Abstract
Purpose: To detect the expression and prognostic clinical significance of heat-shock protein gp96 (HSP gp96) in gallbladder cancer. Methods: Immunohistochemistry was used to detect and compare the rate of HSP gp96 expression in 107 samples of gallbladder cancer, 70 of gallbladder adenoma and 67 of chronic cholecystitis. The association of clinicopathological factors and patients’ survival were calculated by univariate and multivariate (Cox proportional hazard regression method) analysis. Results: The expression positive rate of HSP gp96 was 90.7% (97/107) in gallbladder cancer, 71.4% (50/70) in gallbladder adenoma and 47.76% (32/67) in chronic cholecystitis respectively. The positive rate of HSP gp96 in gallbladder cancer tissues was significantly higher than that in gallbladder adenoma and chronic cholecystitis tissues (P < 0.01). Multivariate and Cox regression analysis showed that positive of HSP gp96 (P = 0.026) expression was an independent poor prognostic predictor in gallbladder cancer. Conclusions: HSP gp96-positive expression is closely correlated with poor survival in gallbladder cancer.
Keywords: HSP gp96, gallbladder cancer, gallbladder adenoma, chronic cholecystitis
Introduction
Gallbladder cancer is a rare gastrointestinal malignancy, but it is the most common malignant tumor of the biliary tract worldwide [1,2]. More than 85% of gallbladder cancers belong to adenocarcinomas, often well or moderately differentiated, and the rest approximately 15% are squamous, adenosquamous or undifferentiated carcinoma. This tumor is traditionally regarded as a highly lethal disease with an overall 5-year survival of less than 5% [3]. To date, surgery is the only potentially curative modality for patients with gallbladder cancer. However, more than 75% gallbladder cancer patients are diagnosed as the disease when it is beyond the limits of resection [1]. Overall median survival for advanced stage gallbladder cancer is 2-5 months [4]. A better understanding of pathogenesis and clinicopathological characteristics of gallbladder cancer may provide insights for prognosis and treatment improvement for this deadly disease.
Heat-shock proteins (HSPs) are a family of highly conserved proteins and grouped together according to sequence homology and molecular size. Recent guidelines according to their molecular size, HSPs have been classified into 9 distinct families-HSPH (HSP110), HSPC (HSP90), HSPA (HSP70), DNAJ (HSP40), HSPB (small HSPs), the chaperoninfamilies HSPD (HSP60), HSPE (HSP10) and CCT (TriC) as well as chaperonin-like genes (MKKS, BBS) [5], Heat-shock protein gp96, also called glucose regulated protein 94 (GRP94), is a member of the HSP90 family of stress proteins. Gp96 has a close relationship with antitumor immunity and its immunological properties have been described [6,7]. In addition, it acts as a molecular chaperone and forms a complex with tumor antigens before the antigen-presenting cells incorporate the antigen [8-10]. A gp96-antigen complex is then incorporated into dendritic cells via the CD91 surface molecule, which is also known as gp96 receptor [11-13]. A number of animal studies documented that gp96 purified from tumors was able to initiate efficient tumor-specific CTL responses and protective immunity. Moreover, gp96 worked in both prophylactic and therapeutic protocols [6,7,14-19]. Therefore, gp96 is a key molecule in antitumor immunity.
Nowadays, it is fever to study tumor immunity function of HSP gp96, but there is no study on gp96 in gallbladder cancer. Up to now, the relationship between gp96 expression and clinicopathological and prognostic significance in gallbladder cancer was still unknown. It is necessary to make a deep research. In the present study, we examined the expression frequency of HSP gp96 in 107 specimens of gallbladder cancer, 70 of gallbladder adenoma and 67 of chronic cholecystitis by means of immunohistochemistry, with attempt to better understand the relationship between HSP gp96 expression and prognostic clinical significance in gallbladder cancer.
Patients and methods
Patients
Dates from all the patients who were diagnosed as gallbladder adenocarcinoma, gallbladder adenoma or chronic cholecystitis after the surgery in the First, Second, Third and Fourth Affiliated Hospital of Harbin Medical University from January 2012 to January 2014 were retrospectively reviewed of the 107 cases with gallbladder cancer, 38 was male (35.5%) and 69 was female (64.5%). Age of patients ranged from 37 to 84 years old with an average 61.02 = 10.03. The pathological types of these cases were all adenocarcinoma, including of 32 cases (29.9%) well-differentiated adenocarcinoma, 40 cases (37.4%) of moderately-differentiated adenocarcinoma and 35 cases (32.7%) of poorly-differentiated adenocarcinoma. There were 58 cases (54.2%) with regional lymph node metastasis and 48 (44.9%) with gallbladder cancer complicated with gallstones. Tumors staging was performed according to the pathological tumor, node, metastasis (pTNM) classification based on the American Joint Committee on Cancer staging manual (AJCC, 7th edition) [20]. They were classified as T1 (n = 6, 5.61%), T2 (n = 35, 32.71%), T3 (n = 35, 32.71%) and T4 (n = 31, 28.97%). All the patients underwent a radical surgery. A total of 70 patients’ gallbladder adenoma tissues with no acute inflammation and another 67 patients’ chronic cholecystitis specimens were also studied as control, in which there were no severe clinical symptoms. All these specimens were fixed by 4% formaldehyde, followed by conventional paraffin-embedded sectioning, this work had discussed and permission by the Harbin Medical University ethics committee.
Immunohistochemical staining
The sections (2 to 4 μm) of formalin-fixed, paraffin-embedded surgical specimens were transferred to glass slides and used for immunohistochemical staining. Paraffin was removed from the sections; next, antigen retrieval was performed with high pressure and high temperature by citric acid then cooled at room temperature at least for 40 min. After washing, samples were treated for internal peroxidase blocking with methanol with 3% hydrogen peroxide in room temperature for 30 min, followed by 5-min washing three times. The primary anti-human gp96 monoclonal antibody (ab54244, Abcam, prediluted, USA) was applied to the sections at 4°C overnight followed by washing. Thereafter, the sections were incubated with second antibody (rat antibody) at room temperature for 20 min followed by washing. Next, sections were treated with counterstained with hematoxylin. Thereafter, samples were dehydrated in ascending concentrations of ethanol, and coverslips were mounted. Samples were observed by two independent, blinded pathologists and immunohistochemical scores were performed using a semiquantitative grading system as previous studies [21]. Sections with no labeling or with < 5% labeled cells were scored as 0, and sections were scored as 1 with labeling of 5-30% of cells, as 2 with 31-70% of cells and as 3 with ≥ 71% of cells. The staining intensity was scored similarly with 0 used for negative staining, 1 for weakly positive, 2 for moderately positive and 3 for strongly positive. The scores for moderately positive tumor cells and for the staining intensity were added to generate an immunoreactive score for each specimen. The product of the quantity scores was calculated such that a final score 0-2 indicated negative and 3-6 score indicated positive.
Statistical analysis
Associations in 2 × 2 tables were evaluated with Fisher’s exact test and other tables were compared by the x2 test. Comparison of the means between groups was performed by x2 test. All survival time was defined as the period from the operation day to death or the last follow-up. Multivariate analyses were applied by the Cox proportional hazard regression analysis to evaluate the correlation between survival time and multiple variables. All tests were two sided and the significance level was set at P < 0.05. SAS version 13.0 software was used for statistical analysis.
Results
Expression of gp96 in gallbladder adenocarcinoma
Immunohistochemical examination was used to analyze gp96 expression in 107 gallbladder adenocarcinoma specimens, 70 gallbladder adenoma specimens, 67 chronic cholecystitis specimens and each was categorized as positive or negative. The positive samples in each group were shown in Figure 1. The positive rate in gallbladder cancer, gallbladder adenoma and chronic cholecystitis were 90.7% (97/107), 71.4% (50/70) and 47.8% (32/67) respectively and they had obviously difference among these three groups (P < 0.0001). In the groups of gallbladder cancer, the gp96-positive rate was evaluated according to several clinicopathologic parameters, which was shown in Table 1. The gp96-positive rates have statistical significance in terms of tumor depth (T1, T2, T3, T4) and lymph node metastasis. No statistical significance was seen in the age, sex, gallstone and the degree of tumor differentiation.
Figure 1.
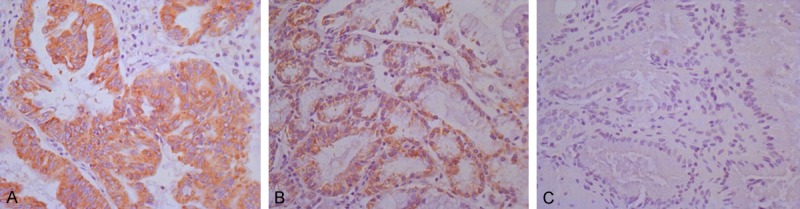
Positive expression of gp96 in gallbladder cancer (A), gallbladder adenoma (B) and chronic cholecystitis (C) (original magnification IHC ×200).
Table 1.
Expression of HSP gp96 with clinicopathologic parameters in gallbladder adenocarcinoma and adenoma
| Group | NO. of cases | HSP gp96-positive (%) | P |
|---|---|---|---|
| Gallbladder adenocarcinoma | |||
| Overall | 107 | 97 (90.7) | |
| Age (years) | |||
| < 61 | 54 | 46 (85.19) | 0.0930 |
| ≥ 61 | 53 | 51 (96.23) | |
| Sex | |||
| Male | 38 | 33 (86.84) | 0.3218 |
| Female | 69 | 64 (92.75) | |
| Differentiated | |||
| Well | 32 | 27 (84.38) | 0.1953 |
| Moderately | 40 | 36 (90.00) | |
| Poorly | 35 | 34 (97.14) | |
| Lymph node metastasis | |||
| Positive | 58 | 57 (98.28) | 0.0051 |
| Negative | 49 | 40 (81.63) | |
| Tumor depth | |||
| T1 | 6 | 4 (66.67) | 0.0096 |
| T2 | 35 | 29 (82.86) | |
| T3 | 35 | 33 (94.29) | |
| T4 | 31 | 31 (100) | |
| Gallstone | |||
| Yes | 48 | 44 (91.67) | 1.000 |
| No | 59 | 53 (89.83) | |
| Gallbladder adenoma | |||
| Overall | 70 | 50 (71.43) | |
| Chronic cholecystitis | |||
| Overall | 67 | 18 (26.87) |
Univariate analysis for gp96 expression in several clinicopathologic parameters
Several clinicopathologic parameters were calculated and the differences were evaluated by univariate analysis, as shown in Table 1. Tumor depth and lymph node metastasis were statistically significant prognostic factors that were related to expression of gp96. On the other hand, age, sex, degree of differentiation and gallstone were not statistically significant with gp96 expression.
Multivariate analysis for survival
Cox proportional regression analysis of the several factors and the survival curves were shown in Figure 2. The results showed that positive expression of gp96, positive metastasis of lymph node, poor differentiated tumor and deeper invasion of tumor were the factors for poor prognosis after surgery. But age, gender, gallstone were not. The factor of gallbladder stone did not meet the conditions of multivariate analysis and was regarded as a stratification variable. The other factors were evaluated by multivariate analysis and the results indicated that gp96 positive was an independent risk factor for poor survival (hazard ratio 0.3920; P = 0.0175; Table 2).
Figure 2.
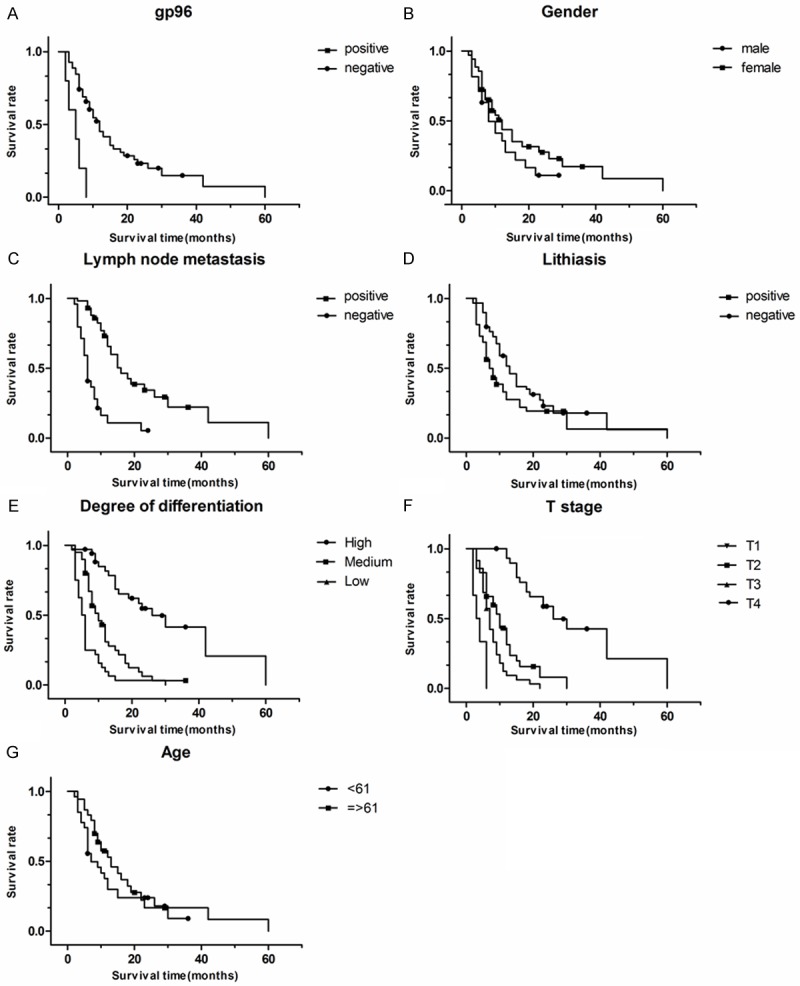
The survival rate was calculated by the Kaplan-Meier method according to gp96 (A), gender (B), lymph node metastasis (C), gall stone (D), degree of differentiation (E), T stage (F), age (G), the statistically significant prognostic factors was gp96 (A), lymph node metastasis (C), degree of differentiation (E), T stage (F).
Table 2.
Multivariate analysis for survival
| Factors | DF | Parameter | SE | Chi-Square | HR | 95% CI of HR | P |
|---|---|---|---|---|---|---|---|
| age | 1 | -0.0190 | 0.0133 | 2.0631 | 0.9810 | 0.9560-1.0070 | 0.1509 |
| gp96 | 1 | -0.9356 | 0.3937 | 5.6481 | 0.3920 | 0.1810-0.8490 | 0.0175* |
| gender | 1 | 0.4377 | 0.2528 | 2.9981 | 1.5490 | 0.9440-2.5430 | 0.0834 |
| Degree of differentiation | 1 | -0.7273 | 0.1837 | 15.6803 | 0.4830 | 0.3370-0.6930 | < .0001* |
| Lymph node metastasis | 1 | 0.6984 | 0.2847 | 6.0170 | 2.0100 | 1.1510-3.5130 | 0.0142* |
| T stage | 1 | 0.7452 | 0.1760 | 17.9208 | 2.1070 | 1.4920-2.9750 | < .0001* |
Statistically significant.
Discussion
HSPs are a family of highly conserved proteins and grouped together according to sequence homology and molecular size, which was widely existed in prokaryotes and eukaryotes, Ritossa in 1962 first discovered in drosophila salivary gland chromosome [22]. Recent guidelines proposed their classification into 9 distinct families [5]. And they are constitutively expressed in mammalian cells, but can be strongly induced under stressful conditions such as elevated temperatures, deprivation of nutrients, heavy metals, toxins, oxidants, bacterial or viral infections and therefore are also known as stress proteins. They constitute a system with essential housekeeping functions for proper folding, translocation, repair and degradation of proteins. And the heat shock protein Gp96 is a member of the family of HSP90, which through variety of pathways involved in cell growth and breeding [23], for example, the cell signal transduction, cell cycle regulation, etc. So far, the cytosolic Hsp70 and the endoplasmic reticulum (ER) resident HSP gp96 have been in the focus for immunization against malignancies. This was the first report HSP gp96 positive expression for gallbladder cancer and it was significantly correlated with gallbladder carcinoma. Similar results were obtained by E. Pick Y [24] in breast cancer, T. Shimamura [25] in lung cancer and Slotta-Huspenina J in Adenocarcinomas of the Esophagus [26], and so on. Some researchers have indicated that gp96 played an important role in antitumor immunity [27]. Although the exact anti-tumor action of HSP gp96 inhibitors remains largely unknown, but we know that it formed the gp96-antigen complex and this complex was incorporated by dendritic (DC) cells, and gp96 isolated from tumor cells has a binding affinity for the inhibitors between 20 and 200 times higher than does HSP gp96 isolated from normal cells. DC cells would be unable to incorporate the tumor antigen and elicit the cytotoxic T lymphocytes in the absence of gp96 expression [28,29]. These findings indicated that the gp96 expression correlated with tumor development or antitumor immunity and it should have an influence on patient prognosis. In this study, almost all the gallbladder cancer patients (90.7%), most gallbladder adenoma patients (71.4%) and fewer chronic cholecystitis patients (47.7%) were positive of gp96 expression. In other words, HSP gp96 expression gradually increased in chronic cholecystitis, gallbladder adenoma and gallbladder adenocarcinoma. This phenomenon may be caused by self-regulation of the body in order to cope with the abnormal proliferation of tumor cell, therefore upregulation of the HSP gp96 expression in tumor. This research implies that HSPgp96 plays a important role on the pathogenesis and development in human gallbladder cancer and there is hope in its application in identifying the degree of malignancy of cancer and utilization as a target for tumor immunity.
HSP gp96 has the capacity to elicit an immunoreaction to cancers and can be an activator of antitumor activity [30]. And the administration of gp96 could suppress tumor growth and enhance the antitumor effects [31]. Some studies have indicated that gp96 ought to be analyzed as a possible cancer vaccine [32-34]. Therefore, in gallbladder cancer, HSP gp96 expression is significant to elicit an effective immunoresponse to cancer cells. In this study, gp96 positive expression correlated closely with the poor prognosis. In our data, the gp96 positive rate was 98.28% (57/58) in lymph node metastasis positive patients, but it was 81.63% (40/49) in lymph node metastasis negative patients and there were statistically significance (P < 0.05). In other words, patients with gp96- positive had more opportunities to induce lymph nodes metastasis. This result indicated that gp96 expression could promote lymph node metastasis to some extent and than induce poor prognosis. HSP gp96 expression could weaken the antitumor effects and may be an available biomarker for prognosis of gallbladder cancer. Some study identified some other biomarkers-such as CD24, CDX2, and hepatocyte antigen. They are biomarker and maybe indicate the prognosis but they do not have immunological significance [35,36]. Gp96 could be a predictable marker for the prognosis and had potential immunoreactivity for patients with gallbladder cancer. Gp96 has many features and functions that remain to be revealed [38,39]. Therefore, further research is necessary. These efforts may improve the outcome of the patients with this fatal gallbladder cancer in the near future, but gallbladder cancer incidence rate is low, fewer patients underwent radical operation and radical operation without strict norms lead to this research have some limitations. In our study, there were 107 patients who were diagnosed as gallbladder adenocarcinoma enrolled in our group and it is necessary to collect more patients to be studied.
In conclusion, this study indicated that HSP gp96 played an important role in the pathogenesis and development in human gallbladder cancer. It also confirmed that positive of HSP gp96 expression was an independent poor prognostic predictor in gallbladder cancer.
Acknowledgements
This work was supported by department of Pathology of the First, Second, Third and Fourth Affiliated Hospital of Harbin Medical University.
Disclosure of conflict of interest
None.
References
- 1.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 2.Alexander S, Lemmens VE, Houterman S, Nollen L, Roumen R, Slooter GD. Gallbladder cancer, a vanishing disease? Cancer Causes Control. 2012;23:1705–1709. doi: 10.1007/s10552-012-0049-0. [DOI] [PubMed] [Google Scholar]
- 3.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 4.Lemrow SM, Perdue DG, Stewart SL, Richardson LC, Jim MA, French HT, Swan J, Edwards BK, Wiggins C, Dickie L, Espey DK. Gallbladder cancer incidence among American Indians and Alaskan natives, US, 1999-2004. Cancer. 2008;113(Suppl 5):1266–73. doi: 10.1002/cncr.23737. [DOI] [PubMed] [Google Scholar]
- 5.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava PK, Das MR. The serologically unique cell surface antigen of Zajdela ascetic hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984;33:417–422. doi: 10.1002/ijc.2910330321. [DOI] [PubMed] [Google Scholar]
- 8.Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER. Cell- secreted Gp96-Ig- peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal Immunol. 2010;3:182–92. doi: 10.1038/mi.2009.127. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, Rammensee HG, Schild H. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones. 2005;10:221–229. doi: 10.1379/CSC-117R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–32. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 12.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder RJ, Han DK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 14.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–5. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 15.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 16.Yedavelli SP, Guo L, Daou ME, Srivastava PK, Mittelman A, Tiwari RK. Preventive and therapeutic effect of tumor derived heat shock protein, gp96, in an experimental prostate cancer model. Int J Mol Med. 1999;4:243–8. doi: 10.3892/ijmm.4.3.243. [DOI] [PubMed] [Google Scholar]
- 17.Arnold-Schild D, Kleist C, Welschof M, Opelz G, Rammensee HG, Schild H, Terness P. One step single-chain Fv recombinant antibody-based purification of gp96 for vaccine development. Cancer Res. 2000;60:4175–8. [PubMed] [Google Scholar]
- 18.Kovalchin JT, Murthy AS, Horattas MC, Guyton DP, Chandawarkar RY. Determinants of efficacy of immunotherapy with tumor-derived heat shock protein gp96. Cancer Immun. 2001;1:7. [PubMed] [Google Scholar]
- 19.Sato K, Torimoto Y, Tamura Y, Shindo M, Shinzaki H, Hirai K, Kohgo Y. Immunotherapy using heat-shock protein preparations of leukemia cells after syngeneic bone marrow transplantation in mice. Blood. 2001;98:1852–7. doi: 10.1182/blood.v98.6.1852. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7th edition. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Zhang J, Feng Y, Li R, Sun X, Du W, Piao X, Wang H, Yang D, Sun Y, Li X, Jiang T, Kang C, Li Y. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol. 2012;44:1711–1717. doi: 10.1016/j.biocel.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Ritossa F. A new puffing pattern induced by heat shock and DNP in Drosophila. Experiential. 1962;18:571–573. [Google Scholar]
- 23.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 24.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 25.Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J Thorac Oncol. 2008;3:S152–159. doi: 10.1097/JTO.0b013e318174ea3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slotta-Huspenina J, Becker KF, Feith M, Walch A, Langer R. Heat Shock Protein 90 (HSP90) and Her2 in Adenocarcinomas of the Esophagus. Cancers (Basel) 2014;6:1382–1393. doi: 10.3390/cancers6031382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–59. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XX, Ding Y, Liu CG, Mikhail S, Yang CS. Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis. 2002;23:123–130. doi: 10.1093/carcin/23.1.123. [DOI] [PubMed] [Google Scholar]
- 29.Wolfram L, Fischbeck A, Frey-Wagner I, Wojtal KA, Lang S, Fried M, Vavricka SR, Hausmann M, Rogler G. Regulation of the expression of chaperone gp96 in macrophages and dendritic cells. PLoS One. 2013;8:e76350. doi: 10.1371/journal.pone.0076350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinagawa N, Yamazaki K, Tamura Y, Imai A, Kikuchi E, Yokouchi H, Hommura F, Oizumi S, Nishimura M. Immunotherapy with dendritic cells pulsed with tumor-derived gp96 against murine lung cancer is effective through immune response of CD8+ cytotoxic T lymphocytes and natural killer cells. Cancer Immunol Immunother. 2008;57:165–74. doi: 10.1007/s00262-007-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Wang H, Yang Z, Kon T, Zhu J, Cao Y, Li F, Kirkpatrick J, Nicchitta CV, Li CY. Enhancement of cancer radiation therapy by use of adenovirus-mediated secretable glucose-regulated protein 94/gp96 expression. Cancer Res. 2005;65:9126–31. doi: 10.1158/0008-5472.CAN-05-0945. [DOI] [PubMed] [Google Scholar]
- 32.Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, Yoneyama Y, Hoshino I, Murakami K, Usui A, Kano M, Ochiai T. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int J Oncol. 2007;31:509–15. [PubMed] [Google Scholar]
- 33.Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52:226–34. doi: 10.1007/s00262-002-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Wang Y, Wu B, Liu S, Wu E, Fan H, Gui M, Chen L, Li C, Ju Y, Zhang W, Meng S. Placenta-derived gp96 as a multivalent prophylactic cancer vaccine. Sci Rep. 2013;3:1947. doi: 10.1038/srep01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li QL, Yang ZL, Liu JQ, Miao XY. Expression of CDX2 and hepatocyte antigen in benign and malignant lesions of gallbladder and its correlation with histopathologic type and clinical outcome. Pathol Oncol Res. 2011;17:561–8. doi: 10.1007/s12253-010-9346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Zhang Y, Liao M, Deng Z, Gong L, Jiang J, Lynn L, Wu K, Miao X. Clinicopathologic and prognostic significance of CD24 in gallbladder carcinoma. Pathol Oncol Res. 2011;17:45–50. doi: 10.1007/s12253-010-9278-2. [DOI] [PubMed] [Google Scholar]
- 37.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25high- Foxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–11. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 38.Kliger Y, Levy O, Oren A, Ashkenazy H, Tiran Z, Novik A, Rosenberg A, Amir A, Wool A, Toporik A, Schreiber E, Eshel D, Levine Z, Cohen Y, Nold-Petry C, Dinarello CA, Borukhov I. Peptides modulating conformational changes in secreted chaperones: from in silico design to preclinical proof of concept. Proc Natl Acad Sci U S A. 2009;106:13797–801. doi: 10.1073/pnas.0906514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM 2nd, Tak PP, Haines GK 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–73. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]


