Abstract
Background
Cognitive control impairments are linked to functional outcome in schizophrenia. The goal of the current study was to investigate precise abnormalities in two aspects of cognitive control: reactively changing a prepared response, and monitoring performance and adjusting behavior accordingly. We adapted an oculomotor task from neurophysiological studies of cellular basis of cognitive control in nonhuman primates.
Methods
16 medicated outpatients with schizophrenia (SZ) and 18 demographically-matched healthy controls performed the modified double-step task. In this task, participants were required to make a saccade to a visual target. Infrequently, the target jumped to a new location and participants were instructed to rapidly inhibit and change their response. A race model provided an estimate of the time needed to cancel a planned movement. Response monitoring was assessed by measuring reaction time (RT) adjustments based on trial history.
Results
SZ patients had normal visually-guided saccadic RTs but required more time to switch the response to the new target location. Additionally, the estimated latency of inhibition was longer in patients and related to employment. Finally, although both groups slowed down on trials that required inhibiting and changing a response, patients showed exaggerated performance-based adjustments in RTs, which was correlated with positive symptom severity.
Conclusions
SZ patients have impairments in rapidly inhibiting eye movements and show idiosyncratic response monitoring. These results are consistent with functional abnormalities in a network involving cortical oculomotor regions, the superior colliculus, and basal ganglia, as described in neurophysiological studies of nonhuman primates using an identical paradigm, and provide a translational bridge for understanding cognitive symptoms of SZ.
Keywords: schizophrenia, saccades, response inhibition, response monitoring, cognitive control, stop-signal
1. Introduction
Cognitive impairments in schizophrenia are omnipresent across domains and are likely closer to disease pathophysiology than the surface manifestation of psychotic symptoms (Elvevag & Goldberg, 2000; Lencz et al., 2006; Sitskoorn et al., 2004). Cognitive control, the ability to control thoughts and actions and respond flexibly to the environment, is particularly affected in schizophrenia and linked to functional outcome (Bilder et al., 2000; Green et al., 2000). Since cognitive control impairments are major treatment targets, understanding their biological underpinnings is of great clinical interest. In exploring these biological mechanisms, it is important to consider that cognitive control is a multifaceted construct (Bilder, 2012; Braver, 2012; Miyake et al., 2000). One pragmatic way of dissecting cognitive control is to separate proactive and reactive control. Proactive control refers to maintaining goal-relevant information in an anticipatory manner in order to prepare for having to override prepotent response tendencies. Reactive control, on the other hand, refers to later recruitment of control processes in response to some external event in order to meet the challenges of cognitively demanding circumstances. As reactive and proactive control are partly dissociable at the level of behavior and brain (Braver, 2012), we can further elucidate the nature and etiology of cognitive control impairments in schizophrenia. Moreover, adopting a translational approach and comparing behavior across species using identical paradigms provides a concrete framework for inferring the cellular basis of impairments in schizophrenia.
One crucial aspect of cognitive control studied extensively in schizophrenia is response inhibition. Most of these studies have focused on proactive inhibition, preparing to inhibit prior to stimulus onset (Clementz, 1998; Gooding & Basso, 2008; Hutton & Ettinger, 2006; Westerhausen et al., 2011). Fewer studies have investigated reactive inhibition, the stimulus-driven process of inhibiting during motor preparation. From the perspective of pharmacological interventions in particular, characterizing reactive in addition to proactive inhibition is crucial, as different pharmacological manipulations in rodents have differing effects on these two functions (Eagle et al., 2008; Eagle et al., 2007). The countermanding, or stop-signal, task is widely used for investigating reactive inhibition (Lappin & Eriksen, 1966; Verbruggen & Logan, 2008). Participants are instructed to respond quickly to a stimulus (GO stimulus). On some trials, a second signal is presented (STOP stimulus), and subjects are instructed to inhibit the prepared response. Performance is described as a race between competing GO and STOP units, and based on this model, the time needed to inhibit a response, the stop-signal reaction time (SSRT), can be estimated (Logan & Cowan, 1984). We recently showed that patients with schizophrenia have longer SSRT in a saccadic countermanding task, which was related to negative symptom severity and unemployment (Thakkar et al., 2011). Based on neurophysiology studies of non-human primates performing the saccadic countermanding task, these findings suggest specific and clinically relevant abnormalities within a network involving frontal eye fields (FEF), superior colliculus (SC), and basal ganglia (BG; Hikosaka et al., 2000; Schall & Boucher, 2007; Schall & Godlove, 2012).
The saccadic countermanding task allows us to examine another aspect of cognitive control—response monitoring, the ability to track ongoing performance and adjust future behavior. In this task, humans and non-human primates slow down following trials in which they must inhibit a response (Bissett & Logan, 2011; Emeric et al., 2007; Nelson et al., 2010). Medial frontal cortex neurons are sensitive to performance history and can implement adjustments in response speed to optimize behavior (Emeric et al., 2008; Emeric et al., 2010; Godlove et al., 2011; Ito et al., 2003; Stuphorn & Schall, 2006; Stuphorn et al., 2000). In our previous countermanding study, we observed idiosyncratic response monitoring in schizophrenia. Patients slowed down more than controls following trials in which inhibition was successful.
The major aim of the current study was to investigate another aspect of reactive cognitive control in schizophrenia and its relationship to functional outcome. In the current study, we probed the ability to rapidly change a prepared response with an oculomotor task used in neurophysiological studies—the modified double-step task (Bissett & Logan, 2013; Camalier et al., 2007; Murthy et al., 2007; Murthy et al., 2009). In this task, participants are instructed to look at a visual target. On a minority of trials, the target jumps to a new location, and participants are instructed to inhibit the prepared saccade and look instead at the new target. This task differs from the countermanding task in that participants are instructed not just to inhibit an inappropriate response outright, but also to replace the old response with a new response rapidly —to change one's mind, as Ramakrishnan et al. (2012) describe it. Although experiments with double-step tasks for movements of eyes (Becker & Jürgens 1979) and limbs (Georgopoulos et al. 1981) have a long history, the mechanisms whereby individuals change plan have gained renewed interest (e.g., Resulaj et al. 2009). The race model can also be applied to double-step task performance (Camalier et al., 2007). Reactive inhibition can be computed from two variables: the estimated speed of inhibition, and reaction time (RT) to the final target location when the first saccade plan was successfully inhibited. Thus, the double-step task allows us to both estimate the speed of inhibition and directly measure the time it takes for subjects to redirect their movement to the new target location.
In addition, we explored trial-by-trial adjustments in behavior. Based on our previous findings, we expected to find clinically-relevant slowing of inhibition in schizophrenia and slower RTs to change the partially planned movement, providing further evidence for poorer reactive control. We also expected exaggerated trial history-based slowing in patients with schizophrenia. These findings may illuminate our understanding of very specific aspects of cognitive control in schizophrenia, resulting in more hypothesis-driven treatment development for cognitive deficits. Because this task has been used in humans and non-human primates under similar experimental conditions, the results provide a translational bridge for understanding the mechanisms of cognitive control impairments.
2. Methods and Materials
2.1 Participants
Diagnostic information is presented in Table 1. Individuals who met DSM-IV criteria for schizophrenia (SZ) were recruited from outpatient psychiatric facilities in Nashville,TN. Diagnoses were confirmed using structured clinical interviews (SCID-IV: First et al., 1995). All patients were taking antipsychotic medication, and half of the patient sample were also medicated with antidepressants, anxiolytics, mood stabilizers, or a combination thereof. Detailed medication status of patients is provided in Supplementary Table 1. Healthy, unmedicated control subjects (HC) without a personal and family history of DSM-IV Axis-I disorders were recruited from the same community by advertisements.
Table 1.
Demographic characteristics of the patient and control groups.
| Controls Mean (s.d.) | SZ Patients Mean (s.d.) | Statistic | p | |
|---|---|---|---|---|
| Age | 37.6 (8.3) | 39.9 (9.4) | t = 0.8 | 0.5 |
| Sex | 7F / 11M | 7F / 9 M | ϕ = 0.08 | 0.8 |
| IQ | 107.7 (2.2) | 101.1 (2.3) | t = 2.0 | 0.05 |
| Education (yrs) | 16.1 (2.1) | 12.9 (1.9) | t = 2.4 | 0.0002 |
| Handedness | 67.8 (62.5) | 54.4 (49.0) | t = 0.7 | 0.5 |
| SFS Total | 156.6 (14.8) | 130.7 (17.9) | t = 4.6 | <0.0001 |
| SFS Employment | 9.9 (0.2) | 4.7 (3.7) | t = 6.0 | <0.0001 |
| Years of Illness | n/a | 19.9 (8.3) | ||
| CPZ Equivalent | n/a | 486.6 (531.6) | ||
| BPRS | n/a | 17.2 (7.0) | ||
| SAPS | n/a | 17.0 (7.8) | ||
| SANS | n/a | 24.8 (14.4) | ||
Group means and standard deviations of demographic characteristics of the patient and control groups. Test statistics and corresponding p-values of group comparisons are presented. Two-tailed independent-sample t-tests were used to compare all variables, except sex, which was compared using a Fisher's Exact Test. Handedness was compared using the Edinburgh Handedness Inventory. Higher scores indicate greater right-handedness. IQ was measured using the National American Adult Reading Test. Abbreviations-SFS Total: Total score for Social Functioning Scale, SFS Employment: Employment subscale score of Social Functioning Scale, CPZ Equivalent: Chlorpromazine equivalent antipsychotic dose, BPRS: Brief Psychiatric Rating Scale, SAPS: Scale for the Assessment of Positive Symptoms, SANS: Scale for the Assessment of Negative Symptoms.
Clinical symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962), Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983). Social and occupational functioning was assessed with the Social Functioning Scale (SFS; Birchwood et al., 1990). IQ was measured with the North American Adult Reading Test (NAART; Blair & Spreen, 1989). Handedness was assessed using the Modified Edinburgh Handedness Inventory (Oldfield, 1971).
Exclusion criteria included substance use, neurological disorders, history of head injury, inability to fixate, and excessive sleepiness. All participants were native English speakers and had normal or corrected-to-normal vision. Three patients were excluded based on task performance, as outlined in the Statistical Methods section, and one patient chose to abort the experiment. Analyses were conducted on the remaining 16 SZ and 18 HC. Nine SZ patients and 9 HC in this sample participated in the previous countermanding study (Thakkar et al., 2011). Groups were matched for age, sex, and handedness. All subjects gave written informed consent approved by the Vanderbilt Institutional Review Board and were paid.
2.2 Apparatus and Stimuli
Eye position was monitored using the EyeLink II eyetracker (SR Research,Canada) at a sampling rate of 500 Hz with average gaze position error <0.5°, noise limited to <0.01° RMS. Saccades were detected on-line using a velocity criterion (35°/sec) and minimum amplitude criterion (2° visual angle). Subjects were seated 57cm from the monitor with their head in a chinrest.
2.3 Design and Procedure
2.3.1 Double-step Task
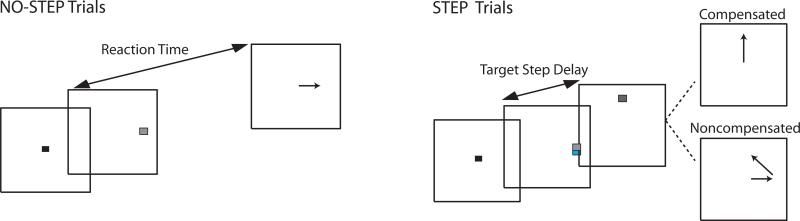
Subjects performed the saccadic double-step task (Figure 1), which comprised randomly interleaved no-step (60%) and step trials (40%). No-step trials required subjects to fixate on a central spot (white square subtending 0.5°) until it disappeared (after a random 500-1000ms delay) and a target (T1), subtending 1°, flashed for 94ms at one of eight positions 12° equidistant from fixation. Subjects were instructed to look at the target as quickly as possible. Step trials were initially identical to the no-step trials, but after a variable delay (target step delay; TSD) following T1 presentation, a second target (T2) flashed for 94ms at a new location1. T1 and T2 were separated by either 90° or 135°. The target step instructed subjects to inhibit a saccade to T1 and instead look towards T2 as quickly as possible. Step trials were labeled compensated or noncompensated based on whether subjects succeeded or failed to look immediately at T2, respectively. T1 and T2 were different isoluminant colors (cyan and magenta, 2.06cd/m2), facilitating detection of target order. Color mapping was counterbalanced across subjects. Response inhibition and redirection become more difficult with increasing TSDs. TSDs were adjusted on-line using a tracking procedure that yielded successful inhibition on approximately 50% of trials (see Supplementary Material for details). Participants performed a practice block of 60 trials, and 4 experimental blocks of 120 trials each.
Figure 1.
Modified double-step task. All trials began after a variable fixation length. In all trials, a target (T1) flashed at a non-central location, and subjects were instructed to saccade to the target as quickly as possible. On step trials, a second target (T2) was flashed at an alternate location at some delay following T1 (target step delay; TSD). On these trials, subjects were instructed to inhibit the planned saccade to T1 and instead redirect gaze towards T2. Trials in which subjects were successful in looking immediately at T2 were referred to as compensated, and trials in which participants erroneously looked first towards T1 were referred to as noncompensated. On the majority of noncompensated trials, subjects made a second corrective saccade to T2. The probability of correctly compensating becomes more difficult with longer TSD; thus, TSD was dynamically altered using a staircase procedure to ensure approximately 50% accuracy on redirect trials.
2.3.1 Double-step task performance evaluation
Performance was evaluated through measurements of RT on no-step, compensated, and noncompensated trials, and TSDs to arrive at four main outcome measures: 1) the speed of response execution; 2) the speed of response inhibition; 3) the ability to trigger an inhibitory response; 4) adjustments in RT as a function of the previous trial. Performance in this task can be accounted for by a mathematical model that assumes a race between independent processes that generate a response to T1 (GO1 process) and inhibit (STOP process) the T1 response (Boucher et al., 2007; Camalier et al., 2007; Logan & Cowan, 1984; Logan et al., 2014; Ramakrishnan et al., 2012). The saccade to T1 is executed or inhibited if GO1 or STOP wins the race, respectively. The speed of response execution can be measured directly from observable RTs, RTs on no-step and non-compensated trials were defined as the time between T1 onset at the onset of the first saccade. RTs on compensated trials were defined as the time between the onset of T2 and the first saccade.
On the other hand, the speed of response inhibition must be estimated. The independent race model provides an estimate of the time needed to respond to T2 and cancel the initially planned movement, referred to as the target step reaction time (TSRT). This measure is analogous to stop signal reaction time (SSRT) in the countermanding task. There are several published methods of calculating SSRT/TSRT (earlier methods reviewd in Band et al., 2003; more recent methods include Logan et al., 2014, Matzke et al., 2013 & Cowan, 1984), the mean method and integration method being the most widely used (see Supplementary Material for SSRT/TSRT calculation methods). In studies where a tracking procedure is used to adjust the delay between the signal to respond and the signal to stop or change that response, the mean method is most frequently applied. However, a recent study found that the mean method tended to overestimate SSRT/TSRT as distribution of RTs on trials in which no stop/step signal was presented became increasingly skewed (Verbruggen et al., 2013), potentially resulting in spurious group differences in SSRT/TSRT. Thus, TSRT was calculated using both the mean and integration method. Skew of each subject's no-step RT distribution was also calculated by fitting an ex-Gaussian distribution (see Supplementary Material) and extracting the estimated parameter, which represents the fall in the right tail of the distribution. Greater values indicate more positively skewed distributions. To investigate whether our previous finding of longer SSRT in SZ (Thakkar et al., 2011), in which we used the mean method, was robust to the calculation method, we re-calculated SSRT using the method of integration and estimated the parameter value from the ex-Gaussian distribution that was fit to the distribution of RTs on trials in which no stop-signal was presented for each participant.
To estimate variability in SSRT and the ability to trigger the inhibitory response, we measured the slope of the compensation function, in which the proportion of noncompensated responses was plotted as a function of the TSD (Logan & Cowan, 1984). The slope of the compensation function is thought to reflect variability in the STOP and GO1 RT and the ability to trigger an inhibitory response. The slope can be corrected for variability in GO RT by applying a Z-transformation to the TSDs (Logan et al., 1984), which expresses them in terms of the latency relative to finishing times of GO1 and STOP processes standardized with respect to variability in GO1 RT using the equation:
Finally, to index response monitoring, RT was examined as a function of trial history. Median RT was computed separately for no-step trials preceding and following no-step trials, compensated step trials, and noncompensated step trials (i.e. errors). RTs on no-step trials preceding and following two consecutive step trials were included in this analysis only if the response on the two step trials was the same. Post-compensated slowing was calculated as the difference between median RT for no-step trials preceding and following a compensated trial. Likewise, post-error slowing was calculated as the difference between median RT for no-step trials preceding and following a noncompensated trial.
2.3.3 Statistical Methods
Fisher's exact tests, independent t-tests, and repeated measures ANOVAs were used where appropriate. Spearman rank-correlation coefficients were used to evaluate the association between clinical symptoms and performance measures in SZ patients. All tests were two-tailed. Subjects were excluded if the adaptive tracking procedure in the double-step task was ineffective, defined by a proportion of successfully inhibited responses lying outside a 95% binomial confidence interval around p=0.5.
3. Results
Task performance in SZ and HC is outlined in Table 2.
Table 2.
Performance measures of the patient and control groups.
| Controls Mean (s.d.) | SZ patients Mean (s.d.) | t-statistic | p | |
|---|---|---|---|---|
| Probability of Inhibition (%) | 47.8 (2.7) | 47.8 (4.0) | 0.01 | 0.99 |
| No-step RT (ms) | 289 (46) | 311 (80) | 1.01 | 0.32 |
| Noncompensated RT(ms) | 256 (36) | 269 (88) | 0.56 | 0.56 |
| Compensated RT (ms) | 284 (58) | 349 (86) | 2.59 | 0.01 |
| TSRT (ms) | ||||
| Mean calculation method | 147 (36) | 186 (47) | 2.76 | 0.009 |
| Integration calculation method | 131 (37) | 163 (57) | 1.96 | 0.059 |
| Post-compensated slowing (ms) | 9 (22) | 28 (58) | 1.26 | 0.22 |
| Post-error slowing (ms) | 21 (20) | 38 (35) | 1.79 | 0.08 |
| Post-step slowing (ms) | 14 (12) | 32 (35) | 2.15 | 0.04 |
Group means and standard deviations of double-step task performance measures in the patient and control groups. Test statistics and corresponding p-values of group comparisons are presented. Two-tailed independent-sample t-tests were used to compare all variables. Abbreviations-TSRT: Target step reaction time.
3.1 Probability of inhibition
The dynamic tracking procedure was successful. The mean percentage of noncompensated trials was 48% and there was no group difference (t(32)=0.01, p=0.99). For each subject, the slope of the compensation function plotted against ZRFT was calculated (Supplementary Figure 1). There was no group difference in the slope (t(32)=0.18, p=0.86), providing evidence for equal variability in the inhibitory process across groups.
3.2 Speed of response execution
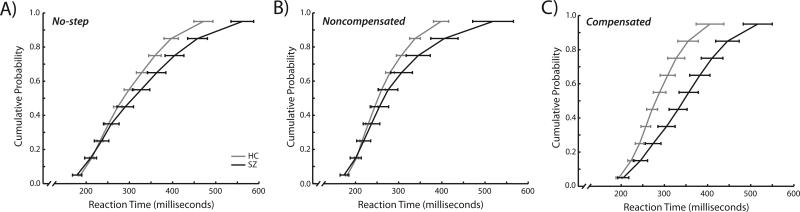
The effect of trial type (no-step, noncompensated, compensated) on median RT of the first saccade was assessed using a repeated measures ANOVA with group as a between-subjects variable and trial type as a within-subjects variable (Figure 2). There was a significant effect of trial type (F(2,64)= 11.67, p<0.0001). Noncompensated RTs were faster than no-step RTs (t(33)=8.2, p<0.0001), consistent with race model predictions (Logan & Cowan, 1984), and compensated RTs (t(33)=4.8, p<0.0001). There was no difference between compensated and no-step RTs (t(33)=1.6, p=0.11). There was no main effect of group (F(1,32)=2.5, p=0.13); however, there was a significant group-by-trial type interaction (F(1,32)=6.0, p=0.004). Planned comparisons indicated longer compensated RTs in SZ patients (t(32)=2.6, p=0.01) but no significant group differences in no-step (t(32)=1.0, p=0.31) or noncompensated (t(32)=1.48, p=0.59) RTs. RTs in SZ were only slowed when required to first inhibit a saccade and then redirect gaze.
Figure 2.
Vincentized reaction time distributions for no-step trials (a), noncompensated trials (b), and compensated trials (c). Healthy controls are depicted in blue and schizophrenia patients are depicted in red. No-step and noncompensated RTs were measured as the time between T1 onset at the onset of the first saccade. Compensated RTs were measured as the time between T2 onset and the onset of the first saccade. For each participant, reaction times for each of the three trial types were binned into deciles. Decile means were averaged across subjects to create the group-averaged vincentized RT distributions. Error bars represent standard error of the mean.
3.3 TSRT
Because a recent study reported that the mean method overestimates SSRT/TSRT as the GO RT distribution becomes increasingly right skewed (Verbruggen et al., 2013), group differences in the right skew of the no-step RT distribution were evaluated by comparing the parameter of the fitted ex-Gaussian distributions using an independent t-test. Although there was a trend for larger parameter estimates in schizophrenia, (t(32)=2.0, p=0.052), the value was not in the range in which the mean method overestimated TSRT (Verbruggen et al., 2013).
To examine the effect of both group and calculation method on TSRT, a repeated-measures ANOVA was conducted with group and calculation method (mean, integration) entered as between- and within-subjects variables, respectively. There was a significant effect of group (F(1,32)=5.6, p=0.02); SZ patients had significantly longer TSRT than HC. There was also a significant effect of TSRT calculation method (F(1,32)=37.8, p<0.0001). The mean method yielded longer TSRT estimates. Importantly, however, there was no significant group-by-calculation method interaction (F(1, 32)=1.1, p=0.30), indicating that longer TSRT in SZ was irrespective of calculation method. We replicated this analysis on our previously published countermanding findings (see Supplementary Material), and found longer SSRT in SZ regardless of calculation method.
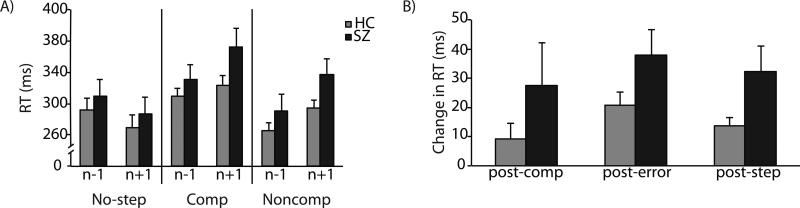
3.4 Trial history effects
See Supplementary Material for detailed analysis of trial history effects. Consistent with previous studies, we observed that relative to the n-1th trial, participants slow down on the no-step trial immediately following step trials, and speed up following consecutive no-step trials (Figure 3). Independent t-tests were used to evaluate group differences in the magnitude of post-compensated and post-error slowing. There was no group difference in either post-compensated (t(32)=1.26, p=0.22) or post-noncompensated (t(32)=1.79, p=0.08) slowing. However, collapsed across noncompensated and compensated trials, patients slowed down significantly more following step trials (t(32)=2.15, p=0.04).
Figure 3.
Mean no-step RT as a function of trial history. A) Mean no-step RT for trials following (n+1) and preceding (n-1) no-step, compensated (Comp) and noncompensated (Noncomp) trials for healthy controls in blue and schizophrenia patients in red. B) Mean post-compensated, post-error, and post-step trial slowing. Error bars represent standard error of the mean.
3.5 Symptom and occupational functioning correlations
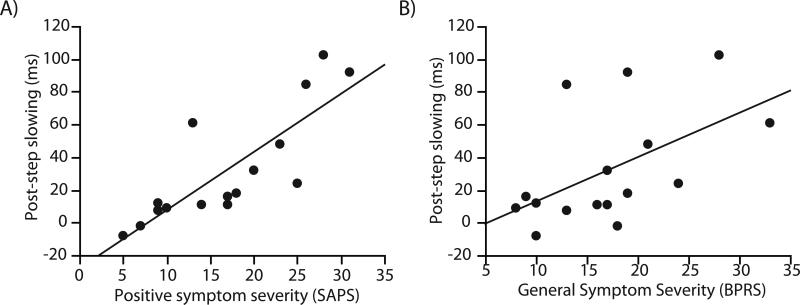
We examined the relationship between SAPS, SANS, and BPRS scores and TSRT, post-compensated slowing, post-error slowing, and post-step slowing. Higher SAPS and BPRS scores were correlated with post-compensated (SAPS: rs=0.7, p=0.002;BPRS: rs=0.6, p=0.007), post-noncompensated (SAPS: rs=0.6, p=0.01,BPRS: rs=0.5, p=0.05), and post-step (SAPS: rs=0.9, p<0.0001,BPRS: rs=0.6, p=0.01; Figure 4) slowing. No other significant symptom correlations were observed.
Figure 4.
Relationship between post-step trial slowing and severity of positive symptoms, indexed by SAPS score (A) and general psychiatric symptoms, as indexed by BPRS score (B).
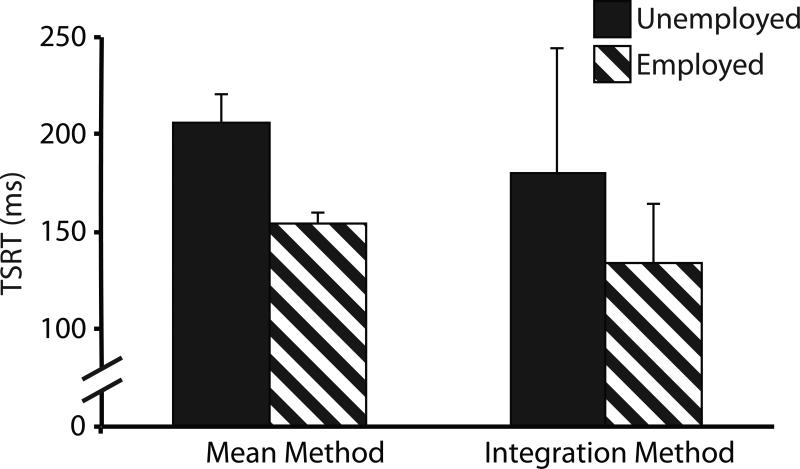
To examine the relationship between employment and task performance, we divided SZ patients by employment status and compared employed and unemployed patients on TSRT and history-based slowing measures. For TSRT, we performed a repeated-measures ANOVA with employment status and estimation method entered as between- and within-subject variables, respectively. Consistent with our previous finding (Thakkar et al., 2011), there was a statistical trend towards a main effect of employment group (F(1,14)=4.2, p=0.059), with unemployed patients having slower TSRT than employed patients (Figure 5). Again larger TSRT estimates were obtained using the mean method (F(1,14)=15.4, p=0.002), but there was no significant employment-by-estimation method interaction (F(1,14)=0.20, p=0.67).
Figure 5.
TSRT for unemployed (red bars) and employed (striped bars) schizophrenia patients, using both the mean method and method of integration for calculating TSRT. Error bars represent standard error of the mean.
4. Discussion
We observed that SZ patients had impairments in reactive control of eye movements. First, patients had poorer efficiency of inhibition, indexed by longer TSRT, consistent with previous studies using key press responding on this task (Huddy et al., 2009; Hughes et al., 2012; Nolan et al., 2011; Shin et al., 2013; but see Badcock et al., 2002, Zandbelt et al., 2011). Longer TSRT was related to employment status. Importantly, data complied with race model assumptions, and longer TSRT in SZ was not dependent on the method used to calculate it. Second, we found a specific impairment in response execution only when required to redirect gaze to a new location. Otherwise, data were consistent with intact visually-guided saccade latencies in schizophrenia (Gale & Holzman, 2000; Holzman et al., 1973). We also observed abnormal response monitoring in SZ. Patients slowed down more than HC following step trials, and the magnitude of slowing was strongly correlated with positive symptom severity. Data from the current study extend findings of saccadic countermanding performance in SZ (Thakkar et al., 2011) and indicate replicable impairments in reactive inhibition and idiosyncratic trial-by-trial adjustments. The implication of these findings for understanding cognitive control disturbances, potential neurobiological mechanisms underlying disturbances, and the clinical relevance of these impairments are discussed.
4.1 Nature of cognitive control disturbances in schizophrenia
These data shed further light on the precise nature of cognitive control disturbances in SZ. Patients are argued to have a prominent impairment in proactive action control and an increased reliance on reactive control mechanisms (e.g. Barch & Ceaser, 2012; Zandbelt et al., 2011). However, an alternative theory of cognitive deficits in SZ has long argued that the general inability to maintain and use mental representations to guide behavior on-line is the core problem, which would affect proactive and reactive cognitive control (Goldman-Rakic, 1994; Lee & Park, 2005; Park et al., 1995). Indeed, longer TSRT and compensated RTs suggest additional reactive control impairments in SZ, and consistent with Goldman-Rakic's theory (1994), the importance of internal representation in guiding behavior proactively as well as reactively cannot be underestimated.
Is it possible, however, that longer TSRT and compensated RTs are products of proactive control deficits? That is, perhaps impairments in maintaining task goals, resulting in a failure to trigger a STOP process, results in poor task performance. A failure to trigger the STOP process, however, would result in a flatter compensation function because on some trials subjects would fail to inhibit regardless of the target step delay (Logan et al., 1984). Because the compensation function slopes are equivalent across groups, we do not think a failure to maintain task goals can fully account for our results. However, abnormal maintenance of the stimulus or the target representation itself, regardless of the maintenance of the task goal, might also play a role in the task performance. In sum, reactive and proactive control interactions merit further exploration in SZ.
A second consideration is that longer TSRT and slower compensated saccade latencies are caused by impairments in low-level perceptual or attentional processes. That is, perhaps patients are not delayed at inhibiting, per se, but rather in processing the cue to stop/change action. This is an intriguing possibility, given modeling work showing that perceptual processing time comprises a large part of SSRT/TSRT (Boucher et al., 2007; Logan et al., 2014; Salinas & Stanford, 2013). However, equal no-step RTs would argue against generally slowed target selection processes, consistent with intact bottom-up, stimulus-driven attentional processes in schizophrenia (Gold et al., 2007; Mayer et al., 2012; Mori et al., 1996). An additional hypothesis is that longer TSRT is due to impaired dual-task performance. These hypotheses warrant explicit investigation in future studies.
With regard to our finding of exaggerated post-step slowing, results are partially consistent with our countermanding study where we observed greater slowing in SZ patients following trials of successful inhibition. Could these larger history-based adjustments be taken as evidence for better response monitoring? In interpreting the significance of these current results, we turn to non-mutually exclusive accounts of post-stop/step signal slowing (Bissett & Logan, 2011). One theory posits that trial history-based slowing arises due to conflict between mutually incompatible responses in the prior trial (Botvinick et al., 2001). Thus, our findings could be explained by greater conflict between going and stopping or a stronger behavioral response to conflict in schizophrenia. This interpretation is consistent with previous studies showing greater slowing following antisaccade production in SZ (Barton et al., 2005; Barton et al., 2006). Although this argument can explain greater slowing following correctly inhibited saccades observed in our previous study, it is less consistent with the general post-step slowing we observed in the current study.
Alternatively, since step trials comprised a minority of trials, greater post-step slowing in SZ could arise from greater attention to infrequent stimuli (Notebaert et al., 2009). Indeed, exaggerated post-error slowing has been observed in SZ, but only when errors were infrequent (Nunez Castellar et al., 2012). That greater post-step slowing in schizophrenia is due to increased orienting towards infrequent events fits neatly with the theory that psychosis arises from aberrant novelty detection (Christensen & Bilder, 2000; Gray et al., 1991; Kapur, 2003). This explanation is bolstered by the strikingly high correlation between positive symptom severity and post-step slowing. Arguing against this hypothesis, however, are findings of increased post-stop slowing with increases in stop-signal and error probability in HC (Bissett & Logan, 2011).
Another possible mechanism for greater post-step slowing in SZ is aberrant probability estimation (Bell et al., 2006). SZ patients are more likely to make probabilistic judgments based on less evidence than HC (Garety, 1991; Garety et al., 1991; Huq et al., 1988) and, importantly, over-adjust based on disconfirmatory evidence (Garety et al., 1991; Langdon et al., 2010; Moritz & Woodward, 2005). That is, they rely on the most recent events to make probabilistic decisions. It is possible that a step/stop signal leads to a reactive shift in the estimated overall proportion of step/stop trials in SZ. Since participants slow down with increasing proportions of stop/step trials in these tasks (Emeric et al., 2007; Logan & Burkell, 1986; Verbruggen & Logan, 2009), this change in estimated step probability could result in greater transient slowing in SZ (Bissett & Logan, 2011). Given the theorized relationship between probabilistic reasoning and delusion development, this explanation is consistent with the correlation between post-step slowing and positive symptoms.
4.2 Neural mechanisms
Neurophysiology work conducted in non-human primates performing the saccadic double-step and countermanding tasks provides us with unique leverage to understand neurobiological underpinnings of cognitive control impairments in SZ. Regarding saccade inhibition, saccade-producing neurons in both the FEF and SC must quickly reduce their activity in order to accomplish response inhibition (Hanes et al., 1998; Murthy et al., 2009; Paré & Hanes, 2003). Neurons capable of inhibiting pre-saccadic activity have been described in FEF, SC and the BG. Contributions of BG circuits for controlling movement initiation have long been apparent (Hikosaka et al., 2000), and BG activation during double-step task performance has been found to correlate with faster TSRT (Thakkar et al., 2014; see also Zandbelt & Vink, 2010). Thus, to the extent that slower TSRT is reflecting inhibition impairments, we can make specific hypotheses about disturbances in a network involving specific BG nuclei and cortical and subcortical oculomotor regions that underlie slower inhibition in this task.
How the brain monitors performance and makes adjustments to saccadic RT has also been investigated in non-human primates, and the medial frontal cortex has been highlighted. Neurons in supplementary eye fields (SEF) are sensitive to errors and conflict between movement and fixation-related activity in the FEF (Stuphorn et al., 2000), and these signals can bias RTs via anatomical connections with cortical and subcortical oculomotor regions (Stuphorn & Schall, 2006). Thus, we can predict that exaggerated post-step slowing in SZ might arise from heightened conflict between movement and fixation-related activity in the FEF, heightened sensitivity to errors and conflict in the SEF, or a larger bias in latency by SEF resulting from the preceding trial.
4.3 Limitations
This study should be considered in light of its limitations. First, patients were medicated; however, we do not think that antipsychotics are causing group differences in performance. Basic RTs were normal in SZ patients, and there was no correlation between task performance and medication dose (see Supplementary Material). Although the effect of medication on reactive control in SZ has not been explicitly tested, antipsychotics improve performance on the related antisaccade task (Harris et al., 2006). With regards to post-error slowing, a single haloperidol dose has no effect on post-error slowing (de Bruijn et al., 2006; Zirnheld et al., 2004). Regardless, medication effects warrant further study. Another caveat in interpreting the current results is the non-replicability of correlations between task performance and clinical symptoms across studies. In this study, we did not replicate the correlation between negative symptom severity and inhibition speed. Additionally, we did not observe a significant correlation between history-based slowing and positive symptom severity in our previous countermanding study. These inconsistencies likely arise from variability in clinical status of study samples, small groups, and limited range of symptom severity within samples. Larger and clinically heterogeneous samples will aid in elucidating putative relationships between cognitive control and symptoms.
4.4 Clinical implications
We observed a robust relationship between trial-by-trial changes in behavior and positive symptoms, which can shed light on specific cognitive neuropsychiatric origins of psychosis. Further, we have now shown a replicable relationship between inhibition efficiency and employment, attesting to the clinical relevance of longer SSRT/TSRT. Importantly, we know from rodent studies that some pharmacological manipulations improve reactive but not proactive inhibition (e.g. psychostimulants), and visa versa (Eagle et al., 2008; Eagle et al., 2007). In addition, the mathematical modeling and neurophysiology data on this task allow us to infer behaviorally-specific and biologically-constrained impairments in schizophrenia. Accordingly, we argue that there is real value in using the double-step or countermanding tasks, alongside proactive control tasks, to facilitate more targeted interventions and to assess potentially specific treatment-related changes in cognitive functioning in clinical trials (Barch et al., 2009).
Supplementary Material
Schizophrenia patients (SZ) and controls performed an inhibitory control task
This task required rapid inhibition and redirection of an eye movement
SZ showed reduced inhibition efficiency, which was related to unemployment
SZ made larger trial history-based adjustments that related to more severe symptoms
Results suggest abnormalities in a specific fronto-striatal oculomotor network
Acknowledgements
We would like to thank Lindsay Gilling McIntosh and Heathman Nichols for their assistance with clinical interviews and Dr. Stefan Heckers for his contributions to subject recruitment. This work was supported in part by F31-MH085405-01 (KNT), Netherlands Organisation for Scientific Research Rubicon grant (KNT), NARSAD (SP), MH073028 (SP), P30-HD015052 (SP, JDS, KNT), FA9550-07-1-0192 (JDS, GDL), P30-EY08126 (JDS), MH055806 (JDS), the E. Bronson Ingram Chair in Neuroscience (JDS) and MH073878 (GDL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
If the TSD was less than 94ms, T1 was only presented for the length of the TSD. At TSDs of 47 or 94 ms, T1 offset and T2 onset were simultaneous.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA.: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA.: 1984. [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol. Med. 2002;32:287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol. (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophr. Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV, Lindgren KA, Goff DC, Manoach DS. What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. J. Abnorm. Psychol. 2005;114:75–84. doi: 10.1037/0021-843X.114.1.75. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Goff DC, Manoach DS. The inter-trial effects of stimulus and saccadic direction on prosaccades and antisaccades, in controls and schizophrenia patients. Exp. Brain Res. 2006;174:487–498. doi: 10.1007/s00221-006-0492-9. [DOI] [PubMed] [Google Scholar]
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn. Sci. 2006;10:219–226. doi: 10.1016/j.tics.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Executive control: balancing stability and flexibility via the duality of evolutionary neuroanatomical trends. Dialogues Clin. Neurosci. 2012;14:39–47. doi: 10.31887/DCNS.2012.14.1/rbilder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am. J. Psych. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br. J. Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2011;37:392–404. doi: 10.1037/a0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Stop before you leap: changing eye and hand movements requires stopping. J. Exp. Psychol. Hum. Percept. Perform. 2013;39:941–946. doi: 10.1037/a0033049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting Premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol. Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camalier CR, Gotler A, Murthy A, Thompson KG, Logan GD, Palmeri TJ, et al. Dynamics of saccade target selection: race model analysis of double step and search step saccade production in human and macaque. Vision Res. 2007;47:2187–2211. doi: 10.1016/j.visres.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BK, Bilder RM. Dual cytoarchitectonic trends: an evolutionary model of frontal lobe functioning and its application to psychopathology. Can. J. Psychiatry. 2000;45:247–256. doi: 10.1177/070674370004500303. [DOI] [PubMed] [Google Scholar]
- Clementz BA. Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology. 1998;35:648–668. [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. J. Psychopharmacol. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. J. Psychopharmacol. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, et al. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res. 2007;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J. Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Leslie MW, Pouget P, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: Supplementary eye field. J. Neurophysiol. 2010;104:1523–1537. doi: 10.1152/jn.01001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. Biometrics Research Department; New York: 1995. [Google Scholar]
- Gale HJ, Holzman PS. A new look at reaction time in schizophrenia. Schizophr. Res. 2000;46:149–165. doi: 10.1016/s0920-9964(00)00006-2. [DOI] [PubMed] [Google Scholar]
- Garety PA. Reasoning and delusions. Br. J. Psychiatry Supplement. 1991:14–18. [PubMed] [Google Scholar]
- Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J. Nerv. Ment. Dis. 1991;179:194–201. doi: 10.1097/00005053-199104000-00003. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Massey JT. Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol. 1981;46:725–743. doi: 10.1152/jn.1981.46.4.725. [DOI] [PubMed] [Google Scholar]
- Godlove DC, Emeric EE, Segovis CM, Young MS, Schall JD, Woodman GF. Event-related potentials elicited by errors during the stop-signal task. i. macaque monkeys. J. Neuro. 2011;31:15640–15649. doi: 10.1523/JNEUROSCI.3349-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophr. Res. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlings JNP, Hemsley DR, Smith AD. The neuropsychology of schizophrenia. Behav. Brain Sci. 1991;14:1–81. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J. Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol. Med. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181:179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- Huddy VC, Aron AR, Harrison M, Barnes TR, Robbins TW, Joyce EM. Impaired conscious and preserved unconscious inhibitory processing in recent onset schizophrenia. Psychol. Med. 2009;39:907–916. doi: 10.1017/S0033291708004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol. Psychol. 2012;89:220–231. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non- deluded subjects. Q. J. Exp. Psych. A. 1988;40:801–812. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psych. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr. Bull. 2010;36:321–330. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin J, Eriksen CW. Use of a delayed signal to stop a visual reaction-time response. J. Exp. Psychol. Gen. 1966;72:805–811. [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J. Abnorm. Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol. Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Logan GD, Burkell J. Dependence and independence in responding to double stimulation: A comparison of stop, change, and dual-task paradigms. J. Exp. Psychol. Hum. Percept. Perform. 1986;12:549–563. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Van Zandt T, Verbruggen F, Wagenmakers EJ. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol. Rev. 2014;121:66–95. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Matzke D, Love J, Wiecki TV, Brown SD, Logan GD, Wagenmakers EJ. Release the BEESTS: Bayesian Estimation of Ex-Gaussian Stop-Signal reaction time distributions. Front. Psychol. 2013;4:918. doi: 10.3389/fpsyg.2013.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7:e48586. doi: 10.1371/journal.pone.0048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Pani P, Ferraina S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. J. Neurophysiol. 2011;106:1454–1466. doi: 10.1152/jn.00995.2010. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka G, Ayaka Y, Michitsuji S, Niwa H, Uemura M, et al. Preattentive and focal attentional processes in schizophrenia: a visual search study. Schizophr. Res. 1996;22:69–76. doi: 10.1016/0920-9964(96)00049-7. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br. J. Clin. Psychol. 2005;44:193–207. doi: 10.1348/014466505X35678. [DOI] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson KG. Frontal eye field contributions to rapid corrective saccades. J. Neurophysiol. 2007;97:1457–1469. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Schall JD, Thompson KG. Neural control of visual search by frontal eye field: effects of unexpected target displacement on visual selection and saccade preparation. J. Neurophysiol. 2009;101:2485–2506. doi: 10.1152/jn.90824.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MJ, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonindependent and nonstationary response times in stopping and stepping saccade tasks. Attent. Percept. Psychophys. 2010;72:1913–1929. doi: 10.3758/APP.72.7.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KA, D'Angelo D, Hoptman MJ. Self-report and laboratory measures of impulsivity in patients with schizophrenia or schizoaffective disorder and healthy controls. Psychiatry Res. 2011;187:301–303. doi: 10.1016/j.psychres.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Nunez Castellar E, Houtman F, Gevers W, Morrens M, Vermeylen S, Sabbe B, et al. Increased orienting to unexpected action outcomes in schizophrenia. Front. Hum. Neurosci. 2012;6:32. doi: 10.3389/fnhum.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropyschologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Report. 1962;10:799–812. [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J. Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch. Gen. Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Sureshbabu R, Murthy A. Understanding how the brain changes its mind: microstimulation in the macaque frontal eye field reveals how saccade plans are changed. J. Neurosci. 2012;32:4457–4472. doi: 10.1523/JNEUROSCI.3668-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Stanford TR. The countermanding task revisited: fast stimulus detection is a key determinant of psychophysical performance. J. Neurosci. 2013;33:5668–5685. doi: 10.1523/JNEUROSCI.3977-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cogn. Affect. Behav. Neurosci. 2007;7:396–412. doi: 10.3758/cabn.7.4.396. [DOI] [PubMed] [Google Scholar]
- Schall JD, Godlove DC. Current advances and pressing problems in studies of stopping. Curr. Opin. Neurobiol. 2012;22:1012–1021. doi: 10.1016/j.conb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YS, Kim SN, Shin NY, Jung WH, Hur JW, Byun MS, et al. Increased intra-individual variability of cognitive processing in subjects at risk mental state and schizophrenia patients. PLoS One. 2013;8:e78354. doi: 10.1371/journal.pone.0078354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr. Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat. Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan G, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia Biol. Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, van den Heiligenberg FM, Kahn RS, Neggers SFW. Frontal- subcortical circuits involved in reactive control and monitoring of gaze. J. Neurosci. 2014;34:8918–8929. doi: 10.1523/JNEUROSCI.0732-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychol. Sci. 2013;24:352–362. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop- signal paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Kompus K, Hugdahl K. Impaired cognitive inhibition in schizophrenia: a meta-analysis of the Stroop interference effect. Schizophr. Res. 2011;133:172–181. doi: 10.1016/j.schres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, van Buuren M, Kahn RS, Vink M. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol. Psychiatry. 2011;70:1151–1158. doi: 10.1016/j.biopsych.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLoS One. 2010;5:e13848. doi: 10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J. Cogn. Neurosci. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.