Abstract
Interleukin-4 (IL-4) and IL-13 are anti-inflammatory and immunoregulatory cytokines that can influence cancer-directed immunosurveillance. However, they are not evaluated as biomarkers for ccRCC outcomes. The aim of this study was to investigate the prognostic value of tumor-derived IL-4 and IL-13 in patients with localized ccRCC after surgery. Our study comprised 194 consecutive patients with localized ccRCC undergoing nephrectomy in a single center. Clinical characteristics, recurrence-free survival (RFS) and overall survival (OS) were recorded. We assessed IL-4 and IL-13 expression as continuous variables and dichotomized as low versus high by immunohistochemistry. For associations with RFS and OS, we used the Kaplan-Meier method and Cox regression models. Concordance index was calculated for predictive accuracy. We found that high expression levels of IL-4 and IL-13 were associated with increased recurrence (P < 0.001 and P = 0.006, respectively) and reduced survival (P = 0.001 and P = 0.016, respectively). Furthermore, multivariate analyses confirmed that combination of IL-4 and IL-13 expression (IL-4/IL-13 signature) was an independent prognostic factor for RFS and OS (P = 0.009 and P = 0.016, respectively). When applied to UISS score, IL-4/IL-13 signature improved the predictive accuracy. Notably, this improvement in prediction was mainly observed in patients with low-risk disease. To conclude, IL-4/IL-13 signature is an independent predictor of outcomes in patients with localized ccRCC, and the prognostic value is more prominent among patients with low-risk disease. Evaluation of IL-4 and IL-13 expression provides the opportunity to optimize postsurgical management and develop novel targeted therapies for ccRCC patients.
Keywords: Renal cell carcinoma, interleukin-4, interleukin-13, biomarker, prognosis
Introduction
Renal cell carcinoma (RCC) accounts for 2%-3% in all of the adult malignances, as the seventh most common cancer in men and the ninth most common cancer in women [1]. Clear-cell RCC (ccRCC) is the most frequent histologic subtype of RCC (70%-85%) [2]. The global incidence of all stages of RCC has increased steadily during last decades especially for localized RCC because of the widespread use of abdominal imaging [3]. Currently, surgery remains the most effective treatment for localized RCC. However, 20%-30% of patients with localized RCC undergoing nephrectomy experience recurrence and metastasis, resulting in incurable disease [4]. Therefore, it is important to stratify patients with localized RCC into different risk levels of recurrence and mortality for individualized therapy and monitoring.
So far several prognostic models have been established to estimate patients who are at a high risk of disease progression after surgery [5]. One widely used model is the University of California integrated staging system (UISS), which incorporates TNM stage, Fuhrman grade, and Eastern Cooperative Oncology Group performance status (ECOG-PS), resulting in three risk levels [low- (LR), intermediate- (IR) and high-risk (HR)][6]. The predictive accuracy of UISS score may be further improved by combining prognostic biomarkers [7].
Interleukin-4 (IL-4) and IL-13, two kinds of Th2 cytokines, are structurally similar multifunctional peptides that exert anti-inflammation and immunomodulation [8]. Several studies have revealed that IL-4 and IL-13 may suppress cancer-directed immunosurveillance and increase tumor metastasis. IL-4 was found to directly induce tumor cell proliferation and mediate resistance to apoptosis to support tumor growth [9,10]. IL-13 may trigger negative regulation of tumor immunosurveillance through the IL-4Rα signaling and activitation of its downstream transcriptional factor STAT6, and IL-13 inhibitors may be a useful tool in cancer immunotherapy [11]. In addition, IL-4 and IL-13, as products of malignant and stromal cells, can stimulate tumor-associated macrophages (TAMs) to be polarized into an M2 phenotype, which promotes proliferation, survival and metastasis of tumor cells [12]. Hence it is plausible that tumor-derived IL-4 and IL-13 can serve as prognostic biomarkers and potential therapeutic targets for RCC patients.
In this study, we assessed IL-4 and IL-13 levels by immunohistochemistry in tumor tissues from 194 ccRCC patients and their correlations with clinicopathologic characteristics and patient outcomes. We further assessed the prognostic significance of the combined analysis of IL-4 and IL-13 expression, and its ability to improve the predictive accuracy of the well-established UISS score.
Materials and methods
Patients
We identified 194 consecutive patients with localized ccRCC (T1-4N0M0) undergoing radical nephrectomy or nephron-sparing surgery between January 2003 and December 2004 at Zhongshan Hospital (Shanghai, China). This study was approved by the hospital’s ethics committee and informed consent was obtained from each patient. The exclusion criteria in this study were: 1) patients with mixed types of primary RCC confirmed by pathology; 2) patients with N1 or M1 disease; 3) patients with a prior history of other malignances; 4) patients who died within the first month after surgery because of surgical complications. Clinicopathologic information was obtained through medical record review, including age at surgery, gender, tumor size, TNM stage, Fuhrman grade, tumor necrosis and ECOG-PS. Patients were staged by radiographic reports and postoperative pathological data, and then were reassigned according to the 2010 American Joint Committee on Cancer TNM classification. The UISS score was stratified these patients into three risk levels [low- (LR), intermediate- (IR) and high-risk (HR)].
Postoperative patients were evaluated with physical examination, laboratory studies, chest imaging and abdominal ultrasound or computed tomographic scans semiannually for the first two years and annually thereafter. Follow-up was terminated on October 2013. RFS and OS were defined as the interval between the surgery and recurrence or death from all causes, respectively.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor specimens from 194 patients were obtained from the Department of Pathology, Zhongshan Hospital, Fudan University. All tissues were reviewed by H&E-staining and 2 representative areas were premarked on each of the paraffin blocks free from necrotic, hemorrhagic and fibrotic materials. Duplicate tissue cores in 1 mm diameter from 2 representative areas of each tissue were used to construct the TMA. Sections from the TMA blocks were cut at 4 μm.
TMA sections were heated at 60°C for 6 hours, dewaxed in xylene, and then hydrated in 100%, 95%, 85% and 75% alcohol successively. After the treatment of 3% H2O2 as endogenous peroxidase inhibitor for 30 minutes, the sections were infiltrated in unmasking solution (0.01 M sodium citrate buffer, pH = 6) and heated in a pressure cooker for 3 minutes. And then the sections were blocked by 10% normal goat serum for 2 hours at 37°C. Primary antibodies against human IL-4 (1:400; Abcam, Cambridge, MA, USA) and IL-13 (1:400; Abcam, Cambridge, MA, USA) were applied overnight at 4°C. Finally, EnVision Detection System (Dako) was used under manufacturer’s instructions.
A semiquantitative method was used for the evaluation of immuostaining by two independent pathologists without knowledge of patient information. Staining intensity was scored (0, no staining; 1, weak; 2, moderate; 3, strong) and the percentage of positive tumor cells was scored (0% to 100%). The final score for each case was recorded by multiplying the score of staining intensity and the percentage of positive tumor cells, which ranges from 0 to 300.
Statistical analysis
The median immunostaining score was applied to obtain the cutoff which separated each cytokine into low and high expression. The Pearson x2 test was used to compare categorical variables and the t tests was used to compare continuous variables. Kaplan-Meier method with log-rank test was used to compare with survival curves. Univariate and multivariate Cox regression models were applied to analyze the impact of prognostic factors on RFS and OS. The predictive accuracy of various Cox regression models was quantified by the Harrell concordance index (C-index). Analysis was performed with SPSS Statistics 21.0 and Stata 12.0. All statistical tests were two-sided and P < 0.05 was considered statistically significant.
Results
Patient characteristics and association with IL-4 and IL-13 expression
Patient characteristics are listed in Table 1. The study included 194 patients with localized ccRCC. The mean age at surgery was 55.2 years (range, 24 to 80 years), and 68.6% of patients were male. The mean tumor size was 4.6 cm (range, 1.0 to 18.0 cm), and 27.8% of cases had T3 or T4 tumors. The tumor necrosis was present in 21.1% of cases and high-grade tumor was distributed in 38.1% of cases. ECOG-PS was evaluated as ≥ 1 in 15.5% of cases. UISS was categorized as LR, IR and HR in 25.1%, 57.2% and 7.7% of cases, respectively. The median follow-up period was 106 months (range, 12 to 120 months). There were 61 (31.4%) patients confirmed with tumor recurrence and 48 (24.7%) patients confirmed dead at last follow-up. The 5- and 10-year RFS rates were 80.9% and 68.6%, respectively. The 5- and 10-year OS rates were 89.2% and 75.3%, respectively.
Table 1.
Patient characteristics and associations with expression of IL-4 and IL-13
| Characteristic | Total patients (n = 194) | IL-4 | IL-13 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| No. | % | Low (n = 109) | High (n = 85) | P | Low (n = 104) | High (n = 90) | P | |
| Mean age, yr* | 55.2 | 54.2 | 56.5 | 0.155 | 55.3 | 55.0 | 0.853 | |
| Gender | 0.339 | 0.673 | ||||||
| Male | 133 | 68.6 | 72 | 63 | 73 | 62 | ||
| Female | 61 | 31.4 | 37 | 24 | 31 | 30 | ||
| Mean tumor size, cm* | 4.6 | 4.6 | 4.5 | 0.918 | 4.6 | 4.5 | 0.843 | |
| Pathologic T stage | 0.491 | 0.367 | ||||||
| T1a | 68 | 35.1 | 37 | 31 | 36 | 32 | ||
| T1b | 54 | 27.8 | 31 | 23 | 29 | 25 | ||
| T2 | 18 | 9.3 | 13 | 5 | 13 | 5 | ||
| T3a + T4 | 54 | 27.8 | 28 | 26 | 26 | 28 | ||
| Fuhrman grade | 0.225 | 0.158 | ||||||
| 1 | 33 | 17.0 | 23 | 10 | 23 | 10 | ||
| 2 | 87 | 44.8 | 50 | 37 | 47 | 40 | ||
| 3 | 54 | 27.8 | 27 | 27 | 25 | 29 | ||
| 4 | 20 | 10.3 | 9 | 11 | 9 | 11 | ||
| Tumor necrosis | 0.153 | 0.161 | ||||||
| Absent | 153 | 78.9 | 90 | 63 | 86 | 67 | ||
| Present | 41 | 21.1 | 19 | 22 | 18 | 23 | ||
| ECOG-PS | 0.052 | 0.407 | ||||||
| 0 | 164 | 84.5 | 97 | 67 | 90 | 74 | ||
| ≥ 1 | 30 | 15.5 | 12 | 18 | 14 | 16 | ||
| UISS score | 0.196 | 0.737 | ||||||
| LR | 68 | 25.1 | 44 | 24 | 39 | 29 | ||
| IR | 111 | 57.2 | 58 | 53 | 57 | 54 | ||
| HR | 15 | 7.7 | 7 | 8 | 8 | 7 | ||
ECOG-PS = Eastern Cooperative Oncology Group performance status; UISS = University of California Los Angeles Integrated Staging System; LR = low-risk; IR = intermediate-risk; HR = high-risk.
Student’s t test; chi-square test for all the other analyses.
IL-4 and IL-13 positive staining was mainly appeared in the cytoplasm of tumor cells (Figure 1). The median immunostaining scores for IL-4 and IL-13 were 180 (range, 0 to 300) and 140 (range, 0 to 300), respectively, which dichotomized the population into 109 patients (56.2%) in IL-4 low expression and 85 patients (43.8%) in IL-4 high expression, and 104 patients (53.6%) in IL-13 low expression and 90 patients (46.4%) in IL-13 high expression. Of note, we found no significant differences between IL-4 and IL-13 expression and clinicopathologic features as summarized in Table 1.
Figure 1.
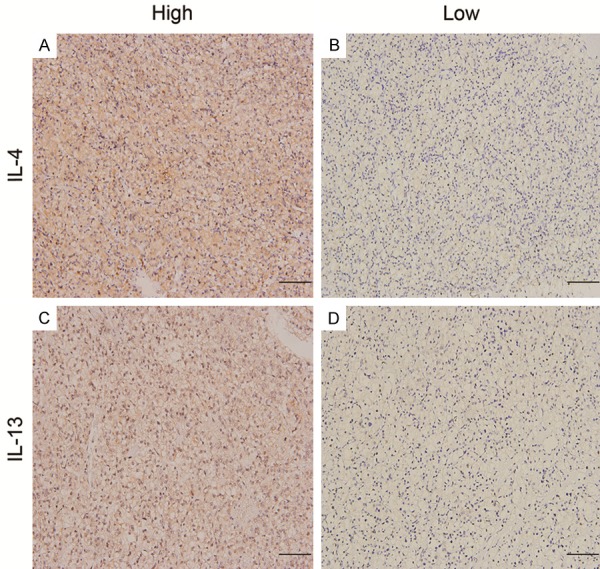
IL-4 and IL-13 expression in ccRCC tissues. Representative photographs of IL-4 (A and B) and IL-13 (C and D) immunostaining in tissue mircoarrays (scale bar, 100 μm).
Prognostic value of IL-4 and IL-13 expression
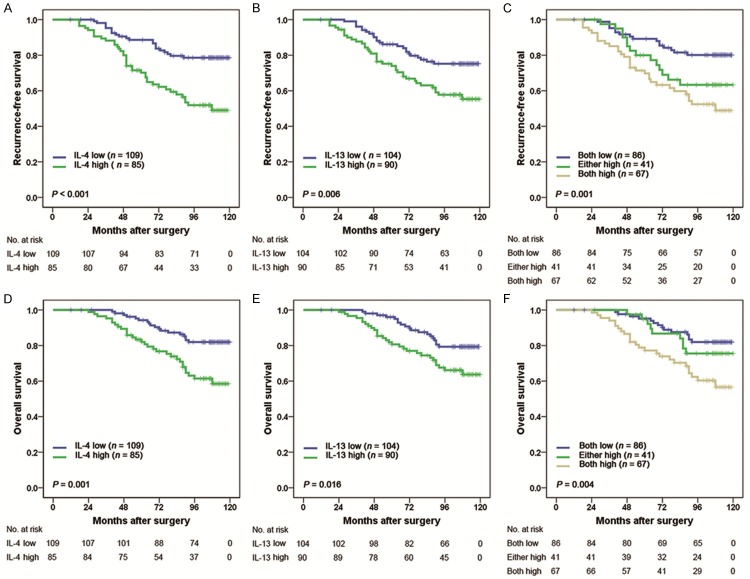
Kaplan-Meier analyses indicated that high expression levels of IL-4 and IL-13 were associated with shorter RFS (P < 0.001 and P = 0.006, respectively; Figure 2A and 2B) and OS (P = 0.001 and P = 0.016, respectively; Figure 2D and 2E). Moreover, we examined whether the combined analysis of IL-4 and IL-13 (named IL-4/IL-13 signature) could be related to outcomes. Patients were divided into three groups based on the levels of IL-4 and IL-13: group I, both low IL-4 and low IL-13 expression; group II, either high IL-4 or high IL-13 expression; group III, both high IL-4 and high IL-13 expression. Kaplan-Meier analysis showed significant difference among the three groups for RFS and OS (P = 0.001 and P = 0.004, respectively; Figure 2C and 2F), and both high IL-4 and high IL-13 expression was associated with worst RFS and OS. The 5-year RFS rates for group I, II and III were 89.5%, 80.5% and 70.1%, respectively. The 5-year OS rates for group I, II and III were 95.3%, 95.1% and 77.6%, respectively.
Figure 2.
Kaplan Meier curves showing RFS (A-C) and OS (D-F) probabilities based on intratumoral IL-4 and IL-13 expression levels. In (C and F), patients were classified into 3 groups: group I, both low IL-4 and low IL-13 expression; group II, either high IL-4 or high IL-13 expression; group III, both high IL-4 and high IL-13 expression.
Further on, we evaluated whether the IL-4/IL-13 signature was an independent predictor for RFS and OS using univariate and multivariate Cox regression analyses. As shown in Table 2, after adjusting for tumor size, pT stage, Fuhrman grade, tumor necrosis and ECOG-PS, the multivariate analysis demonstrated that IL-4/IL-13 signature was an independent prognostic predictor for RFS (HR, 2.277; 95% CI, 1.233-4.204; P = 0.009 for patients with both high vs. both low) and OS (HR, 2.268; 95% CI, 1.163-4.423; P = 0.016 for patients with both high vs. both low) for patients with localized ccRCC after surgery.
Table 2.
Univariate and multivariate cox regression analyses for recurrence-free survival and overall survival
| Variable | Recurrence-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Multivariate | Multivariate | |||||
|
|
|
|||||
| Univariate P | HR (95% CI) | P | Univariate P | HR (95% CI) | P | |
| Age, yr | 0.133 | 0.122 | ||||
| Gender (male vs female*) | 0.641 | 0.489 | ||||
| Tumor size, cm | < 0.001 | 1.103 (1.004-1.212) | 0.042 | < 0.001 | 1.118 (1.005-1.243) | 0.040 |
| Pathologic T stage | ||||||
| T3+T4 vs T1+T2* | < 0.001 | 2.642 (1.564-4.463) | < 0.001 | < 0.001 | 2.767 (1.545-4.957) | 0.001 |
| Fuhrman grade (3+4 vs 1+2*) | < 0.001 | 2.459 (1.472-4.108) | 0.001 | 0.004 | 2.138 (1.204-3.796) | 0.009 |
| Necrosis (present vs absent*) | 0.018 | 1.145 (0.632-2.007) | 0.655 | 0.012 | 1.312 (0.684-2.517) | 0.413 |
| ECOG PS (≥ 1 vs. 0*) | 0.001 | 1.587 (0.875-2.878) | 0.129 | 0.002 | 1.593 (0.818-3.100) | 0.171 |
| Combination of IL-4 and IL-13a | 0.001 | 0.029 | 0.006 | 0.051 | ||
| Either high vs both low* | 0.053 | 1.931 (0.929-4.015) | 0.078 | 0.417 | 1.435 (0.610-3.377) | 0.408 |
| Both high vs both low* | < 0.001 | 2.277 (1.233-4.204) | 0.009 | 0.002 | 2.268 (1.163-4.423) | 0.016 |
HR = hazard ratio; CI = confidence interval; ECOG-PS = Eastern Cooperative Oncology Group performance status.
Reference group.
Overall P value is provided, and HR, 95% CI, and P values are provided for each level.
Extension of UISS prognostic model with IL-4/IL-13 signature
The UISS score is widely applied to estimate prognosis after surgery for RCC patients. The 5-year RFS rates of UISS LR, IR and HR group were 91.2%, 78.4%, 53.3%, respectively. The 5-year OS rates of UISS LR, IR, and HR group were 94.1%, 88.3% and 73.3%, respectively. We calculated the C-index to appraise whether combination of IL-4/IL-13 signature with UISS score would improve the predictive accuracy. The C-indices of the original UISS score were 0.660 for RFS and 0.665 for OS, and improved to 0.712 for RFS and 0.715 for OS when IL-4/IL-13 signature was added. Then we performed a stratification analysis with the IL-4/IL-13 signature in each risk level of UISS score. For patients in low-risk group (n = 68), a significant statistical difference was observed for RFS and OS (P < 0.001, P = 0.004, respectively). The 5-year RFS rates for both low IL-4 and low IL-13 expression in UISS low-risk patients were 100% in contrast to 68.4% for both high IL-4 and high IL-13 expression (Figure 3A). Similarly, the 5-year OS rates were 100% and 78.9% for both low and both high expression group, respectively (Figure 3B). In UISS intermediate-/high-risk group (n = 126), the IL-4/IL-13 signature failed to reach statistical significance for RFS or OS (P = 0.093 and P = 0.204, respectively; Figure 4A and 4B).
Figure 3.
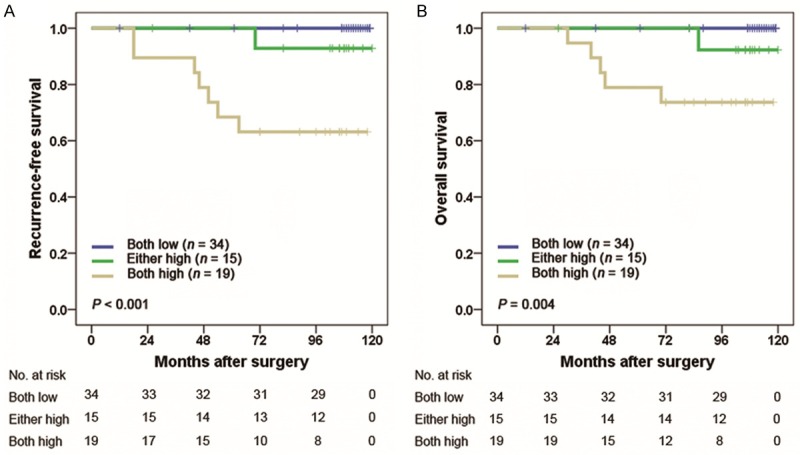
Kaplan Meier curves showing RFS (A) and OS (B) probabilities based on the combination of intratumoral IL-4 and IL-13 expression in UISS low-risk patients. UISS, University of California Los Angeles Integrated Staging System.
Figure 4.
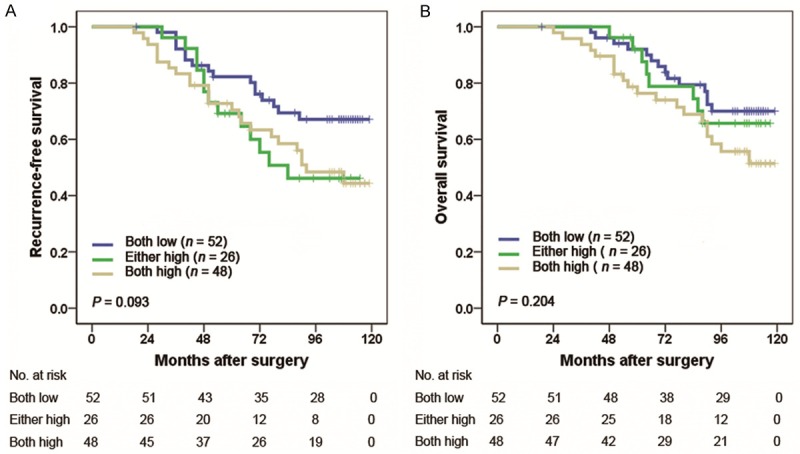
Kaplan Meier curves showing RFS (A) and OS (B) probabilities based on the combination of intratumoral IL-4 and IL-13 expression in UISS intermediate-/high-risk patients. UISS, University of California Los Angeles Integrated Staging System.
Discussion
In this study, we demonstrated that IL-4/IL-13 signature was an independent prognostic factor for RFS and OS in patients with localized ccRCC following surgery. Moreover, when incorporated into the well-established UISS score, the IL-4/IL-13 signature could help improve the predictive accuracy of this model and particularly represented a good discriminatory power in UISS low-risk patients as a complementary risk factor. Thereby, we propose that the inflammatory tumor microenvironment is important for understanding the mechanism of RCC progression and developing novel targeted therapies for prevention of recurrence after surgery.
Numerous studies showed that IL-4 and IL-13 expression were increased in many kinds of tumor tissues and peripheral blood including RCC [8,13]. In the present study, high levels of IL-4 and IL-13 in tumor tissues were correlated with adverse outcomes, indicating their participating in tumor progression and promising therapeutic values. Todaro et al. found that human colon and breast cancer cells could produce IL-4 which upregulated expressions of antiapoptotic proteins to protect against death ligand-induced apoptosis, and treating subcutaneous colon and breast cancers in mice with IL-4 neutralizing antibody modestly decreased the tumor growth and sensitized them to chemotherapy through downregulation of the antiapoptotic factors [14]. IL-13 receptors have two subtypes, IL-13Rα1 and IL-13Rα2. The latter is mainly expressed and activated on tumor cell surface [8]. Barderas et al. found that addition of IL-13 significantly increased colorectal cancer cell adhesion, migration, invasion and metastasis, but not in the IL-13Rα2-silenced cells by preparing stable shRNA transfectants, suggesting IL-13 effects were mediated by IL-13Rα2 [15]. Moreover, IL-4 and IL-13 are well-recognized to polarize TAMs into an M2 phenotype exerting protumoral actions [12]. Our group previously identified that intratumoral M2-polarized macrophages infiltration was predictive of short cancer-specific survival, denoted by the immune profile of low CD11c+ and high CD206+ TAM densities [16]. Taken together, IL-4 and IL-13 could either directly act on tumor growth or indirectly polarize TAMs into M2-skewed phenotype to facilitate tumor initiation and progression. Therefore, it is noteworthy to explore the inhibition of IL-4 and IL-13 signaling pathways which could activate tumor cell and TAMs as novel therapies to RCC.
The proportion of small and incidental RCC has significantly increased globally due to widespread use of abdominal imaging techniques [17]. Hence, there is an urgency to quest for a reliable and inexpensive clinical tool in postoperative management of the patients with low-risk disease. In this study, we demonstrated that the incorporation of IL-4/IL-13 signature into UISS score improved the predictive accuracy and this improvement in prediction largely took place in UISS low-risk patients. Park et al. advocated for the sequential or stepwise use of biomarkers on an as-needed basis in determining ccRCC outcomes after surgery [18]. Under this principle, we propose that postsurgical patients with localized ccRCC who are classified into the UISS low-risk group need to be further assessed by IL-4/IL-13 signature test in order to provide early interventions and close follow-up.
To date, many clinically useful biomarkers of RCC have been identified. The genetic polymorphisms of IL-4 receptor α were demonstrated to be associated with an increasing risk and a poor prognosis of patients with sporadic RCC [19]. The presence of intratumoral neutrophils was found to be an independent prognostic factor of poor outcomes in patients with localized and metastatic RCC, respectively [20,21]. Kapur et al. showed the overall survival was shorter in BAP1-mutant group than PBRM1-mutant group and patients with mutations in both BAP1 and PBRM1 had the worst prognosis, indicating mutation-defined subtypes of ccRCC with distinct clinical outcomes [22]. Another study showed that higher expression of type IIa topoisomerase (TOPOIIa) was independently correlated with increased risk of cancer death among patients with ccRCC undergoing nephrectomy [23]. On the whole, intensive molecular research over the past years has provided substantial insight into the biology of RCC and is beginning to shape clinical practice in terms of predicting tumor behavior and thereby improving patient outcomes. In light of the complexity of molecular pathways involved in RCC behavior, even more comprehensive biomarkers are needed to allow robust fingerprinting of RCC and host biological behavior leading to a customized therapy and surveillance [24].
There are a few limitations of this study that warrant further discussion. Firstly, the prognostic significance of IL-4 and IL-13 expression needs to be validated in other populations and larger cohorts. Secondly, the predictive value of IL-4 and IL-13 expression in metastatic RCC (mRCC) is not analyzed owing to limited cases with metastasis. Further assessment in mRCC needs to be performed. Thirdly, this is a retrospective study, blood samples from patients are not available. Thus the associations between serum-based IL-4 and IL-13 levels and patient outcome are not established. Further examination in blood samples should be done. At last, as many studies described, IL-4 and IL-13 receptors had important roles in tumor microenvironment, for an example of IL-13Rα2 [15]. In the future, the prognostic value of IL-4 and IL-13 receptors needs to be evaluated in RCC and the underlying mechanisms behind these associations need to be elucidated.
In conclusion, our results indicate that the concurrence of high expression of IL-4 and IL-13 in tumor tissues correlates with increased recurrence and reduced survival in patients with localized ccRCC after surgery. Moreover, this association is more pronounced in patients with low-risk disease. This finding provides a novel independent predictor for prognosis and could improve the widely-used UISS score in patient stratification, selecting patients for adjuvant treatment, and planning postsurgical follow-up.
Acknowledgements
We thank Ms. Haiying Zeng (Department of Pathology, Zhongshan Hospital, Fudan University) for technical assistance. This study was funded by grants from National Basic Research Program of China (2012CB822104), National Key Projects for Infectious Diseases of China (2012ZX10002-012), National Natural Science Foundation of China (31100629, 31270863, 81372755, 81471621, 81472227, 81402082, 81402085), Program for New Century Excellent Talents in University (NCET-13-0146) and Shanghai Rising-Star Program (13QA1400300). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Disclosure of conflict of interest
None.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J. Clin. Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Chiong E, Tay MH, Tan MH, Kumar S, Sim HG, Teh BT, Umbas R, Chau NM. Management of kidney cancer in Asia: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol. 2012;13:e482–491. doi: 10.1016/S1470-2045(12)70433-3. [DOI] [PubMed] [Google Scholar]
- 4.Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Can J Urol. 2008;15:3954–3966. [PubMed] [Google Scholar]
- 5.Meskawi M, Sun M, Trinh QD, Bianchi M, Hansen J, Tian Z, Rink M, Ismail S, Shariat SF, Montorsi F, Perrotte P, Karakiewicz PI. A review of integrated staging systems for renal cell carcinoma. Eur Urol. 2012;62:303–314. doi: 10.1016/j.eururo.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, deKernion JB, Figlin RA, Belldegrun AS. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J. Clin. Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, Pantuck AJ, Zigeuner R, Karakiewicz PI. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Hallett MA, Venmar KT, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012;72:6338–6343. doi: 10.1158/0008-5472.CAN-12-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Jiang J, Wang Z, Zhang J, Xiao M, Wang C, Lu Y, Qin Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008;68:8687–8694. doi: 10.1158/0008-5472.CAN-08-0449. [DOI] [PubMed] [Google Scholar]
- 10.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 12.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onishi T, Ohishi Y, Goto H, Tomita M, Abe K. An assessment of the immunological status of patients with renal cell carcinoma based on the relative abundance of T-helper 1- and -2 cytokine-producing CD4+ cells in peripheral blood. BJU Int. 2001;87:755–759. doi: 10.1046/j.1464-410x.2001.02210.x. [DOI] [PubMed] [Google Scholar]
- 14.Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, Di Stefano AB, Di Bernardo C, Agrusa A, Condorelli G, Walczak H, Stassi G. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762–772. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- 15.Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780–2790. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Zhu Y, Chen L, An H, Zhang W, Wang G, Lin Z, Xu J. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol. 2014;21:3142–3150. doi: 10.1245/s10434-014-3601-1. [DOI] [PubMed] [Google Scholar]
- 17.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Eberhard K, Hoefler G, Pummer K, Zigeuner R. Trends of stage, grade, histology and tumour necrosis in renal cell carcinoma in a European centre surgical series from 1984 to 2010. J Clin Pathol. 2012;65:721–724. doi: 10.1136/jclinpath-2012-200797. [DOI] [PubMed] [Google Scholar]
- 18.Parker AS, Leibovich BC, Lohse CM, Sheinin Y, Kuntz SM, Eckel-Passow JE, Blute ML, Kwon ED. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115:2092–2103. doi: 10.1002/cncr.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura E, Megumi Y, Kobayashi T, Kamoto T, Ishitoya S, Terachi T, Tachibana M, Matsushiro H, Habuchi T, Kakehi Y, Ogawa O. Genetic polymorphisms of the interleukin-4 receptor alpha gene are associated with an increasing risk and a poor prognosis of sporadic renal cell carcinoma in a Japanese population. Clin Cancer Res. 2002;8:2620–2625. [PubMed] [Google Scholar]
- 20.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 21.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 22.Kapur P, Pena-Llopis S, Christie A, Zhrebker L, Pavia-Jimenez A, Rathmell WK, Xie XJ, Brugarolas J. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker AS, Eckel-Passow JE, Serie D, Hilton T, Parasramka M, Joseph RW, Wu KJ, Cheville JC, Leibovich BC. Higher Expression of Topoisomerase II Alpha Is an Independent Marker of Increased Risk of Cancer-specific Death in Patients with Clear Cell Renal Cell Carcinoma. Eur Urol. 2013;66:929–935. doi: 10.1016/j.eururo.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shariat SF, Xylinas E. Biomarkers in personalised treatment of renal-cell carcinoma. Lancet Oncol. 2012;13:751–752. doi: 10.1016/S1470-2045(12)70292-9. [DOI] [PubMed] [Google Scholar]