Abstract
Objective: To investigate the Lgr5 (Leucine-rich repeat-containing G protein-coupled receptor 5) expression in cervical carcinoma and to estimate its clinical significance. Methods: The expression of Lgr5 mRNA was evaluated by Real-time PCR in 8 pairs of surgically removed cervical cancer and adjacent normal cervical tissues. Lgr5 protein expression was evaluated by immunohistochemistry in 94 paraffin-embedded cervical carcinoma specimens. The correlation between Lgr5 expression and clinicopathological features were statistically analyzed. Results: Lgr5 expression was significantly higher in cervical cancer tissues compared with that in adjacent normal cervix. High Lgr5 expression was positively correlated with tumor size (P = 0.025) and parametrial infiltration (P = 0.027). Moreover, high levels of Lgr5 was associated with lower overall survival (P = 0.021) and recurrent-free survival (P = 0.008), especially in stage II patients (P = 0.035). Multivariate analysis showed that the expression of Lgr5 was an independent factor of recurrent-free survival for the patients with cervical carcinoma (P = 0.135). Conclusion: Lgr5 may play an important role in the development and progression of cervical carcinoma, and may be a potential therapeutic target for the treatment of cervical carcinoma.
Keywords: Lgr5, prognosis, cervical carcinoma
Introduction
Cervical carcinoma is the third most prevalent malignant gynecologic malignancies worldwide and the one of the most frequent cause of cancer-related death in developing countries [1]. Although the prognosis of cervical carcinoma has been greatly improved due to early screening over the past decades, the 5-year survival of patients with advanced tumors remains less than 40% [2]. One of the crucial problems is the presence of advanced disease at diagnosis and recurrent or metastatic diseases. More than 30% patients die from metastatic or recurrent disease and the lack of treatment options [3]. Therefore, it is necessary to develop new biomarkers with the potential to predict the tumor progression and to evaluate the efficacy of therapeutic strategies.
Cancer stem cells (CSCs) have recently been the focus in cancer related studies. CSCs are defined as having the capacity to both self-renewal and give rise to the heterogeneous progeny of cancer cells that drive tumorigenesis, which may play critical roles in the development and maintenance of a malignant tumor [4]. Recently, the importance of CSCs in tumorgenesis and development has been firmly established in numerous tumors, and CSCs has been proposed to be a potential target for elucidation of mechanisms on carcinogenesis and exploration of new therapeutic strategies [5,6]. It is generally accepted that Wnt/β-catenin signaling pathway plays a pivotal role in the CSCs maintenance and tumorigenesis. Therefore, various Wnt-related molecules are being investigated and proposed as putative markers of CSCs [7]. CD133-positive progenitor cells have been shown to initiate growth and metastasis of colorectal cancer cells and to be related to poor prognosis [8]. CD44 has been described to possess cancer stem cell-like properties and correlates to clinical endpoints with increased risk of recurrence and shorter progression-free survival in patients with ovarian cancer [9]. ALDH1 has been reported to be positively correlated with the stage, grade and clinical prognosis in patients with non-small cell lung cancer [10].
In this retrospective study, we investigated the expression of CSC marker Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) in human cervical cancer. Then, we analyzed the correlation of the Lgr5 expression levels and clinical clinicopathological in cervical cancer patients. The relationship between Lgr5 expression and the clinical prognosis was also investigated. The findings from this study will provide novel insights into the carcinogenic process of cervical cancer from the perspective of the cancer stem cell origin hypothesis.
Materials and methods
Patients and tissue specimens
Primary tumor specimens from 94 paraffin-embedded primary cervical squamous cell carcinoma tissues were obtained from The First Affiliated Hospital of Shenzhen University from January 2004 to December 2007. The clinical and pathologic parameters were reviewed from impatient medical records and presented in Table 1. The median follow-up period was 46 months (range, 0.5-60 months). In addition, 8 pairs of cervical cancer tissues and matched adjacent normal tissues were dissected and frozen liquid nitrogen until further use. Informed consent was obtained from all patients for the use of their resected tumor specimens and approval from the Institutional Research Ethics Committee was obtained.
Table 1.
Association of Lgr5 expression with clinicopathologic features of cervical cancer
| Variable | Category | No. | Lgr5 expression | P | |
|---|---|---|---|---|---|
|
| |||||
| high | low | ||||
| Age (y) | ≤ 50 | 69 | 39 | 30 | 0.516 |
| > 50 | 25 | 16 | 9 | ||
| Tumor stage | I | 62 | 37 | 25 | 0.749 |
| II | 32 | 18 | 14 | ||
| Differentiation | 1/2 | 40 | 21 | 19 | 0.309 |
| 3 | 54 | 34 | 20 | ||
| Tumor size | ≤ 4 cm | 65 | 33 | 32 | 0.025 |
| > 4 cm | 29 | 22 | 7 | ||
| Parametrial infiltration | No | 77 | 41 | 36 | 0.027 |
| Yes | 17 | 14 | 3 | ||
| LN Metastasis | No | 81 | 47 | 34 | 0.811 |
| Yes | 13 | 8 | 5 | ||
RNA extraction and Real-time PCR
The mRNA expression of Lgr5 in cervical cancer and matched cervical tissues was quantified by Real-time PCR. Approximate 100 mg tissues were used for RNA extraction using the Trizol Reagent (Invitrogen) according to manufacturer’s instructions. The RNA was pretreated with RNase-free DNase (Promega), and cDNA was synthesized from 1 μg RNA. Real-time PCR was performed using a Thermal Cycler Dice® Real-time System TP800 (Takara Bio Inc., Otsu, Japan) system. Sequences of the primers are: Lgr5 forward primer 5’-GATGTTGCTCAGGGTGGACT-3’, backward primer 5’-GGGAGCAGCTGACTGATGTT-3’; Actin forward primer 5’-GCACCCAGCACAATGAAGA-3’, backward primer 5’-CGATCCACACGGAGTACTTG-3’.
Immunohistochemical staining
Paraffin-embedded samples were obtained from 94 patients for immunohistochemical analysis. In brief, slides were cut to a thickness of 4 μm, deparaffinized in xylene, and rehydrated in a descending ethanol-to-water gradient series. Endogenous peroxidase was blocked by exposure to 3% H2O2, followed by antigen retrieval via pressurized heating in 10 mmol/L of citrate buffer (pH 6.0). Then sections were incubated with 5% serum to avoid the non-specific binding. Lgr5 immunodetection was carried out by incubating with primary rabbit polyclonal antibody at dilutions for Lgr5 (Abcam, USA; 1:100). After washing with phosphate-buffered saline (PBS), the slides were incubated with hoseradishperoxidase-conjugated goat-anti-rabbit secondary antibody, followed by reaction with diaminobenzidine, and counterstaining with Mayer hematoxylin. For blank controls, the primary antibody was omitted. Negative controls were created using the same procedure but replacing the primary antibody to nonimmune serum.
Evaluation of immunohistochemical staining
The processed immunostained sections were examined by two pathologists, working independently and blinded to the corresponding clinical data. The proportion of positive tumor cells was scored according to the following criteria: 0 (no positive tumor cells); 1 (< 10% positive tumor cells); 2 (10-50% positive tumor cells); 3 (51-80% positive tumor cells), and 4 (> 80% positive tumor cells). Staining intensity was graded as follows: 1 (weak staining = light yellow); 2 (moderate staining = yellow brown) and 3 (strong staining = brown). Staining index was calculated by multiplying the staining intensity score and the proportion of positive tumor cells. The cut-off value for distinguishing high and low Lgr5 expression was set as an staining index of 6.
Statistical analysis
All statistical analyses were carried out using SPSS version 16.0 (SPSS, Chicago, IL). The Chi-square test was performed to evaluate the correlation of Lgr5 expression with clinicopathological characteristics. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazard model. Statistical significance was considered at a value of P < 0.05.
Results
Expression of Lgr5 in cervical cancer
Real-time PCR was performed to analyze Lgr5 mRNA expression levels in cervical cancer tissues. As shown in Figure 1, the expression level of Lgr5 was higher in cervical cancer samples compared to matched adjacent normal cervical tissues. The immunohistochemical data showed that Lgr5 protein was predominantly localized in the cytoplasm or on cell membrane. The representative immunostaining of Lgr5 in cervical carcinoma tissues was shown in Figure 2A-D.
Figure 1.
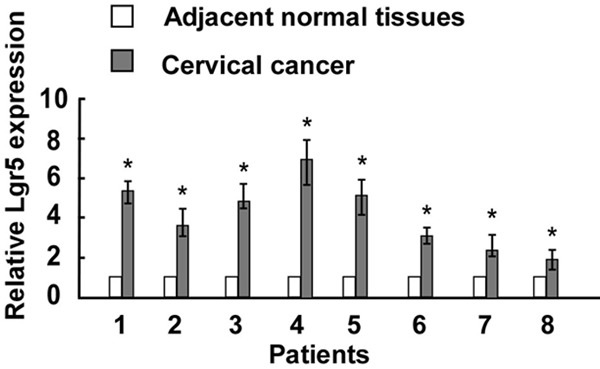
Relative expression levels of Lgr5 in cervical cancer specimens was detected by Real-time PCR (n = 8) compared with matched adjacent normal tissues. Asterisks, P < 0.05.
Figure 2.
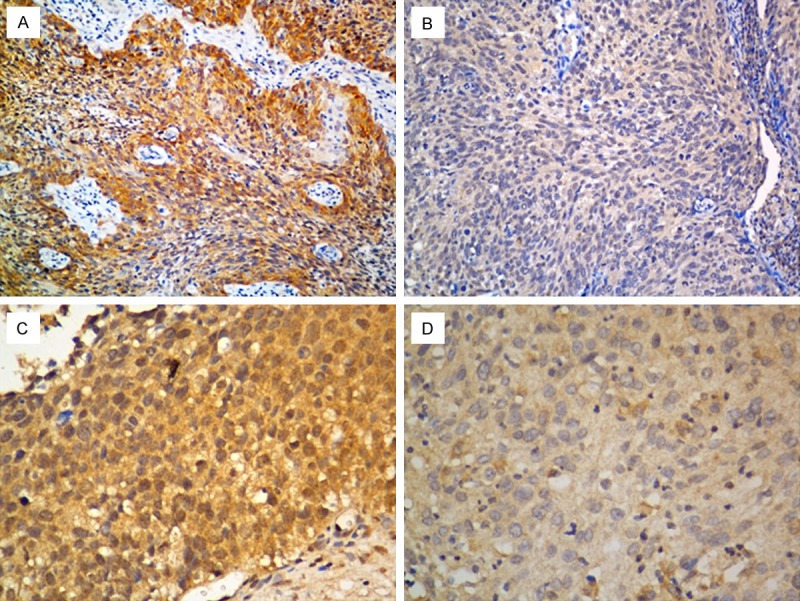
Representative immunohistochemical staining of Lgr5 in cervical cancer tissues. A and C: High expression of Lgr5; B and D: Low expression of Lgr5 (A-B, 200X, C-D, 400 × magnification).
Association of Lgr5 expression with clinicopathological feature
In order to investigate the clinical significance of Lgr5 expression in cervical carcinoma, the relationship between Lgr5 expression and clinicopathological features was further analyzed. As shown in Table 1, Lgr5 expression was significantly correlated with tumor size (P = 0.025) and parametrial infiltration (P = 0.027), but not correlated with patients’ age, tumor stage, tumor differentiation, or lymph node metastasis.
Association of Lgr5 expression with clinical prognosis
Survival analysis showed that patients with high level of Lgr5 expression had significantly shorter overall survival time (Figure 3A, P = 0.021) and recurrent-free survival time (Figure 3B, P = 0.008) than patients with low level of Lgr5 expression. Among patients with stage II cervical cancer, the 5-year survival rate was significantly lower in patients with high Lgr5 tumors than in those with low Lgr5 tumors (Figure 3C, P = 0.035). However, no difference was observed in the survival of patients with stage IB cervical carcinoma according to Lgr5 status (Figure 3D, P = 0.203). Lgr5 expression was further found to be an independent prognostic factor by the Cox proportional hazard model (Table 2).
Figure 3.
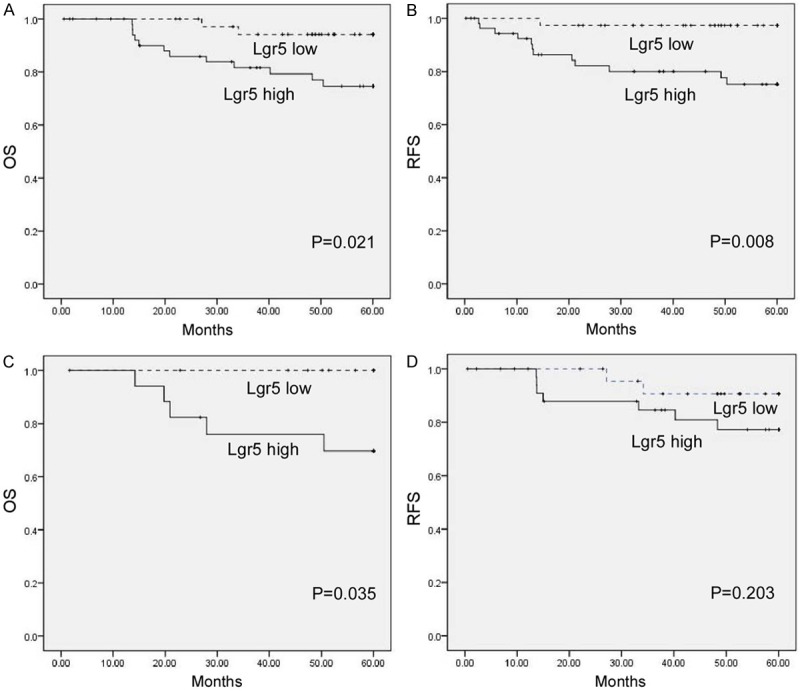
Correlation between expression levels of Lgr5 and patients’ survival. Patients with higher Lgr5 expression were closely correlated with poorer overall (A) and recurrence- free survival (B) than patients with tumor with lower Lgr5 expression. (C-D) Overall survival in relation to the Lgr5 status in stage II (C) and stage I (D) patients.
Table 2.
Multivariate Cox regression analysis of OS and RFS in cervical cancer patients
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (> 50 vs. ≤ 50) | 1.134 (0.261-4.038) | 0.627 | 1.428 (0.158-5.045) | 0.572 |
| Tumor Stage (II vs. I) | 1.833 (0.418-7.126) | 0.326 | 1.724 (0.473-6.265) | 0.247 |
| Differentiation (Grade 3 vs. 1/2) | 1.256 (0.721-4.356) | 0.835 | 1.245 (0.763-4.843) | 0.824 |
| Tumor size (> 4 cm vs. ≤ 4 cm) | 1.462 (0.247-5.624) | 0.072 | 1.674 (0.425-6.845) | 0.089 |
| Parametrial infiltration (+ vs. -) | 2.456 (1.422-5.529) | 0.065 | 1.934 (0.523-7.542) | 0.095 |
| LN Metastasis (+ vs. -) | 2.736 (0.153-9.012) | 0.030 | 2.245 (0.534-6.635) | 0.043 |
| Lgr5 expression (high vs. low) | 2.126 (0.754-5.164) | 0.069 | 3.763 (1.534-9.527) | 0.135 |
Discussion
To develop specific and sensitive molecular biomarkers is a major challenge in the management of cancers. In the present study, we observed a different expression of Lgr5 mRNA in cervical cancer tissues compared with matched adjacent normal cervical tissues. Moreover, the clinical clinicopathological significance of Lgr5 expression in cervical cancer was demonstrated, and tumor size and parametrial infiltration were found to be correlated with Lgr5 expression. Furthermore, statistical analysis showed that high levels of Lgr5 expression were associated with poor prognosis in patients with cervical cancer. Our data suggest a critical role of Lgr5 in the progression of human cervical carcinoma.
Lgr5 is a member of G-protein coupled receptor class A orphan receptor protein family. The LGR5 gene is 144, 810 bases long and located at chromosome 12 at position 12q22-q23 [11]. The Lgr5 protein contains seven transmembrane domains and highly conserved in the mammalian clade [12]. It’s expressed across numerous tissues such as in the intestine epithelium, placenta, central nervous system, muscle, and as a marker of adult stem cells in several tissues [13]. Recent findings revealed that Lgr5 is upregulated in a variety of human cancers including colorectal cancer, hepatocellular carcinoma, endometrial cancer, and ovarian cancer, indicating the possible role of Lgr5 expression cells in the progression and development of tumors [14]. Zheng et al. reported that enhanced Lgr5 is related to dedifferentiation and metastasis of gastric cancer, indicating its potential as an early diagnostic and prognostic biomarker in gastric cancer [15]. Liu et al. demonstrated that overexpression of Lgr5 might be a general characteristics and is correlated with poor survival of colon cancer both in mice and in patients [16]. Ryuge et al. described Lgr5 is expressed in a subset of lung adenocarcinoma and its expression is related to some clinicopathological parameters and poorer clinical prognosis [17]. However, the role of Lgr5 in the development of cervical cancer remains unknown.
Herein, in our study, we firstly observed significantly higher Lgr5 expression in cervical cancer tissues. Increased expression of Lgr5 was significantly correlated with parametrial infiltration. Besides, higher Lgr5 expression was seen more frequently in advanced cervical cancer. These results suggest that Lgr5 may play an important role in the progression and development of this tumor. In agreement with our results, Gao et al. suggested that Lgr5 overexpression is positively related to tumor progression in advanced colorectal cancer, and might be involve in proliferation, invasion, and distant and regional metastasis of colorectal cancer cells [18]. Similarly, Kleist et al. reported that Lgr5 is expressed in the metastatic cascade of colorectal cancer and these cells might be biologically powerful in the metastatic process of cancer subsets [19]. Rather, as Wnt signaling has been considered to be involved in the regulation of cancer stem cells and tumor progression, Garcia et al. suggested that Lgr5 is a negative regulator of the Wnt signaling in the developing intestine [20]. These studies indicate the complex roles of Lgr5 in tumorigenesis and tumor progression. Further studies are needed to investigate the underlying molecular mechanism.
Intriguingly, we found that Lgr5 expression was associated with poor clinical survival of patients with cervical cancer, especially in Stage II patients, indicating that high Lgr5 expression cells might be more aggressive and progress more quickly [21]. Saigusa et al. shown that patients with high expression levels of both LKB1 and LGR5 had a significantly lower recurrence-free survival in locally advanced rectal cancer [22]. Wu et al. demonstrated that Lgr5 is a potential marker of colorectal carcinoma stem cells that correlates with patient survival [23]. Conversely, Bu et al. investigated that LGR5 expression was more frequently expressed in lower staged colorectal cancer [24]. Takeda et al. also reported that Lgr5 expression in luminal surface showed a negative association with the progressive grade of colorectal tumors [25]. Together these findings underline that the prognostic role of Lgr5 might be diverse in different tissues.
In conclusion, Lgr5, a putative cancer stem cell marker, is frequently over-expressed in cervical cancer tissues. The Lgr5 may play an important role in cervical tumorigenesis and tumor development, and would be a powerful biomarker to predict the prognosis of cervical cancer patients, especially of advanced stage patients. Our study indicates that Lgr5 could be a candidate target for future diagnosis and therapy in cervical cancer management.
Disclosure of conflict of interest
None.
References
- 1.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 3.Tao X, Hu W, Ramirez PT, Kavanagh JJ. Chemotherapy for recurrent and metastatic cervical cancer. Gynecol Oncol. 2008;110:S67–71. doi: 10.1016/j.ygyno.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, Gollin SM, Gamblin TC, Geller DA, Lagasse E. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–6941. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 6.Delude C. Tumorigenesis: Testing ground for cancer stem cells. Nature. 2011;480:S43–45. doi: 10.1038/480S43a. [DOI] [PubMed] [Google Scholar]
- 7.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 8.Kemper K, Versloot M, Cameron K, Colak S, de Sousa e Melo F, de Jong JH, Bleackley J, Vermeulen L, Versteeg R, Koster J, Medema JP. Mutations in the Ras-Raf Axis underlie the prognostic value of CD133 in colorectal cancer. Clin Cancer Res. 2012;18:3132–3141. doi: 10.1158/1078-0432.CCR-11-3066. [DOI] [PubMed] [Google Scholar]
- 9.Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012;29:939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- 10.Dimou A, Neumeister V, Agarwal S, Anagnostou V, Syrigos K, Rimm DL. Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer. Am J Pathol. 2012;181:1436–1442. doi: 10.1016/j.ajpath.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 11.McDonald T, Wang R, Bailey W, Xie G, Chen F, Caskey CT, Liu Q. Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily. Biochem Biophys Res Commun. 1998;247:266–270. doi: 10.1006/bbrc.1998.8774. [DOI] [PubMed] [Google Scholar]
- 12.McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–426. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- 13.Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- 14.Sanders MA, Majumdar AP. Colon cancer stem cells: implications in carcinogenesis. Front Biosci (Landmark Ed) 2011;16:1651–1662. doi: 10.2741/3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng ZX, Sun Y, Bu ZD, Zhang LH, Li ZY, Wu AW, Wu XJ, Wang XH, Cheng XJ, Xing XF, Du H, Ji JF. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J Gastroenterol. 2013;19:8714–8721. doi: 10.3748/wjg.v19.i46.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Dai W, Jiang L, Cheng Y. Over-expression of LGR5 correlates with poor survival of colon cancer in mice as well as in patients. Neoplasma. 2014;61:177–185. doi: 10.4149/neo_2014_016. [DOI] [PubMed] [Google Scholar]
- 17.Ryuge S, Sato Y, Jiang SX, Wang G, Kobayashi M, Nagashio R, Katono K, Iyoda A, Satoh Y, Masuda N. The clinicopathological significance of Lgr5 expression in lung adenocarcinoma. Lung Cancer. 2013;82:143–148. doi: 10.1016/j.lungcan.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Gao FJ, Chen JY, Wu HY, Shi J, Chen M, Fan XS, Huang Q. Lgr5 over-expression is positively related to the tumor progression and HER2 expression in stage pTNM IV colorectal cancer. Int J Clin Exp Pathol. 2014;7:1572–1579. [PMC free article] [PubMed] [Google Scholar]
- 19.Kleist B, Xu L, Li G, Kersten C. Expression of the adult intestinal stem cell marker Lgr5 in the metastatic cascade of colorectal cancer. Int J Clin Exp Pathol. 2011;4:327–335. [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Simon E, Petke D, Boger C, Behrens HM, Warneke V, Ebert M, Rocken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One. 2012;7:e35486. doi: 10.1371/journal.pone.0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saigusa S, Inoue Y, Tanaka K, Toiyama Y, Kawamura M, Okugawa Y, Okigami M, Hiro J, Uchida K, Mohri Y, Kusunoki M. Significant correlation between LKB1 and LGR5 gene expression and the association with poor recurrence-free survival in rectal cancer after preoperative chemoradiotherapy. J Cancer Res Clin Oncol. 2013;139:131–138. doi: 10.1007/s00432-012-1308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu XS, Xi HQ, Chen L. Lgr5 is a potential marker of colorectal carcinoma stem cells that correlates with patient survival. World J Surg Oncol. 2012;10:244. doi: 10.1186/1477-7819-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bu Z, Zheng Z, Zhang L, Li Z, Sun Y, Dong B, Wu A, Wu X, Wang X, Cheng X, Xing X, Li Y, Du H, Ji J. LGR5 is a promising biomarker for patients with stage I and II gastric cancer. Chin J Cancer Res. 2013;25:79–89. doi: 10.3978/j.issn.1000-9604.2013.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Kinoshita I, Shimizu Y, Matsuno Y, Shichinohe T, Dosaka-Akita H. Expression of LGR5, an intestinal stem cell marker, during each stage of colorectal tumorigenesis. Anticancer Res. 2011;31:263–270. [PubMed] [Google Scholar]


