assessing porosity.(30, 31)
It is important to note that the improvements in the cortical bone parameters observed in the combination group in the present study do not represent a mixture of the teriparatide and denosumab-associated effects, but rather they represent an advantage over both monotherapies. The HR-pQCT changes described in the denosumab monotherapy group are also similar to those described in prior studies. For example, in a recent study of 83 women with osteoporosis treated with denosumab for 12 months, total vBMD, cortical vBMD, and cortical thickness all increased significantly.(7) A notable finding of the present study is that in both the tibia and radius, denosumab completely inhibits the commonly observed teriparatide-induced increase in cortical porosity.
Several limitations of our study deserve mention. First, it should be noted that the lack of a placebo control group limits the interpretation of within-group changes. Given that many of the effects of are large magnitude, however, we feel that the within group changes, particularly in cortical parameters, are likely to be related to the specific treatment. Second, HR-pQCT is limited to measurements at the appendicular skeleton, and may not be consistent with changes in the axial skeletal. Additionally, motion artifact is a limitation of HR-pQCT and differences in the quality of serial scans could add to the error associated with these measurements. As suggested by Pialat et al in a report examining impact of scan quality on HR-pQCT measures, developing standardized quality control criteria will be important for HR-pQCT studies in the future.(32) We minimized this effect by censoring scans with severe motion artifact. Another potential limitation is that changes in bone size would not be detectable by HR-pQCT because matching of serial VOI was performed by assuming constant periosteal contours and cross-sectional areas among a participant’s serial measurements. This technical limitation makes it impossible to assess if an increase in cortical porosity in the teriparatide group is offset by changes in bone size through periosteal expansion. Lastly, the clinical implications of the µFEA estimates of bone strength are not yet fully established as there are no prospective longitudinal studies assessing the relationship between changes in µFEA-estimated bone strength and fracture. Moreover, our µFEA analysis assumed fixed, homogeneous material properties although we observed that cortical TMD increased with combination therapy. Prior work has shown that bone tissue mineral density is related directly to the intrinsic material properties of bone (e.g. the elastic modulus), thus the µFEA-measured stiffness and failure load in the combination group may have been underestimated.(33)
In summary, the combination of denosumab and teriparatide appears to result in the most favorable changes in peripheral bone compartmental vBMD, microstructure and estimated strength as compared to either drug alone. Along with the previously reported superior efficacy of increases in DXA-derived BMD at the hip and spine, these changes may translate to greater fracture resistance and support the consideration of combination therapy in patients with severe osteoporosis.
Supplementary Material
Acknowledgements
We thank the study volunteers for their participation. This work is supported by the Harvard Clinical and Translational Science Center, the National Center for Research Resources (grant number 1 UL1 RR025758-04), Eli Lilly, and Amgen.
Footnotes
Disclosures
RMN is a consultant for Eli Lilly and BZL is a consultant for Eli Lilly, Amgen Inc, Merck, and Radius.
Contributors
Authors’ roles: Study design: HL, RMN, BZL. Study conduct: JNT, AVU, SMB, RMN, YZ, ND, BZL. Data collection: JNT, YZ, ND. Data analysis: HL. Data interpretation: JNT, HL, MLB, BZL. Drafting manuscript: JNT. All authors edited and approved the report for final submission. JNT and BZL take responsibility for the integrity of the data analysis.
References
- 1.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. The New England journal of medicine. 2003 Sep 25;349(13):1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, Eriksen EF, Recknor C, Miller PD, Guanabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Mar;26(3):503–511. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. The New England journal of medicine. 2003 Sep 25;349(13):1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. The Journal of clinical endocrinology and metabolism. 2010 Apr;95(4):1838–1845. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013 Jul 6;382(9886):50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Dec;25(12):2558–2571. doi: 10.1002/jbmr.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Aug;25(8):1886–1894. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzoli R, Laroche M, Krieg MA, Frieling I, Thomas T, Delmas P, et al. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatology international. 2010 Aug;30(10):1341–1348. doi: 10.1007/s00296-010-1542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 Jan;22(1):357–362. doi: 10.1007/s00198-010-1226-1. [DOI] [PubMed] [Google Scholar]
- 10.Rizzoli R, Chapurlat RD, Laroche JM, Krieg MA, Thomas T, Frieling I, et al. Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Results of a 2-year study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 Jan;23(1):305–315. doi: 10.1007/s00198-011-1758-z. [DOI] [PubMed] [Google Scholar]
- 11.Chapurlat RD, Laroche M, Thomas T, Rouanet S, Delmas PD, de Vernejoul MC. Effect of oral monthly ibandronate on bone microarchitecture in women with osteopenia-a randomized placebo-controlled trial. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Jan;24(1):311–320. doi: 10.1007/s00198-012-1947-4. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 Apr;28(4):736–745. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 13.Bala Y, Chapurlat R, Cheung AM, Felsenberg D, LaRoche M, Morris E, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014 Feb;29(2):380–388. doi: 10.1002/jbmr.2101. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama KK, Cohen A, Young P, Wang J, Lappe JM, Guo XE, et al. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: A pilot HR-pQCT study. The Journal of clinical endocrinology and metabolism. 2014 Mar 31; doi: 10.1210/jc.2014-1041. jc20141041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. The Journal of bone and joint surgery American volume. 1993 Aug;75(8):1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Bousson V, Meunier A, Bergot C, Vicaut E, Rocha MA, Morais MH, et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001 Jul;16(7):1308–1317. doi: 10.1359/jbmr.2001.16.7.1308. [DOI] [PubMed] [Google Scholar]
- 17.Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999 Jan;24(1):57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 18.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Mar;23(3):392–399. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 19.Vilayphiou N, Boutroy S, Sornay-Rendu E, Van Rietbergen B, Munoz F, Delmas PD, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in postmenopausal women. Bone. 2010 Apr;46(4):1030–1037. doi: 10.1016/j.bone.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Vilayphiou N, Boutroy S, Szulc P, van Rietbergen B, Munoz F, Delmas PD, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 May;26(5):965–973. doi: 10.1002/jbmr.297. [DOI] [PubMed] [Google Scholar]
- 21.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. The Journal of clinical endocrinology and metabolism. 2011 Jul;96(7):2041–2048. doi: 10.1210/jc.2011-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2001;12(7):519–528. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 23.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 May;25(5):983–993. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002 Jun;30(6):842–848. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 25.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010 Sep;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Jee WS, Yuan Z, Wei W, Chen H, Pun S, et al. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999 Mar;14(3):439–448. doi: 10.1359/jbmr.1999.14.3.439. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Anabolic effects of human biosynthetic parathyroid hormone fragment (1–34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999 Apr;14(4):536–545. doi: 10.1359/jbmr.1999.14.4.536. [DOI] [PubMed] [Google Scholar]
- 28.Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone(1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001 Jan;16(1):157–165. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- 29.Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY. Effects of daily treatment with parathyroid hormone 1–84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone. 2007 Sep;41(3):321–330. doi: 10.1016/j.bone.2007.04.197. [DOI] [PubMed] [Google Scholar]
- 30.Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013 May;54(1):8–20. doi: 10.1016/j.bone.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014 Feb;59:173–179. doi: 10.1016/j.bone.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012 Jan;50(1):111–118. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. Journal of biomechanics. 1988;21(2):131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.