Abstract
Aims: To investigate the prognostic role of stomatin-like protein 2 (STOML2) in cervical cancer. Methods: The expression of STOML2 in 8 pairs of cervical cancer and adjacent normal cervical tissues were detected by Real-time PCR. Immunohistochemistry was performed to evaluation of STOML2 expression in 94 paraffin-embedded cervical cancer samples. The correlation between STOML2 expression and cervical cancer progression and prognosis was analyzed statistically. Results: STOML2 expression was upregulated in cervical cancer tissues compared with adjacent normal cervical tissues. Of the 94 cervical cancer cases, high STOML2 expression was detected in 54 cases (57.4%). STOML2 expression was significantly related to tumor stage (P = 0.013) and tumor size (P = 0.025). Moreover, patients with high expression of STOML2 had a significant shorter overall survival and recurrent free survival time compared with those with low STOML2 expression in cervical cancer (P = 0.001 and P = 0.017, respectively). Multivariate analysis revealed that STOML2 was an independent prognostic factor (P = 0.022) for the overall survival in cervical cancer. Conclusion: Our study showed STOML2 was correlated to progression in cervical cancer, and implicated it as a potential predictive factor for the prognosis of cervical cancer.
Keywords: STOML2, cervical cancer, overall survival, recurrent free survival
Introduction
Cervical cancer is one of the most common and aggressive carcinoma in female reproductive system, with about 15,000 new cases in the United States per year [1]. More than 80 percent of all cervical cancers are squamous cell carcinomas, while about 15 percent are classified as adenocarcinomas [2]. Most cervical cancers are correlated with human papillomavirus (HPV), the infections of which could be passed between persons through sexual contact [3]. Other risk factors include age, smoking, sex in early age, and number of sexual partners [4]. During the past decades, a number of outstanding improvements have been achieved in cervical cancer prevention, while there are few breakthroughs in predicting the prognosis of cervical cancer.
Stomatin is an erythrocyte membrane protein of unknown function. It has been implicated to play essential roles in mechanoreception, ion channel permeability, and lipid domain organization [5]. Several stomatin homologues have been identified in C. elegans, including MEC-2, UNC-24, and UNC-1. These homologues as well as the stomatin proteins share a characteristic NH2-terminal hydrophobic domain and a cognate stomatin signature sequence that defines the stomatin family [6]. Studies have shown that stomatin family members are involved in tumor as mitochondrial component, as mitochondrial malfunction, such as mitochondria membrane potential alteration, mitochondrial activity defects, and ATP production disturbance, frequently occurred during tumorigenesis [7]. Normal expression of stomatin family proteins is essential to prevent mitochondrial malfunction and carcinogenesis. Recently, it was reported that stomatin-like protein 2 (STOML2), which was one of the stomatin family members, is widely expressed in several cancer tissues [8]. STOML2 was initially identified as a novel plasma membrane protein in human erythrocyte. It was originally considered as a cytoplasmic protein and associates with the cortical spectrin-actin cytoskeleton and probably with other membrane proteins [9]. It appears to exist at least partially as an oligomeric protein complex in the erythrocyte membrane. It has also been postulated that STOML2 expression may act as a linker between PHBs and mitochondrial biogenesis [10].
Recent research has shown that STOML2 is upregulated in several cancers, and ectopic overexpression of STOML2 is correlated with tumor progression [11]. Cao et al. demonstrated that expression of STOML2 was associated with the invasion of esophageal squamous cell carcinoma [8]. Wang et al. proposed that downregulation of STOML2 inhibits tumor cell motility, proliferation and enhances cell sensitivity to chemotherapeutic reagents [12]. Song found that overexpression of STOML2 is associated with glioma aggressiveness and may represent an independent prognostic factor for the outcome of glioma patients [13]. However, less is known about the expression pattern of STOML2 in cervical cancer. In the current study, we analyzed the STOML2 expression in cervical cancer tissue and examined the correlation and mechanism between STOML2 and the clinical prognosis of cervical cancer patients.
Materials and methods
Patients and tissue specimens
A total of 94 paraffin-embedded tumor specimens, obtained from cervical cancer patients, were collected for this study; they had been histopathologically and clinically diagnosed at the First People’s Hospital of Chenzhou City from 2004 to 2009 by at least two independent pathologists separately. All the 94 cases of cervical cancer collected for this study were primary tumors. For the use of these clinical materials for research purposes, prior patients’ consents and approvals from the Institutional Research Ethics Committee were obtained. Clinical information of the samples is described in detail in Table 1. The disease stages of all the patients were classified according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines for clinical staging. Eight pairs of cervical cancer tissues and the matched adjacent non-cancerous cervical tissues were frozen and stored in liquid nitrogen until further use.
Table 1.
Distribution of STOML2 expression in cervical cancer patients according to clinicopathologic characteristics
| Charecrestic | No. | STOML2 | P | |
|---|---|---|---|---|
|
| ||||
| + | - | |||
| Age (y) | 0.762 | |||
| ≤ 40 | 44 | 26 | 18 | |
| > 40 | 50 | 28 | 22 | |
| FIGO Stage | 0.013 | |||
| IB | 62 | 30 | 32 | |
| > IB | 32 | 24 | 8 | |
| Tumor size | 0.025 | |||
| ≤ 4 cm | 57 | 38 | 19 | |
| > 4 cm | 37 | 16 | 21 | |
| Differentiation | 0.356 | |||
| Grade 1/2 | 38 | 24 | 14 | |
| Grade 3 | 56 | 30 | 26 | |
| LN Metastasis | 0.808 | |||
| No | 73 | 41 | 32 | |
| Yes | 21 | 13 | 9 | |
| Total NO. of patients | 94 | 54 | 40 | |
RNA extraction and real-time PCR
Total RNA from cervical cancer tissues was extracted using the Trizol Reagent (Invitrogen), according to the manufacturer’s instructions. The RNA was pretreated with RNase-free DNase (Promega), and 1 μg RNA was used for cDNA synthesis. Real-time PCR was performed using a Thermal Cycler Dice® Real-time System TP800 (Takara Bio Inc., Otsu, Japan) system. Sequences of the primers are: STOML2, forward, 5’-ACTTCCACCCTTCAGTCCAGGTCG-3’ and reverse, 5’-ACTTGGATTCTGTGAAAGCAGACAC-3’; GAPDH, forward, 5’-CTCCTCCTGTTCGACAGTCAGC-3’, reverse, 5’-CCCAATACGACCAAATCCGTT-3’.
Immunohistochemistry
Immunohistochemical analysis was performed to study the expression of STOML2 in 94 cervical cancer specimens. In brief, paraffin-embedded specimens were cut into 5 um sections and baked at 60°C for 3 h followed by deparaffinization with xylene and rehydrated. After antigenic retrieval, the sections were treated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity, followed by incubation with 1% bovine serum albumin to block nonspecific binding. Sections were incubated with monoclonal mouse anti-STOML2 (Abcam, USA; 1:200). After washing with PBS, the slides were incubated with prediluted secondary antibody (Abcam), followed by further incubation with diaminobenzidine (DAB). Finally, the sections were counterstained with 10% Mayer’s hematoxylin, dehydrated, and moun- ted.
The degree of immunostaining was scored independently by two observers based on the proportion of positively stained tumor cells and intensity of staining. Tumor cell proportion was scored as follows: 0 (no positive tumor cells), 1 (< 10% positive tumor cells), 2 (10-30% positive tumor cells), 3 (31-80% positive tumor cells), and 4 (> 80% positive tumor cells). Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). Staining index was calculated as the product of staining intensity score and the proportion of positive tumor cells. Using this method of assessment, we evaluated STOML2 expression by determining the staining index, with scores of 0, 1, 2, 3, 4, 6, 9, or 12. The cutoff value for high and low expression level was chosen based on a measure of heterogeneity with the log-rank test statistical analysis with respect to overall survival (OS) and recurrent-free survival (RFS). An optimal cutoff value was identified: a staining index score of ≥ 6 was used to define tumors with high STOML2 expression and a staining index score of ≤ 4 was used to indicate low STOML2 expression.
Statistical analysis
All statistical analyses were carried out using the SPSS 16.0 statistical software package. The chi-square test was used to analyze the relationship between STOML2 expression and clinicopathological characteristics. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Survival data were evaluated using multivariate Cox regression analyses. In all cases, P < 0.05 was considered significant.
Results
Up-regulation of STOML2 in cervical cancer tissues
To determine the expression of STOML2 mRNA in cervical cancer tissues, Real-time PCR analysis was done in paired cervical cancer tissues and adjacent normal tissues, with each pair taken from the same patient. STOML2 was significantly overexpressed in eight examined samples paired with adjacent non-cancerous tissues from the same patients (Figure 1).
Figure 1.
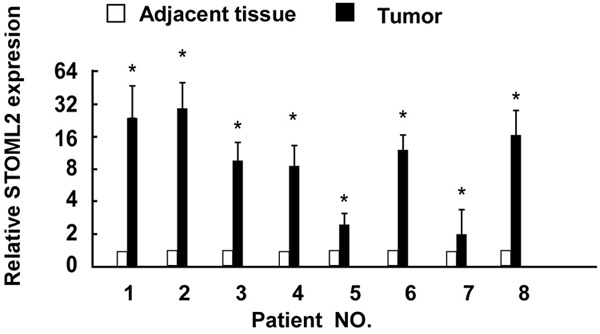
Real time-PCR analysis of STOML2 expression in each of the cervical cancer and paired adjacent normal cervical tissues, asterisks, P < 0.05.
Increased expression of STOML2 correlates with the clinical features of cervical cancer
To investigate the potential roles of STOML2 in the development of cervical cancer, immunohistochemistry was performed to measure STOML2 expression in 94 archived cervical cancer samples. The representative immunostaining of STOML2 in cervical cancer was shown in Figure 2A, 2B. Immunohistochemical staining of STOML2 levels was statistically analyzed to determine their relationship with the clinical features of cervical cancer. As shown in Table 1, STOML2 expression significantly correlated with tumor size (P = 0.025) and FIGO stage (P = 0.013). Taken together, our data revealed a relationship between the expression of STOML2 and tumor progression of cervical cancer.
Figure 2.

Representative images of STOML2 from immunohistochemistry assays in cervical cancer specimens (high expression for A, and low expression for B) 400 ×.
STOML2 expression is associated with the prognosis of cervical cancer patients
Survival analysis indicated the close correlation between STOML2 expression and the clinical survival time in patients with cervical cancer. The length of survival time was different between patients with low and high STOML2 expression, with the high STOML2 group having a shorter overall survival time (Figure 3A, P = 0.001) and recurrent-free survival time (Figure 3B, P = 0.017). Multivariate analysis indicated that STOML2 expression was an independent prognostic factor of patient overall survival (Table 2). Taken together, these results suggest that STOML2 has a strong correlation with the prognosis of patients and represents a novel prognostic marker for cervical cancer.
Figure 3.
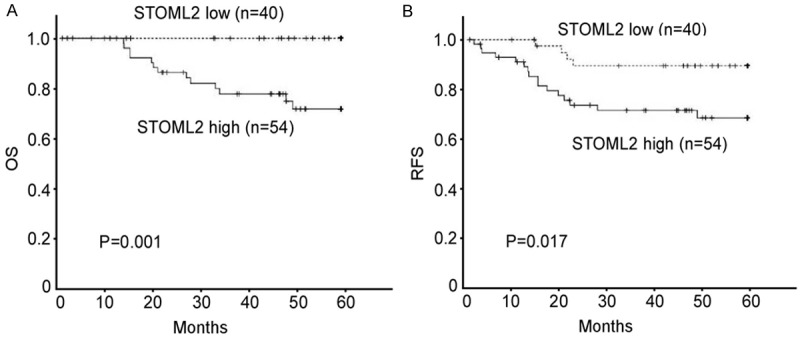
Kaplan-Meier curves of overall survival (A) and recurrent free survival (B) in relation to STOML2 expression in 94 cervical cancer patients.
Table 2.
Multivariate Cox regression analysis of overall survival (OS) and recurrence-free survival (RFS) in patients with cervical cancer
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age (> 40 vs. ≤ 40) | 1.967 (0.262-7.738) | 0.648 | 1.595 (0.417-6.497) | 0.334 |
| FIGO Stage (> IB vs. IB) | 2.253 (0.522-5.784) | 0.109 | 2.726 (0.427-6.492) | 0.112 |
| Tumor size (> 4 cm vs. ≤ 4 cm) | 2.474 (1.865-7.115) | 0.167 | 1.712 (0.342-7.997) | 0.185 |
| Differentiation (Grade 3 vs. 1/2) | 1.513 (0.395-3.395) | 0.547 | 1.142 (0.470-3.745) | 0.821 |
| LN Metastasis (Yes vs. No) | 1.418 (0.130-5.914) | 0.900 | 1.212 (0.195-4.194) | 0.789 |
| STOML2 expression (High vs. Low) | 4.765 (1.727-15.448) | 0.022 | 4.093 (1.946-14.467) | 0.054 |
Discussion
In this study, we revealed that STOML2 expression is up-regulated in cervical cancer tissues in compared with in adjecent normal tissues. High expression of STOML2 protein was found in 57.4% of cervical cancer specimens, and the expression level of STOML2 was found to be significantly correlated with the tumor development and progression. Moreover, statistical analysis showed that patients with higher levels of STOML2 had poorer clinical survival. Our study indicates that STOML2 might be used as a novel prognostic marker for the prognosis in patients with cervical cancer.
The human STOML2 gene, with stomatin consensus signature sequence, had been characterized as a member of the stomatin superfamily. However, the sequences of stomatin and STOML2 have very low homology, and STOML2 gene is lack of a typical N-terminal transmembrane domain, which is present in other stomatin family members [5]. By observing that STOML2 was colocalized with mitochondrial markers both in immunocytochemical and cell fractionation experiments, Hajek et al. suggested that STOML2 is a mitochondrial intermembrane space/inner membrane localized protein [14]. The exact function of STOML2 is unknown, while several recent studies have reported that STOML2 is involved in the progression and development of cancers. Zhang et al. found that STOML2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma [15]. Cui et al. reported that STOML2 is overexpressed and related to cell growth in human endometrial adenocarcinoma [16]. Liu et al. proposed that STOML2 is associated with the clinicopathological features of human papillary thyroid cancer and is regulated by TGF-β in thyroid cancer cells [17]. In this study, we found that STOML2 was overexpressed in cervical cancer tissues in compared with normal tissues at transcriptional level, indicating that the vital role of STOML2 in cervical tumorigenesis and progression.
Based on our present work, high expression of STOML2 protein was found in 57.4% of cervical cancer specimens. In addition, elevated levels of STOML2 were closely associated with clinical overall survival and recurrent free survival in cervical cancer patients. Moreover, multivariate analysis implied that STOML2 may be a useful prognostic indicator in patients with cervical cancer. Recently, the prognostic role of STOML2 has been studied in several cancers. Chang et al. demonstrated that STOML2 Cao et al. suggested that STOML2 overexpression could be used as a prognostic factor in node positive and HER2 negative breast cancer [18]. Liu et al. found that high levels of STOML2 expression were significantly correlated with the depth of invasion, lymph node metastasis, distant metastasis, and prognosis in gastric cancer [19]. Our results are in agreement with previous studies in that STOML2 may serve as a significant prognostic factor in human cancers.
Tumor progression remains a major cause of mortality and morbidity in locally advanced cervical cancer patients. In our study, we found that STOML2 overexpression is correlated with the tumor size and tumor stage of cervical cancer. Our result is in consistent with previous studies demonstrating that stomatin is capable of mediating several biological events associated with cancer development [20], which is supported by Arkhipova, who and colleagues found aberrant expression of stomatin in non-small cell lung cancer and soft tissue sarcomas [21]. Keenan et al. also reported increased expression of stomatin protein in drug-resistant variants derived from the poorly differentiated squamous cell lung carcinoma [22]. These studies together suggested an oncogenic role of STOML2 in cervical cancer development.
In conclusion, this study has shown that STOML2 might play important roles in the development and progression of cervical carcinoma. The expression of STOML2 protein is associated with clinical prognosis of patients with cervical cancer. Understanding the biological function of STOML2 in cervical cancer progression will advance our knowledge of the mechanisms underlying cervical carcinogenesis.
Disclosure of conflict of interest
None.
References
- 1.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Forman D, O'Brien M, Ferlay J, Center M, Parkin DM. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Bergeron C, von Knebel Doeberitz M, Gravitt P, Jeronimo J, Lorincz AT, J L M Meijer C, Sankaranarayanan R, J F Snijders P, Szarewski A. New technologies and procedures for cervical cancer screening. Vaccine. 2012;30(Suppl 5):F107–116. doi: 10.1016/j.vaccine.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 4.Ault KA. Cervical cancer prevention: better tests, better tools, and more equitable outcomes. J Natl Cancer Inst. 2011;103:1352–1353. doi: 10.1093/jnci/djr330. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Morrow JS. Identification and characterization of human SLP-2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J Biol Chem. 2000;275:8062–8071. doi: 10.1074/jbc.275.11.8062. [DOI] [PubMed] [Google Scholar]
- 6.Seidel G, Prohaska R. Molecular cloning of hSLP-1, a novel human brain-specific member of the band 7/MEC-2 family similar to Caenorhabditis elegans UNC-24. Gene. 1998;225:23–29. doi: 10.1016/s0378-1119(98)00532-0. [DOI] [PubMed] [Google Scholar]
- 7.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Cao W, Zhang B, Ding F, Zhang W, Sun B, Liu Z. Expression of SLP-2 was associated with invasion of esophageal squamous cell carcinoma. PLoS One. 2013;8:e63890. doi: 10.1371/journal.pone.0063890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owczarek CM, Treutlein HR, Portbury KJ, Gulluyan LM, Kola I, Hertzog PJ. A novel member of the STOMATIN/EPB72/mec-2 family, stomatin-like 2 (STOML2), is ubiquitously expressed and localizes to HSA chromosome 9p13.1. Cytogenet Cell Genet. 2001;92:196–203. doi: 10.1159/000056902. [DOI] [PubMed] [Google Scholar]
- 10.Christie DA, Lemke CD, Elias IM, Chau LA, Kirchhof MG, Li B, Ball EH, Dunn SD, Hatch GM, Madrenas J. Stomatin-like protein 2 binds cardiolipin and regulates mitochondrial biogenesis and function. Mol Cell Biol. 2011;31:3845–3856. doi: 10.1128/MCB.05393-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W, Zhang B, Liu Y, Li H, Zhang S, Fu L, Niu Y, Ning L, Cao X, Liu Z, Sun B. High-level SLP-2 expression and HER-2/neu protein expression are associated with decreased breast cancer patient survival. Am J Clin Pathol. 2007;128:430–436. doi: 10.1309/C6X54HRB580EP2NQ. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Cao W, Yu Z, Liu Z. Downregulation of a mitochondria associated protein SLP-2 inhibits tumor cell motility, proliferation and enhances cell sensitivity to chemotherapeutic reagents. Cancer Biol Ther. 2009;8:1651–1658. doi: 10.4161/cbt.8.17.9283. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Liu L, Wu Z, Lin C, Dai T, Yu C, Wang X, Wu J, Li M, Li J. Knockdown of stomatin-like protein 2 (STOML2) reduces the invasive ability of glioma cells through inhibition of the NF-kappaB/MMP-9 pathway. J Pathol. 2012;226:534–543. doi: 10.1002/path.3008. [DOI] [PubMed] [Google Scholar]
- 14.Hajek P, Chomyn A, Attardi G. Identification of a novel mitochondrial complex containing mitofusin 2 and stomatin-like protein 2. J Biol Chem. 2007;282:5670–5681. doi: 10.1074/jbc.M608168200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ding F, Cao W, Liu Z, Liu W, Yu Z, Wu Y, Li W, Li Y. Stomatin-like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:1639–1646. doi: 10.1158/1078-0432.CCR-05-1858. [DOI] [PubMed] [Google Scholar]
- 16.Cui Z, Zhang L, Hua Z, Cao W, Feng W, Liu Z. Stomatin-like protein 2 is overexpressed and related to cell growth in human endometrial adenocarcinoma. Oncol Rep. 2007;17:829–833. [PubMed] [Google Scholar]
- 17.Liu Z, Yang Y, Zhang Y, Ye X, Wang L, Xu G. Stomatin-like protein 2 is associated with the clinicopathological features of human papillary thyroid cancer and is regulated by TGF-beta in thyroid cancer cells. Oncol Rep. 2014;31:153–160. doi: 10.3892/or.2013.2833. [DOI] [PubMed] [Google Scholar]
- 18.Cao W, Zhang B, Li J, Liu Y, Liu Z, Sun B. SLP-2 overexpression could serve as a prognostic factor in node positive and HER2 negative breast cancer. Pathology. 2011;43:713–718. doi: 10.1097/PAT.0b013e32834c34ed. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Zhang L, Shen Z, Tan F, Hu Y, Yu J, Li G. Increased levels of SLP-2 correlate with poor prognosis in gastric cancer. Gastric Cancer. 2013;16:498–504. doi: 10.1007/s10120-013-0232-3. [DOI] [PubMed] [Google Scholar]
- 20.Green JB, Young JP. Slipins: ancient origin, duplication and diversification of the stomatin protein family. BMC Evol Biol. 2008;8:44. doi: 10.1186/1471-2148-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arkhipova KA, Sheyderman AN, Laktionov KK, Mochalnikova VV, Zborovskaya IB. Simultaneous expression of flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell lung cancer and soft tissue sarcomas. BMC Cancer. 2014;14:100. doi: 10.1186/1471-2407-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keenan J, Murphy L, Henry M, Meleady P, Clynes M. Proteomic analysis of multidrug-resistance mechanisms in adriamycin-resistant variants of DLKP, a squamous lung cancer cell line. Proteomics. 2009;9:1556–1566. doi: 10.1002/pmic.200800633. [DOI] [PubMed] [Google Scholar]


