Abstract
A number of cancers show increased expression of paxillin which plays a central role in tumor progression, including colorectal cancer. However, the mechanisms causing paxillin upregulation remains unclear. In our study, bioinformatics analyses suggested that paxillin is predicted to be a direct target of miR-145. We firstly identified paxillin as a new target of miR-145 and demonstrated that miR-145 inhibits paxillin expression by binding to the paxillin mRNA 3’UTR. Therefore, we assume overexpression of paxillin induced by suppression of miR-145 may promote cell migration and invasion. We detected the expression of paxillin and miR-145 in human colorectal cancer tissues by real-time quantitative PCR. Higher expression of paxillin and lower expression of miR-145 was observed in colorectal cancer tissues than corresponding paracancerous tissue. Moreover, the expression of paxillin was negatively correlated with miR-145 expression. A dual-luciferase reporter assay was used to confirm that paxillin was a direct target of miR-145. In CRC cell lines, overexpression of miR-145 could downregulate paxillin protein expression levels, and ectopic overexpression of miR-145 mimics or inhibitor could inhibit or promote cell migration, invasion, proliferation and clone formation in vitro. Taken together, these data suggested that miR-145 plays a pivotal role in colon cancer through inhibiting cell proliferation migration and invasion, and miR-145 may serve as a tumor suppressor by targeting paxillin gene.
Keywords: miR-145, paxillin, colorectal cancer, proliferation, migration
Introduction
Colorectal cancer (CRC) is currently a major cause of morbidity and mortality throughout the world. It is the third most common malignancies worldwide and the fourth most common cause of cancer-related mortality [1]. The pathological process and underlying mechanism of colorectal cancer is very complicated and has not been elucidated yet. Previous studies have indicated that colorectal cancer is caused by the accumulation of mutations in many genes, these include alterations in oncogenes and tumor suppresser genes leading to the activation of oncogenes and inactivation of tumor suppressor genes [2]. However, the precise molecular biomarkers that can predict prognosis and recurrence have not yet been determined.
In recent years, microRNAs (miRNAs), a class of endogenous small noncoding RNAs with 18-22 nucleotides in length, are believed to have important regulatory roles in tumorigenesis and metastasis by interfering with the translation of mRNAs to regulate gene expression, and act as oncogenes or tumor suppressor genes depending on the targets they regulate [3]. Recently, there have been many reports of aberrant miRNAs expression in CRC [4-6], suggesting a close correlation between miRNAs expression and the occurrence, progression, metastasis and prognosis of CRC. MiR-145 has been firstly found to be reduced in CRC in 2003, and has been considered to be a tumor suppressor gene [4]. Several studies reported that miR-145 participate in CRC by modulating a set of certain genes expressions in response to tumorigenesis and metastasis [7,8]. The paxillin, one of the key focal adhesion proteins, are believed to to participate in tumor progression and metastasis by forming a structural link between the extracellular matrix and actin cytoskeleton to integrate multiple signals from cell surface receptors, integrins and growth factors [9]. Paxillin has been found highly expressed and act as an oncogene in many malignancies, such as lung cancer [10], prostate cancer [11], gastric cancer [12] and oral cavity squamous cell carcinoma [13]. In CRC, the leves of paxillin expression were higher in CRC tissues than in corresponding normal tissues [14]. In our previous study, overexpression of paxillin could stimulate migration, invasion and adhesion abilities of CRC cells, whereas downregulation of paxillin by small interfering RNA (siRNA) inhibited these capacities [15].
However, the underlying molecular mechanism of paxillin upregulation is unclear. In this study, we firstly detected the expression level of paxillin and miR-145 in tumor tissues surgically resected from patients with CRC by real time quantitative PCR. We found that the expression of paxillin protein was inversely correlated with miR-145 expression in CRC cell lines and human CRC tissues. We further demonstrated that miR-145 directly targeted paxillin in CRC cell lines. Moreover, we found that down-regulation of paxillin induced by overexpression of miR-145 contributed to inhibit cell proliferation, migration and invasion abilities in colon cancer cell lines.
Material and methods
Cell lines and tissue samples
Five human CRC cell lines (HCT-8, HT-29, SW480, SW620, LoVo, HCT-116) were acquired from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) or RPMI-1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Cells were cultured at 37°C in a humidified incubator containing 5% CO2.
Tissue samples from human colorectal cancer and adjacent normal colorectal tissues were obtained from 24 patients who underwent surgery at Affiliated Hospital of Nantong University (Jiangsu, China). None of the patients received chemotherapy or radiotherapy before surgery. Samples were collected and stored at -80°C. All the patients had a histological diagnosis of CRC. Before the resected specimens were collected and used, informed written consent was obtained from all subjects and/or their guardians and approval was obtained from the ethics committee of Affiliated Hospital of Nantong University.
Total RNA extraction and miRNA detection
Total RNA, including the small RNA fraction, was extracted from cultured tissues and CRC cells with TRIZOL Reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Asheville, NC). Validation of the expression miR-145 in the CRC tissues and matched adjacent non-cancerous tissues was performed by real time PCR on a Life Technologies 7500 instrument using TaqMan miRNA assays with TaqMan® Universal Master Mix II according to standard protocol (Life Technologies, Foster City, CA, USA). To ensure sensitivity, 10 ng of total RNA was added to each well before reverse transcription with a miRNA specific primer. One-tenth of the RT reaction was used in a qPCR reaction using the TaqMan 2 × universal master mix and the following cycle conditions: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. All samples were run in duplicate and the resulting Ct values averaged. U6 snRNA was used as the endogenous control. For PAX mRNA detection, reverse transcription was performed using the PrimeScript RT Master Mix (Perfect Real Time, TaKaRa). Quantitative PCR was performed using TaqMan 2 × universal master mix and the following cycle conditions: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds; β-actin mRNA was used as the endogenous control. Forward and reverse primers for paxillin were 5’-ACGTCTACAGCTTCCCCAACAA-3’ and 5’-AGCAGGCGGTCGAGTTCA-3’, and β-actin was 5’-TGGATCAGCAAGCAGGAGTA-3’ and 5’-TCGGCCACATTGTGAACTTT-3’. The qPCR results were analysed relative to miRNA or mRNA expression levels by using the 2-ΔΔCT method.
Reporter constructs, transfections and luciferase activity assay
A miR-145 expression plasmid (pcDNA6.2-miR-145) was constructed with synthetic miR-145 oligonucleotides and the pcDNA6.2-GW/EmGFP vector. A paxillin expression plasmid (pcDNA3.1-paxillin) containing the coding sequence was constructed using PCR-generated fragments and pcDNA3.1 (+) vector. The 3’UTR of paxillin mRNA and a mutant variant were generated by PCR fragments and cloned to the XbaI site of a pGL3-basic vector (Promega) and termed paxillin-wt-3’UTR and paxillin-mt-3’UTR, respectively. All constructs were confirmed by DNA sequence analysis. For the luciferase assay, SW620 cells were seeded in six-well plates the day before transfection and were infected with the pGL3 reporter plasmid (250 ng/well), pRL-TK luciferase reporter plasmid (25 ng/well) and pcDNA6.2-miR-145 (750 ng/well) or a negative-control (NC) plasmid using lipofectamine 3000 (Invitrogen). Luciferase activities were measured with a Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
Western blot analysis
Total protein was extracted by lysis buffer containing protease inhibitors (Promega, Madison, WI). Equal amounts (30 μg) of protein separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane. Nonspecific binding was blocked for 2 h with 5% nonfat milk in TBST (Tris-buffered saline containing 0.1% Tween-20). After incubation with rabbit anti Paxillin Polyclonal antibody at 1:1,000 dilution (Abcam, Cambridge, UK) or a rabbit anti-β-actin as internal reference, at 1:3,000 dilution (Sigma-Aldrich, St. Louis, MO, USA), membranes were washed three times in TBST for 5 min and subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary anti body (1:2,000 dilution, Sigma-Aldrich) for 2 h at room temperature, and signals were detected using by an enhanced chemiluminescence system (ECL).
Oligonucleotide transfection
The miR-145 mimic or inhibitor and NC oligonucleotides were purchased from GenePharm (Shanghai, China) and transfected into the cells with Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol. GenePharm also synthesized a siRNA duplex oligonucleotides targeting human paxillin mRNA (paxillin-siRNA) acorroding to our previous study [15]. The targeting sequence was paxillin-siRNA: CGCGCTGCTACTACTGCAA; CRC cells were grown in six-well dishes for 24 h before transfection. For PCR, western blot and other functional assays, miR-145 inhibitor was transfected into HCT-8, which had high expression level of miR-145; miR-145 mimic was transfected into SW620, which had low expression level of miR-145. Inhibitor negative control and mimic negative control groups were also set up. Cells at 80% confluency were transfected with Lipofectamine 3000 (Invitrogen, Carlsbad, CA) reagent according to the manufacturer’s instructions.
Cell proliferation assays
Cell Counting Kit-8 (CCK-8) Assay was performed to test cell viability. Cells were plated in 96-well plates at 5000 cells/well in complete medium and cultured for 24 h. The medium was then replaced with medium containing 10% FBS, with or without treatment. After incubation, 10 μL of CCK-8 was added to each well, and the plates were further incubated for 4 h in an incubator. The spectrophotometric absorbance at 490 nm was measured for each sample; all the experiments were repeated three times in triplicate and the mean was calculated.
Transwell assay and wound-healing assay
Cell invasion was performed by transwell assay (BD Biosciences) according to the manufacturer’s instructions. Control miRNA, mimics or inhibitor of miR-145 transfected cells were harvested 24 h after transfection. Then 3.0 × 105 transfected cells or untreated cells in serum-free medium were added to each upper insert pre-coated with Matrigel matrix. To the matched lower chamber, 600 μL of 10% FBS medium was added. After incubation, non-invasive cells were removed from the upper surface of the transwell membrane with a cotton swab, and the invasive cells on the lower membrane surface were fixed in methanol, stained with 0.1% crystal violet, photographed, and counted.
In addition, wound healing assay was also performed for analysis of cell migration in vitro. Briefly, SW620 cells transfected with either miR-145 mimics or negative control miRNA and HCT-8 transfected miR-145 inhibitor or negative control miRNA were cultured in six-well plates (5 × 105 cells per well) and incubated overnight. Culture inserts were removed after appropriate cell attachment and washed twice with phosphate-buffered saline (PBS), and complete RPMI medium with 10% FBS was added. Cell migration toward the wounded area was observed and photographed after 24 h. Wound closure (%) was calculated as the area of migrated cells divided by wounded area at 0 h.
Colony formation assay
HCT-8 and SW620 cells (5 × 104/well) were plated in a 24-well plate and transfected with miR-145 mimics, miR-145 inhibitor or control RNA. After 24 h, the cells collected and seeded (1 000-1 500/well) in a fresh 6-well plate for 12 days. Surviving colonies (> 50 cells per colony) were counted after fixed with methanol/acetone (1:1) and stained with 5% Gentian Violet (ICM Pharma, Singapore, Singapore). The experiment was carried out in triplicate wells for three times.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences version 17.0 software (SPSS, Chicago, IL). Data are expressed as mean ± SD. A two-tailed, unpaired Student’s test was used to compare between two groups. A probability of < 0.05 was considered statistically significant.
Results
MiR-145 is a putative regulator targeting paxillin via binding to its 3’UTR
MiRNAs regulate their targets via binding to their 3’ untranslated region (3’UTR). By computer-based sequence analysis using Target Scan, PicTar and MiRanda, we found that the paxillin mRNA 3’UTR (nucleotides 87-105) that is highly conserved among mammals for miR-145 was predicted to be a potential target of miR-145. Therefore, miR-145 was selected for further biological characterization. To test the specific regulation through the predicted binding sites, we constructed a reporter vector which consists of the luciferase coding sequence followed by the 3’UTR of paxillin (Luc-paxillin-3’UTR). Wild type (Luc-paxillin-3’UTR) or mutated sequence (Luc-paxillin-mut 3’UTR) within the putative binding sites was cloned into the pGL3-REPORT vector (Figure 1A, 1B). These reporter constructs were co-transfected into SW620 cells with either miR-145 mimics or miR scramble. Increased expression of miR-145 upon infection of miR-145 mimics significantly decreased the luciferase activity expression derived from reporter constructs containing wild type Luc-paxillin-3’UTR (P < 0.05) comparing to cells co-transfected with miR scramble. This suppressive effect was abolished when Luc-paxillin-mut 3’UTR mRNAs, in which the binding sites for miR-145 were inactivated by site-directed mutagenesis, were co-infected with miR-145 (Figure 1C). Therefore, the results from above confirm that paxillin is a target of miR-145.
Figure 1.
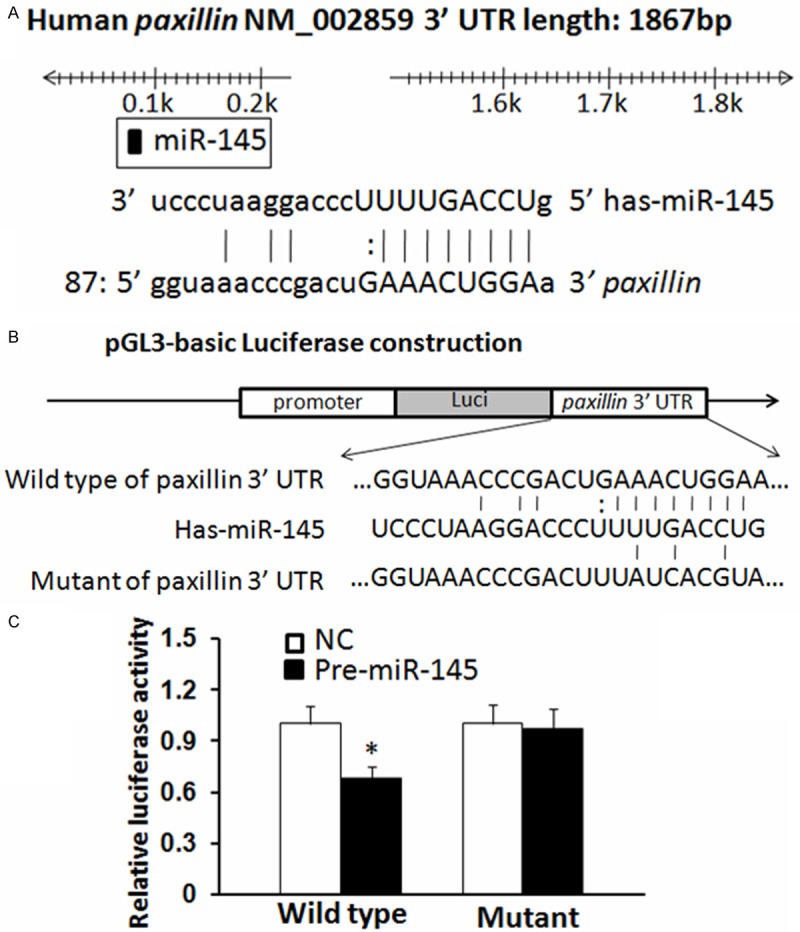
Paxillin is a direct target of miR-145 in CRC. A. Human paxillin 3’UTR binding site for miR-145. B. The miR-145 wild type binding sequence or its mutated form was inserted into C-terminal of the luciferase gene to generate pGL3-paxillin-3’UTR or pGL3-paxillin-mut-3’UTR, respectively. C. miR-145 targeted the wild-type but not the mutant 3’UTR of paxillin (*P < 0.05). The data are means ± SD.
Expression levels of miR-145 and paxillin in colorectal cancer tissues and cell lines
The basal expression levels of miR-145 and paxillin mRNA were measured by qRT-PCR in colorectal cancer cell lines HCT-8, HT-29, SW480, SW620, LoVo, HCT-116. SW620 cells had higher levels of paxillin and lower levels of miR-145, whereas HCT-8 cells had the lower paxillin expression, and the higher miR-145 level, followed by HT-29, HCT-116, LoVo, and SW480 cells (Figure 2A, 2B). A significant inverse correlation between the expression of miR-145 and paxillin mRNA was observed (Figure 2A right). By miR-145 mimics or paxillin-siRNA, overexpression of miR-145 suppressed paxillin protein levels in SW620 cells compared with that of the NC cells (P < 0.05, Figure 3A, 3B), as detected by western blot analysis, respectively, which mimics the effect of interference of paxillin. On the other hand, transfection with miR-145 inhibitor increased paxillin protein levels in HCT-8 cells (P < 0.05, Figure 3C).
Figure 2.

Paxillin mRNA levels were inversely correlated with miR-145 levels in CRC cell lines and patient samples. A-C. Relative paxillin mRNA (normalized to β-actin) and miR-145 (normalized to U6) expression levels were detected by real-time RT-PCR in six CRC cell lines. Data are presented as means ± SD. The expression of miR-145 was inversely correlated with paxillin expression in CRC cell lines. D and E. miR-145 and paxillin mRNA levels were assayed in 24 surgical specimens of human colorectal cancer tissues and adjacent normal colorectal tissues. Significantly upregulated paxillin levels in colorectal cancer tissues are shown relative to paxillin levels in adjacent normal colorectal tissues (P = 0.0032); significantly downregulated miR-145 levels in colorectal cancer tissues are shown relative to miR-145 levels in adjacent normal colorectal tissues (P = 0.0098). Paxillin mRNA levels were assayed by real-time quantitative PCR and normalized to β-actin. The miR-145 levels were assayed by real-time quantitative RT-PCR and normalized to U6. F. The expression of miR-145 was inversely correlated with paxillin expression in CRC tissues. For comparison of 2 groups, a two-tailed, unpaired t-test was used.
Figure 3.

The effects of miR-145 on paxillin expression. A and B. Expression of paxillin in SW620 cell after transfection with miR-145 mimics or paxillin-siRNA compared to negative control cells (NC). Representative bands (left) and the quantification (right) from different treatments (*P < 0.05, **P < 0.01). C. Western blotting of paxillin in HCT-8 cell line after transfection with different concentrations of miR-145 inhibitors or negative control cells (*P < 0.05, **P < 0.01).
In addition, we extended our investigation to samples from colorectal cancer patients. Our results showed that paxillin expression was significantly increased in cancer tissues when compared with that in the paired adjacent normal tissues of 24 colorectal cancer patients (Figure 2E), which was consistent with other findings [16]. In addition, we found the cancerous tissue showed a notable loss of miR-145 as compared with the adjacent normal colorectal cancer tissues of 24 colorectal cancer patients (Figure 2D). We observed an inverse correlation between miR-145 and paxillin expression in CRC tissues (Figure 2F).
MiR-145 expression and paxillin knockdown show similar phenotypes in inhibiting cell proliferation, migration and invasion abilities in vitro
Our previous studies show that paxillin play a key role in the regulation of proliferation of CRC cells [15]. Based on the results listed above, we hypothesized that overexpression of paxillin by a reduced miR-145 expression may affect the proliferation, migration and invasion ability of CRC cell lines. The SW620 cell line was transfected with paxillin siRNA or miR-145 mimics. The CCK-8 assay showed that knockdown of paxillin or ectopic expression of miR-145 could significantly inhibit the proliferative ability in SW620 cells (P < 0.05, Figure 4A). Likewise, colony formation ability was suppressed after downregulation of paxillin or overexpression of miR-145 (P < 0.05, Figure 4C, 4D). Thus, miR-145 mimicked paxillin expression knockdown in SW620 cells, resulting in similar inhibiting growth phenotypes to that by paxillin siRNA.
Figure 4.
Effects of miR-145 mimics or paxillin siRNA and miR-145 inhibitor on the proliferation of CRC cells. A and B. Proliferation rate of SW620 cells with miR-145 mimics or paxillin-siRNA and assay of HCT-8 cells with miR-145 inhibitor on the basis of CCK-8 assay compared with NC (*P < 0.05). C-F. Clone formation rate of SW620 cells with miR-145 mimics or paxillin-siRNA and assay of HCT-8 cells with miR-145 inhibitor by the colony formation assay (*P < 0.05).
In addition to proliferation regulation, our previous studies also show that paxillin is involved in the invasive phenotype of tumor cells that may contribute to the morbidity and mortality of cancer patients [15]. To determine whether miR-145 may regulate invasion through paxillin, SW620 were transfected with paxillin siRNA and miR-145 mimics. Cell migration and invasion was measured by transwell assays and wound healing assay. In above-mentioned assay, we found a significant decrease in invasive activity of miR-145-transfected cells, mimicking that of si-paxillin-transfected cells (Figure 5A, 5C, 5E, 5G). Taken together, our results indicate a role for miR-145 in the regulation of cell proliferation, migration and invasion and this may, at least in part, be attributed to its targeting effect on paxillin.
Figure 5.
Effects of miR-145 mimics or paxillin siRNA and miR-145 inhibitor on the migration and invasion of CRC cells. A and B. Representative micrographs of wound-healing assay of SW620 cells with miR-145 mimics or paxillin-siRNA and miR-145 inhibitor and assay of HCT-8 cells with miR-145 inhibitor compared with NC. C and D. Quantitative analysis of SW620 and HCT-8 wound-healing rate (*P < 0.05). E and F. The stained invasive cells were photographed under an inverted light microscope (100 × magnification). G and H. Quantitative analysis of SW620 and HCT-8 invasion cell numbers (*P < 0.05).
MiR-145 inhibitor mimics paxillin expression in promoting cell proliferation, migration and invasion in vitro
To determine whether miR-145 inhibitor may have a similar effect to paxillin overexpression on cell proliferation, migration and invasion in CRC cells, HCT-8 cell line were transiently transfected with miR-145 inhibitor or a control inhibitor. Contrary to the results of miR-145 expression, suppression of miR-145 by miR-145 inhibitor markedly promoted the proliferation capacity of the HCT-8 cells was stimulated (Figure 4B, 4E, 4F). Furthermore, suppression of miR-145 in CRC cells significantly enhanced the migration and invasion in CRC cells (Figure 5B, 5D, 5F, 5H). These data further support that miR-145-paxillin signaling axis regulates proliferation, migration, and invasion in the CRC cells.
Discussion
Recent studies have shown that miRNAs are frequently deregulated in many human cancers, including colorectal cancer, but their function and mechanisms in tumorigenesis remain elusive. More and more researches have documented that miRNAs have essential roles in multiple biological processes, including tumor cell proliferation, angiogenesis, invasion and migration. In this study, we focused on miR-145, it has been reported that miR-145 acts as a tumor suppressor, which is downregulated in various tumors, including esophageal cancer [17], bladder cancer [18], breast cancer [19], oral cancer [20], and gastric [21], colorectal cancers [22]. Some studies also shows that miR-145 targets ADAM17 and suppresses cell invasion in hepatocellular [23] and head and neck cancers [24]. Moreover, miR-145 overexpression directly targets AKT-3 in thyroid cancer and silences the MUC13 expression and regulates the expression of its critical tumor target genes that are involved in pancreatic pathogenesis [25]. In addition, miR-145 has previously been associated with cancer-relevant biological processes, such as growth, proliferation and inhibition of apoptosis, or with clinical outcome in CRC [7,22]. Indeed previous reports have shown that miR-145 leads to tumor suppress effect in non-metastatic CRC cell lines (DLD1, HCT116, SW480, LS174T), and in colon cancer cells [26,27], miR-145 is directly bound to the insulin receptor substrate-1 (IRS-1) 3’-untranslated region and downregulates IRS-1 protein, inhibiting the growth of colon cancer cells [28].
Identification of cancer-specific miRNAs as well as their target genes is important for elucidating the roles of miRNAs in tumorigenesis and may provide promising therapeutic targets. To further investigate the mechanisms of miR-145 in CRC, we predicted putative targets of miR-145 by bioinformatic analysis. Among numerous genes, paxillin was picked out, as it has been shown to be closely involved in the tumorigenesis and cell migration of CRC [29]. The target role of paxillin was identified by the luciferase reporter assays, as transfection of miR-145 caused a substantial reduction of luciferase activity by the luciferase expression constructs carrying the target paxillin mRNA 3’UTR fragment, and overexpression of miR-145 suppressed paxillin protein levels in SW620 cells, which presented a similar tumor-repressive effect of paxillin knockdown in CRC cell lines, meanwhile inhibition of miR-145 increased paxillin protein levels in HCT-8 cells, which indicated that miR-145 regulates paxillin primarily through translational repression. We also found that levels of miR-145 in patient colorectal tumor tissues were much lower than in adjacent normal tissues, and that miR-145 expression inversely correlated with the expression of paxillin mRNA in CRC cell lines and patient samples in mRNA levels. These studies further confirmed that paxillin is a target of miR-145.
Paxillin is a multi-domain adapter focal adhesions protein that functions as a molecular scaffold for protein recruitment to focal adhesions and thereby facilitates protein networking and efficient signal transmission [9,30]. Thus, paxillin may be involved in signal transduction, regulation of cell morphology and the recruitment of structural and signaling molecules to focal adhesions to control cell spread and migration [31,32]. Consistent with its function as a molecular scaffold protein, paxillin comprises multiple discrete protein binding modules, including numerous tyrosine as well as Ser/Thr phosphorylation sites that, when modified, contribute further to the complexity of the protein interactome [30]. The serine phosphorylation of paxillin at residues 188 and 190 has been shown to stimulate cell migration, and presumably adhesion turnover, by preventing the polyubiquitination of paxillin and its subsequent degradation [33]. Given its roles in cell adhesion and migration, paxillin is thought to play an important role in tumor migration, invasion, and metastasis [34]. Furthermore, in capillary endothelial cells, paxillin rapidly becomes phosphorylated in response to treatment with the tumor-associated vascular endothelial growth factor (VEGF), so paxillin might play a role in governing directional migration of capillary endothelial cells during tumor angiogenesis [35]. Some studies has been reported that paxillin changes in protein expression are also associated with alterations in the malignant progression of many tumors, including breast [36], lung [37], ovarian cancer [38], prostate [39], hepatocellular carcinoma [40], melanoma [41], and esophageal squamous cell carcinoma [42]. In addition, it was identified that paxillin is overexpressed in colorectal carcinoma tissues compared with normal adjacent tissues [16], and overexpression of wild-type paxillin plasmids promoted cell proliferation and also enhanced migration, invasive capacity and metastasis of the colorectal cancer cells [15]. These studies mentioned above strongly suggest the oncogene role of paxillin in tumors, especially CRC. In this study, we found that knockdown of paxillin gene expression by paxillin siRNA or miR-145 mimics reversed miR-145-RNAi-mediated suppression of SW620 tumor cell proliferation, migration and invasion. We further analyze whether paxillin plays an important role in miR-145-mediated suppression of cell proliferation, migration and invasion and found that restored expression of paxillin with transfection of miR-145 inhibitor could promote HCT-8 cell proliferation, migration and invasion. Taken all together, up-regulation of miR-145 in CRC cells may contribute to the reduced expression of paxillin at the posttranscriptional level and in turn facilitate the CRC proliferation and metastasis, which also provide new insight into the mechanism of paxillin function in cancer.
In summary, our present study suggests that miR-145 negatively regulates paxillin expression at the posttranscriptional level by binding to its 3’UTR and regulates its expression and activity. More evidence is required to show that paxillin upregulation in response to miR-145 reduction may enhance tumor proliferation and metastasis in CRC. These results indicate that our results are clinically significant: miR-145 and paxillin are significant biomarkers for proliferation and metastasis and could be targets for the development of anti-proliferation and anti-metastasis strategy in the therapeutic interventions of CRC in the future.
Acknowledgements
This work is supported by the grants from the Jiangsu Province Postdoctoral Science Foundation and the Department of Health of Jiangsu Province of China (NO. H201214).
Disclosure of conflict of interest
None.
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 4.Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 5.Du M, Liu S, Gu D, Wang Q, Zhu L, Kang M, Shi D, Chu H, Tong N, Chen J, Adams TS, Zhang Z, Wang M. Clinical potential role of circulating microRNAs in early diagnosis of colorectal cancer patients. Carcinogenesis. 2014;35:2723–30. doi: 10.1093/carcin/bgu189. [DOI] [PubMed] [Google Scholar]
- 6.de Krijger I, Mekenkamp LJ, Punt CJ, Nagtegaal ID. MicroRNAs in colorectal cancer metastasis. J Pathol. 2011;224:438–447. doi: 10.1002/path.2922. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Zhu J, Ou C, Deng Z, Chen M, Huang W, Li L. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br J Cancer. 2014;110:2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Schepeler T, Holm A, Halvey P, Nordentoft I, Lamy P, Riising EM, Christensen LL, Thorsen K, Liebler DC, Helin K, Orntoft TF, Andersen CL. Attenuation of the beta-catenin/TCF4 complex in colorectal cancer cells induces several growth-suppressive microRNAs that target cancer promoting genes. Oncogene. 2012;31:2750–2760. doi: 10.1038/onc.2011.453. [DOI] [PubMed] [Google Scholar]
- 9.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 10.Kawada I, Hasina R, Lennon FE, Bindokas VP, Usatyuk P, Tan YH, Krishnaswamy S, Arif Q, Carey G, Hseu RD, Robinson M, Tretiakova M, Brand TM, Iida M, Ferguson MK, Wheeler DL, Husain AN, Natarajan V, Vokes EE, Singleton PA, Salgia R. Paxillin mutations affect focal adhesions and lead to altered mitochondrial dynamics: relevance to lung cancer. Cancer Biol Ther. 2013;14:679–691. doi: 10.4161/cbt.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ketscher A, Jilg CA, Willmann D, Hummel B, Imhof A, Russeler V, Holz S, Metzger E, Muller JM, Schule R. LSD1 controls metastasis of androgen-independent prostate cancer cells through PXN and LPAR6. Oncogenesis. 2014;3:e120. doi: 10.1038/oncsis.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, Wang F, Qiu MZ, Bai L, Zhang DS, Wang FH, Li YH, Xu RH. Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Transl Med. 2013;11:277. doi: 10.1186/1479-5876-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu DW, Chuang CY, Lin WL, Sung WW, Cheng YW, Lee H. Paxillin promotes tumor progression and predicts survival and relapse in oral cavity squamous cell carcinoma by microRNA-218 targeting. Carcinogenesis. 2014;35:1823–1829. doi: 10.1093/carcin/bgu102. [DOI] [PubMed] [Google Scholar]
- 14.Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY, Qiu MZ, Ren C, Zhang DS, Wang ZQ, Wang FH, Li YH, Kang TB, Xu RH. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis. 2013;34:803–811. doi: 10.1093/carcin/bgs400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun Q, Zhiwei W, Lilin M, Jing K, Qichao N. Effects of paxillin on HCT-8 human colorectal cancer cells. Hepatogastroenterology. 2011;58:1951–1955. doi: 10.5754/hge11352. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Zhang Q, Wang X, Li T, Wan Y, Liu Y, Zhu J. Role of paxillin in colorectal carcinoma and its relationship to clinicopathological features. Chin Med J (Engl) 2014;127:423–429. [PubMed] [Google Scholar]
- 17.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M, Matsubara H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 18.Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N, Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–891. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spizzo R, Nicoloso MS, Lupini L, Lu Y, Fogarty J, Rossi S, Zagatti B, Fabbri M, Veronese A, Liu X, Davuluri R, Croce CM, Mills G, Negrini M, Calin GA. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010;17:246–254. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Zhang SQ, Quan F, Zhang PF, Wu SL. MicroRNA-145 inhibits the proliferation, migration and invasion of the human TCA8113 oral cancer line. Oncol Lett. 2013;6:1636–1640. doi: 10.3892/ol.2013.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- 22.Gregersen LH, Jacobsen AB, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS One. 2010;5:e8836. doi: 10.1371/journal.pone.0008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XW, Zhang LJ, Huang XH, Chen LZ, Su Q, Zeng WT, Li W, Wang Q. miR-145 suppresses cell invasion in hepatocellular carcinoma cells: miR-145 targets ADAM17. Hepatol Res. 2014;44:551–559. doi: 10.1111/hepr.12152. [DOI] [PubMed] [Google Scholar]
- 24.Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL, Chang YC, Chiou GY, Chou MY, Chiou SH. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013;73:3425–3440. doi: 10.1158/0008-5472.CAN-12-3840. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, Yacoubian AM, Behrman SW, Zafar N, Kumar D, Thompson PA, Jaggi M, Chauhan SC. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget. 2014;5:7599–7609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep. 2006;16:845–850. [PubMed] [Google Scholar]
- 27.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 28.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007;282:32582–32590. doi: 10.1074/jbc.M702806200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Zhang X, Guda K, Lawrence E, Sun Q, Watanabe T, Iwakura Y, Asano M, Wei L, Yang Z, Zheng W, Dawson D, Willis J, Markowitz SD, Satake M, Wang Z. Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T. Proc Natl Acad Sci U S A. 2010;107:2592–2597. doi: 10.1073/pnas.0914884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner CE. Paxillin interactions. J Cell Sci. 2000;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 31.Sattler M, Pisick E, Morrison PT, Salgia R. Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog. 2000;11:63–76. [PubMed] [Google Scholar]
- 32.Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004;3:4–6. [PubMed] [Google Scholar]
- 33.Abou Zeid N, Valles AM, Boyer B. Serine phosphorylation regulates paxillin turnover during cell migration. Cell Commun Signal. 2006;4:8. doi: 10.1186/1478-811X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 35.German AE, Mammoto T, Jiang E, Ingber DE, Mammoto A. Paxillin controls endothelial cell migration and tumor angiogenesis by altering neuropilin 2 expression. J Cell Sci. 2014;127:1672–1683. doi: 10.1242/jcs.132316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37:9–15. doi: 10.1016/j.humpath.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, Loganathan S, Kanteti R, Reichman T, Nallasura V, Schwartz S, Faoro L, Wang YC, Girard L, Tretiakova MS, Ahmed S, Zumba O, Soulii L, Bindokas VP, Szeto LL, Gordon GJ, Bueno R, Sugarbaker D, Lingen MW, Sattler M, Krausz T, Vigneswaran W, Natarajan V, Minna J, Vokes EE, Ferguson MK, Husain AN, Salgia R. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008;68:132–142. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim G, Davidson B, Henning R, Wang J, Yu M, Annunziata C, Hetland T, Kohn EC. Adhesion molecule protein signature in ovarian cancer effusions is prognostic of patient outcome. Cancer. 2012;118:1543–1553. doi: 10.1002/cncr.26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, Raj GV, Rossi R, Hammes SR. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469–2481. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li HG, Xie DR, Shen XM, Li HH, Zeng H, Zeng YJ. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J Gastroenterol. 2005;11:1445–1451. doi: 10.3748/wjg.v11.i10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velasco-Velazquez MA, Salinas-Jazmin N, Mendoza-Patino N, Mandoki JJ. Reduced paxillin expression contributes to the antimetastatic effect of 4-hydroxycoumarin on B16F10 melanoma cells. Cancer Cell Int. 2008;8:8. doi: 10.1186/1475-2867-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li BZ, Lei W, Zhang CY, Zhou F, Li N, Shi SS, Feng XL, Chen ZL, Hang J, Qiu B, Wan JT, Shao K, Xing XZ, Tan XG, Wang Z, Xiong MH, He J. Increased expression of paxillin is found in human oesophageal squamous cell carcinoma: a tissue microarray study. J Int Med Res. 2008;36:273–278. doi: 10.1177/147323000803600209. [DOI] [PubMed] [Google Scholar]




