Abstract
ALK rearrangement is a very rare subset of squamous cell cancer of lung and the efficacy of crizotinib treatment for these patients is lack of data. Here we report a case with squamous cell cancer of lung that harbored the ALK rearrangement was given crizotinib in the second-line therapy. A 55-year-old female patient was diagnosed with squamous cell carcinoma of lung by bronchoscopy biopsy with stage IV. The patient was given two cycles of chemotherapy and the response was progressive disease. After failure to chemotherapy, genotype testing showed wild-type EGFR/KRAS and ALK rearrangement positive. The patient was administered with crizotinib and had a partial response, and the progression-free survival was 6 months. The side effects were tolerable.
Keywords: ALK rearrangement, squamous cell carcinoma, crizotinib
Introduction
In 2007, Soda et al [1] discovered the echinoderm microtubule-associated protein-like 4 and the anaplastic lymphoma kinase (EML4-ALK) in a patient with lung adenocarcinoma. Clinicopathological analysis have showed that the frequency of patients with NSCLC harboring the EML4-ALK fusion gene is approximately 5% in Western world and 3-11% in Chinese populations and this fusion gene is often observed in younger patients, patients who never or light smoke, and those with adenocarcinoma type without epidermal growth factor receptor (EGFR) mutations [2-6]. Crizotinib treatment, as the first oral ALK inhibitor, has been demonstrated higher efficacy (65% vs. 20%, respectively) and longer PFS (7.7 vs. 3.0 months, respectively) in the crizotinib arm than in the chemotherapy arm for patients with advanced ALK-positive non-small-cell lung cancer (NSCLC) previous treated with platinum-based chemotherapy (PROFILE 1007) [7]. Patients with squamous cell carcinoma harbored the ALK rearrangement are extremely rare (only 1%) [8], and the responding to crizotinib is not very clear. We reported the response to crizotinib for a case of ALK-rearranged squamous cell carcinoma with lung in the second-line therapy.
Case presentation
A 55-year-old Asian woman presented with cough in September 2013 for about a month. On examination, no positive signs were found. She had no history of smoking. Computed tomography (CT) of the chest showed a nodule, 1.2 cm by 1.1 cm, in the upper lobe of the left lung. MRI of the brain revealed metastatic nodules in the left cerebellum and the left frontal lobe. Bone scan demonstrated marked accumulation of 99mTc in the vertebrae L2 and the ninth rib. The bronchoscopy was performed and a neoplasm in the left upper bronchus was found. The pathologic diagnosis of biopsy under bronchoscopy was squamous cell carcinoma and the histologic type of carcinoma was assessed by immunohistochemistry analysis including P40, P63, cytokeratin 5/6, CK7, thyroid transcription factor 1, Napsin-A. The tumor cells showed a strong expression of P40, P63, cytokeratin 5/6 (CK), while thyroid transcription factor 1 (TTF-1), CK7, and Napsin-A were negative (Figure 1). The results confirmed the histologic type was squamous cell carcinoma. The clinical stage was T1aN1M1b with score 1 of performance status. In the first-line chemotherapy, the patient was administered with 1250 mg/m2 of gemcitabine on days 1, 8 and 75 mg/m2 of cisplatin on day 1, which was repeated every 21 days. After two cycles of chemotherapy, the pain due to bone metastasis became more severely and ultrasonography displayed new metastatic nodules in the right liver. The patient had progressive disease (PD). Then, the patient underwent genotype testing. No EGFR and KRAS mutation was found. ALK testing using IHC (Ventana anti-ALK (D5F3) rabbit monoclonal primary antibody (Cell Signal Technology, US), the Optiview DAB IHC detection kit, the Optiview Amplification kit) confirmed the presence of the ALK rearrangement (Figure 1). The PS was 2 due to the pain of bone metastasis. The patient received crizotinib treatment (250 mg, bid, orally) on Jan 22, 2014. The chest CT scan demonstrated a decrease in tumor size after twenty days treatment (Figure 2). According to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version1.1), the response to crizotinib belonged to a partial response (PR). Grade 3 neutropenia was observed 2 weeks later and grade 2 elevated liver aminotransferase was observed after 2-monthcrizotinb treatment. And wild edema of feet was also seen. No other side effects were observed. These adverse events have been corrected quickly without reduction of crizotinib. The follow-up chest CT scan revealed a stable reduction in primary tumor size. It was a pity that ultrasonography displayed new metastatic nodules in the left adrenal gland after a six-month crizotinib treatment, which directly leaded to drug discontinuance of crizotinib. The patient is alive and has been receiving best supportive care since the failure of crizotinib.
Figure 1.
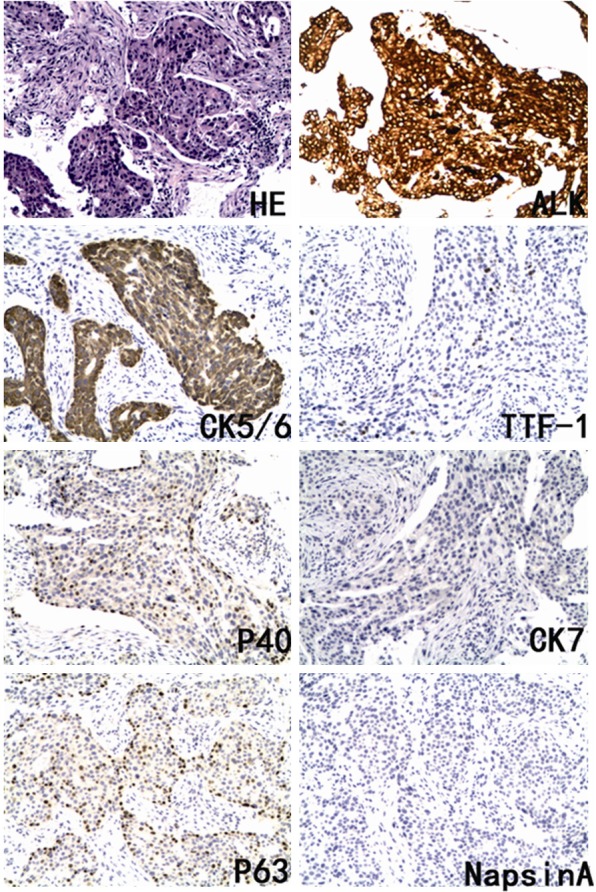
Pathological images. HE staining, immunohistochemistry staining of P40, P63, CK5/6, CK7, TTF-1, Napsin-A, Ventana IHC staining of ALK (D5F3).
Figure 2.
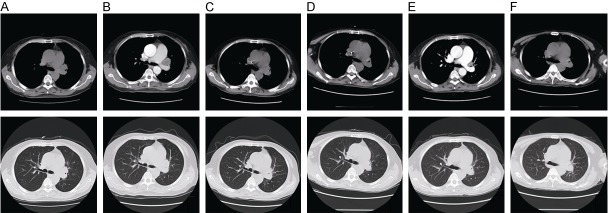
Chest computed tomography (CT) scans. The chest CT showed changes of the primary carcinoma prior to first-line chemotherapy (A), prior to crizotinib treatment (B), twenty days later withcrizotinib treatment (C), two months later withcrizotinib treatment (D), three and a half month later with crizotinib treatment (E), five months later with crizotinib treatment (F).
Discussion
The China Food and Drug Administration (CFDA) have granted full approval to crizotinib for the treatment of patient with ALK-positive NSCLC on Jan 22, 2013 [9], and Ventana IHC (D5F3) has been approved by the CFDA as an aid in identifying patients who are eligible for treatment with crizotinib. Crizotinib was well tolerated and resulted in rapid and durable responses in patients with ALK-positive NSCLC in phase I study [10]. But this study enrolled only one case of squamous cell carcinoma with lung. In PROFILE 1007 study, only 5 non-adenocarcinoma patients were enrolled [7]. Caliò A et al [11] showed that ALK rearrangements may be found in lung pure squamous cell carcinomas and the frequency was 2.5% (one of 40 cases) on biopsy samples harboring squamous cell carcinoma differentiation. Because of the low frequency in squamous lung cancer, the ALK fusion gene testing is not routinely recommended in the National Comprehensive Cancer Network guideline for the treatment of NSCLC. The therapeutic effect of crizotinib to patients with squamous cell carcinoma was not clear too. Our article describes a case of pure squamous lung cancer with poor PS responding to crizotinib after the failure to the first-line chemotherapy. After crizotinib treatment, the symptoms and the quality of life were improved significantly. The progression-free survival was 6months,a little shorter than the data of previous studies in lung adenocarcinoma. Grade 3 neutropenia, grade 2 elevated liver aminotransferase, and mild edema of feet were observed and could be corrected quickly without reduction of crizotinib. No other treatment-related adverse event was observed. Based on our experience, we suggest that crizotinib treatment could be an effective option to patients with squamous cell lung cancer harbored ALK rearrangements, especially to those with poor PS.
Conclusions
Crizotinib is effective to patients with lung squamous cell carcinoma harbored the ALK rearrangement. ALK testing may be considered for patients with squamous carcinoma after the failure to chemotherapy, especially with poor PS.
Disclosure of conflict of interest
None.
References
- 1.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Inamura K, Takeuchi K, Togashi Y, Hatano S, Ninomiya H, Motoi N, Mun MY, Sakao Y, Okumura S, Nakagawa K, Soda M, Choi YL, Mano H, Ishikawa Y. EML4-ALKlung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009;22:508–515. doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, Settleman J, Kobayashi S, Mark EJ, Rodig SJ, Chirieac LR, Kwak EL, Lynch TJ, Iafrate AJ. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, An S, Lin J, Chen S, Xie Z, Zhu M, Zhang X, Wu YL. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. doi: 10.1186/1476-4598-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li Y, Yang T, Wei S, Wang J, Wang M, Wang Y, Zhou Q, Liu H, Chen J. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One. 2013;8:e52093. doi: 10.1371/journal.pone.0052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Dong Y, Cai Y, Zhou L, Wu S, Liu G, Su D, Li X, Qin N, Nong J, Jia H, Zhang Q, Mu J, Zeng X, Zhang H, Zhang S, Zhang Z. Clinicopathologic characteristics of ALK rearrangements in primary lung adenocarcinoma with identified EGFR and KRAS status. J Cancer Res Clin Oncol. 2014;140:453–60. doi: 10.1007/s00432-014-1584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O’Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18:2443–51. doi: 10.1158/1078-0432.CCR-11-2370. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Association of Oncologists Chinese Society for Clinical Cancer Chemotherapy. The Diagnosis and Treatment Guideline of Chinese Patients with EGFR Mutation and ALK Fusion Gene-Positive Non-Small Cell Lung Cancer (2013 Version) Zhonghua Zhong Liu Za Zhi. 2013;35:478–80. [PubMed] [Google Scholar]
- 10.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SH, Kim DW, Salgia R, Fidias P, Engelman JA, Gandhi L, Jänne PA, Costa DB, Shapiro GI, Lorusso P, Ruffner K, Stephenson P, Tang Y, Wilner K, Clark JW, Shaw AT. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caliò A, Nottegar A, Gilioli E, Bria E, Pilotto S, Peretti U, Kinspergher S, Simionato F, Pedron S, Knuutila S, Tortora G, Eccher A, Santo A, Tondulli L, Inghirami G, Tabbò F, Martignoni G, Chilosi M, Scarpa A, Brunelli M. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol. 2014;9:729–32. doi: 10.1097/JTO.0000000000000109. [DOI] [PubMed] [Google Scholar]


