Abstract
Drug combination therapies are common practice in the treatment of cancer. In this study, we evaluated the anticancer effects of myricetin (MYR), methyl eugenol (MEG) and cisplatin (CP) both separately as well as in combination against cervical cancer (HeLa) cells. To demonstrate whether MYR and MEG enhance the anticancer activity of CP against cervical cancer cells, we treated HeLa cells with MYR and MEG alone or in combination with cisplatin and evaluated cell growth and apoptosis using MTT (3 (4, 5 dimethyl thiazol 2yl) 2, 5 diphenyltetrazolium bromide) assay, LDH release assay, flow cytometry and fluorescence microscopy. The results revealed that, as compared to single drug treatment, the combination of MYR or MEG with CP resulted in greater effect in inhibiting cancer cell growth and inducing apoptosis. Cell apoptosis induction, Caspase-3 activity, cell cycle arrest and mitochondrial membrane potential loss were systematically studied to reveal the mechanisms of synergy between MYR, MEG and CP. Combination of MYR or MEG with CP resulted in more potent apoptosis induction as revealed by fluorescence microscopy using Hoechst 33258 and AO-ETBR staining. The combination treatment also increased the number of cells in G0/G1 phase dramatically as compared to single drug treatment. Mitochondrial membrane potential loss (ΛΨm) as well as Caspase-3 activity was much higher in combination treatment as compared to single drug treatment. Findings of this investigation suggest that MYR and MEG combined with cisplatin is a potential clinical chemotherapeutic approach in human cervical cancer.
Keywords: Cervical cancer, combination therapy, apoptosis, myricetin, methyl eugenol, cell cycle
Introduction
Cervical cancer is a cancer arising from the cervix. It is due to the uncontrolled growth of cells that have the capability to attack or spread to other parts of the body. Initially there are typically no symptoms. Later symptoms may include: abnormal vaginal bleeding, pelvic pain or pain during sexual intercourse. Cervical cancer is the second most common malignancy in women with an estimated 493,000 new cases and 274,000 deaths in 2002 [1]. Although radiotherapy represents effective treatment modality, up to one third of patients will grow progressive or recurrent tumors, the pelvis being the most common site of failure [2-4]. The relapse rate of cervical cancer ranges between 11 and 22% in FIGO stages Ib-IIa and between 28 and 64% in FIGO stages IIb-IVa [5]. Human papillomavirus (HPV) infection seems to have a key role in the development of more than 90% of cases. However, most people who have had HPV infections do not develop cervical cancer. Other risk factors include: smoking, a weak immune system, birth control pills. [6-8]. Cervical cancer is of different types including squamous cell carcinomas which constitute about 90% of the cases followed by adenocarcinoma constituting 10% of the cases. Cervical cancer treatment consists of a combination of surgery, chemotherapy and radiotherapy. In the United States, there are around 68% of the five year survival rates and this rate greatly depends on how early the cancer is diagnosed. Larger early stage tumors may be treated with radiation therapy and cisplatin-based chemotherapy, hysterectomy (which then usually requires adjuvant radiation therapy), or cisplatin chemotherapy followed by hysterectomy [9-11]. Recently FDA has approved the use of a combination of two chemotherapy drugs namely hycamtin and cisplatin for women with late stage cervical cancer. However, this combination treatment is accompanied with serious side effects including neutropenia, anemia, and thrombocytopenia [12]. Keeping in view this fact, the objective of the current study was to identify some natural products from plant sources which can enhance the anticancer activity of cisplatin against cervical cancer (HeLa) cells. In this study we found that myricetin and methyl Eugenol enhanced the anticancer activity of HeLa cancer cells in vitro along with enhancing the induction of apoptosis, cell cycle arrest and mitochondrial membrane potential loss. To investigate the mechanism further by which the combination of cisplatin with myricetin and methyl Eugenol induces apoptosis, effect on Caspase-3 was studied which indicated that these combinations enhanced activation of caspase-3 remarkably.
Materials and methods
Cell culture and myricetin and methyl eugenol treatment
HeLa (cervical cancer cells) were procured from the Shanghai Institute of Cell Biology (Shanghai, China). MTT was purchased from Sigma Chemical Co., (St. Louis, MO, USA). The cells were cultured in Dulbecco’s modified Eagle’s media supplemented with 10% fetal bovine serum (Lonza Biologics, Singapore) and 100 U/mL penicillin and 100 μg/mL streptomycin (Vega Pharma Limited, Zhejiang, China). The cells were kept at 37°C in a humidified atmosphere containing 5% CO2. In cell proliferation experiments, HeLa cells were treated with either cisplatin or myricetin and methyl eugenol alone, or vehiclealone for 12, 24 and 48 h. For apoptosis assay, cells weretreated with cisplatin or myricetin and methyl eugenol either alone or combined, or vehicle alone for 48 h. Myricetin and methyl eugenol and cisplatin was purchased from Sigma Chemical Co. (St. Louis, MO, USA) and dissolved in DMSO (Sigma Chemical Co.) (Final concentration 0.2% in medium). Cytotoxicity Detection Kit (LDH) was purchased from Roche Chemical Co.
Cell proliferation assay
MTT assay (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) was used to measure the inhibition of cell proliferation. MTT was added in cells exposed to either cisplatin or myricetin and methyl eugenol or their combinations. Three hours later, the formazan precipitate was dissolved in 100 μL dimethyl sulfoxide, and then the absorbance was measured in an ELISA reader (Thermo Molecular Devices Co., Union City, USA) at 570 nm.
The cell viability ratio was calculated by the following formula:
Inhibitory ratio (%) = (OD control - OD treated)/OD control × 100%.
Cytotoxicity was expressed as the concentration of capillarisin inhibiting cell growth by 50% (IC50 value).
Lactate dehydrogenase (LDH) leakage assay for assessing cell cytotoxicity
Leakage of enzymes such as LDH into the culture medium is a well-known indicator of damage or injury to the cell membrane. Briefly, 1 × 105 cells/well of HeLa cells was transferred to 96-well plates. The plates were incubated overnight at 37°C to allow the cells to attach and proliferate. On the next day, 300 μl of fresh medium containing drug concentrations (myricetin, methyl eugenol and cisplatin or their combinations) were added to each well, and the plates were incubated at 37°C in 5% CO2. All drug concentrations were tested at least in triplicate wells and the assays were repeated independently three times. After 48 h, the plates were removed from the incubator and then 100 μl of medium from each well was carefully transferred to new plates. 100 μl of LDH substrate prepared according to the manufacturer’s direction (Cytotoxicity Detection Kit, Roche Chemical Co.) was added to each well. After 20 min shaking at room temperature, the enzymatic reaction was arrested by adding 50 μl of 1 M hydrochloric acid.
Lactate dehydrogenase activity was determined by change in absorbance at 490 nm. For the purpose of calculating percent cytotoxicity values, background LDH release from culture cells was considered as low control and triton-X 100 (0.01%) treated cells as high control.
Leakage (%) = [A490 (sample) - A490 (low control)/A490 (high control) - A490 (low control)] × 100%
Hoechst 33258 staining to detect apoptosis
HeLa cervical cancer cells were fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 20 min before staining with 5 μg/mL of Hoechst 33258 (Shanghai Hanhong Chemical Co., Ltd., China) at 37°C for 20 min. HeLa cells were treated with cisplatin (1 μM), myricetin (60 μM) and methyl eugenol (60 μM) or their combinations for 48 hours. Then, the cells were washed once with PBS, and then observed under a fluorescence microscope (Nikon). The condensed DNA of apoptotic cells was recognized by intense local staining in the nucleus, in contrast to diffused staining of DNA in normal cells. A minimum of 600 cells was counted, and each experiment was performed in triplicate.
AO-ETBR staining
After incubation of HeLa cells for 3 hours, a staining method using acridine orange (AO) and ethidium bromide (EB) (Sigma, St. Louis, MO, USA) was done. HeLa cells were treated with cisplatin (1 μM), myricetin (60 μM) and methyl eugenol (60 μM) or their combinations for 48 hours. Then, cells on coverslips were collected, washed with PBS twice, stained by AO/EB solution (30 μg/ml both), and then photographed using a fluorescence microscope (Nikon Tokyo Japan).
Cell cycle analysis by flow cytometry
Cervical cancer cells (HeLa) (1 × 105) in a 60 mm dish were subjected to cisplatin (1 μM), myricetin (60 μM) and methyl eugenol (60 μM) or their combinations for 48 hours. The cells were collected by trypsinization and washed twice with PBS (Sigma Chemical Co.). Cells were incubated in 50% ethanol at -20°C overnight and then treated with 40 μg/ml RNase A (Guangzhou Geneshun Biotech Ltd. China), then stained with 10 μg/ml of propidium iodide (PI) (Guangzhou Geneshun Biotech Ltd.China). Finally, the stained cells were analyzed by using FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Cell apoptosis and quantification of apoptosis assay
To verify the synergistic enhancing effect of MYR and MEG combinations, the induction of apoptosis caused by MYR + CP and MEG + CP HeLa cancer cells was carried out. Apoptosis detection was performed using the Annexin V-FITC and PI apoptosis kit (Bestbio, Shanghai, China). HeLa cells were plated at a density of 2 × 105 cells/well into 12-well plates and incubated overnight. Apoptosis was induced by treating cells with MYR, MEG, CP or combinations of MYR and MEG with CP solution (final concentrations of MYR, MEG and CP were 60, 60 and 1 μM, respectively). Cells grown in media containing an equivalent amount of DMSO without any drug were served as control. After 48 h of incubation, the cells with different treatments were harvested and the final samples were measured on a FACS Calibur flow cytometry (Becton Dickinson, San Jose, CA, USA).
Effect on mitochondrial membrane potential loss (ΛΨm)
Mitochondrial membrane potential (ΛΨm) in cervical cancer cells (HeLa) was measured by Rhodamine-123 dye (Zouping Mingxing Chemical Co., Ltd.). HeLa cells (5 × 106) were treated with cisplatin (1 μM), myricetin (60 μM) and methyl eugenol (60 μM) or their combinations for 48 hours and mitochondrial membrane potential was measured by flow-cytometry. Rhodamine-123 (20 mM) was added 2 h before the end of experiment. After it, the cells were washed with PBS and incubated with PI (10 μg/ml) for 30 min. Finally the cells were analyzed by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Caspase-3 activity analysis
HeLa cells were collected by trypsinization and lysed with lysis buffer (1% Triton X-100, 0.40 M sucrose, 10 mM EDTA, 5 mM Tris-HCl, pH 7.5, 4 mM DTT, 2 mM PMSF, 2 μg/ml aprotinin, 1 mg/ml leupeptin) (Guangzhou Geneshun Biotech Ltd. China). Thereafter, the lysates were transferred to wells in a 96-well flat-bottom plate. A peptide with the caspase-3 target motif DEVD bound to the chromophore p-nitroanilide (Santa Cruz, CA, USA) was added and incubated at 37°C for 1 h. The intensity of the developed color was read at 405 nm in a microplate reader (Thermo Molecular Devices Co., Union City, USA).
Statistical analyses
All statistical analyses were done using SPSS® software (version 19.0) and were conducted by one-way analysis of the variance (ANOVA) and Tukey test. Datawere expressed as the mean ± SEM and P values < 0.05 was considered significant.
Results
Effects of myricetin (MYR), methyl eugenol (MEG) and cisplatin (CP) combination on cell proliferation (MTT assay)
The results of the cell viability with different treatments (MYR, MEG, and CP or their combinations) are shown in Figures 1, 2 and 3. For making combinations of MYR and MEG with CP, 60 μM each of MYR and MEG were combined with 1 μM concentration of CP. Comparing with MYR, MEG or CP, their combinations (MYR + CP and MEG + CP) showed much lower cell viabilities at all given concentrations, indicating a strong potential for combination treatment. MEG + CP combination showed higher potent growth inhibition of HeLa cells as compared to MYR + CP combination.
Figure 1.
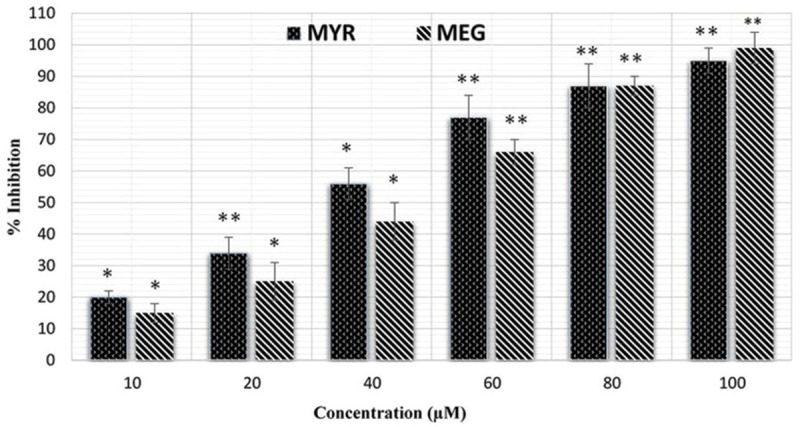
Growth inhibition of the cervical cancer cell line (HeLa) by the two natural products, myricetin (MYR) and methyleugenol (MEG). *P < 0.05 vs. control group. **P < 0.01 vs. control group.
Figure 2.
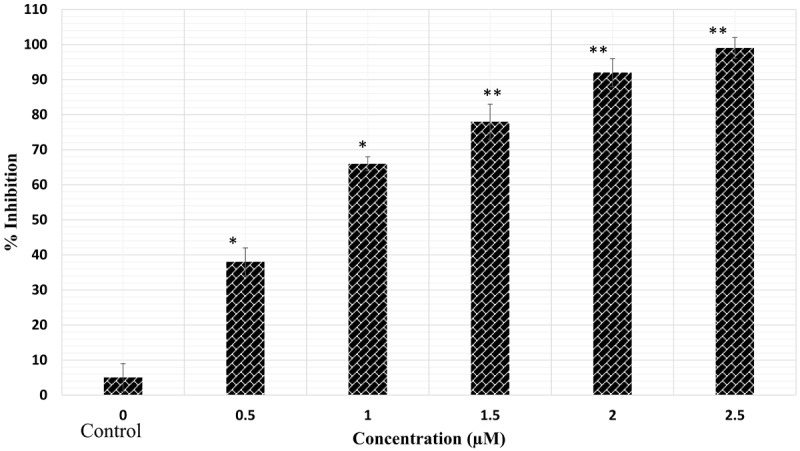
The anticancer effects of cisplatin (CP) on cervical cancer cells (HeLa). *P < 0.05 vs. control group. **P < 0.01 vs. control group.
Figure 3.
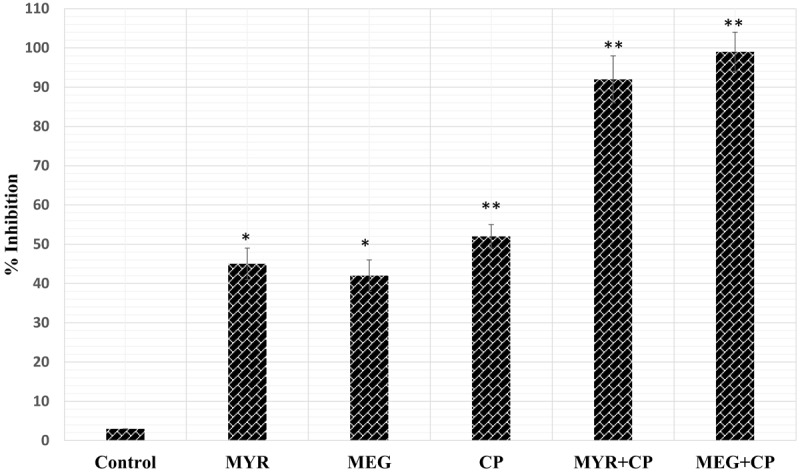
Combined effect of Myricetin (MYR 60 μM), methyl eugenol (MEG 60 μM) and cisplatin (CP 1 μM) on HeLa cell proliferation. *P < 0.05 vs. control group. **P < 0.01 vs. control group.
Evaluation of the cytotoxic effect of myricetin (MYR), methyl eugenol (MEG) and cisplatin (CP) combination by lactate dehydrogenase (LDH) leakage assay
The effects of MYR, MEG, CP and their combinations on lactate dehydrogenase release are shown in Figure 4. As compared to the control treatment, MYR, MEG or CP treatments induced a significant release of LDH from the HeLa cells. However, the effect of the combinations of MYR, MEG with CP was much more pronounced and induced significant cytotoxic effects against cervical cancer cells. Control wells were treated with equivalent amount of media alone. This assay along with MTT assay confirm that the combinations of MYR and MEG with CP produce effects which is much more potent as compared to the effects produced by these compounds alone.
Figure 4.
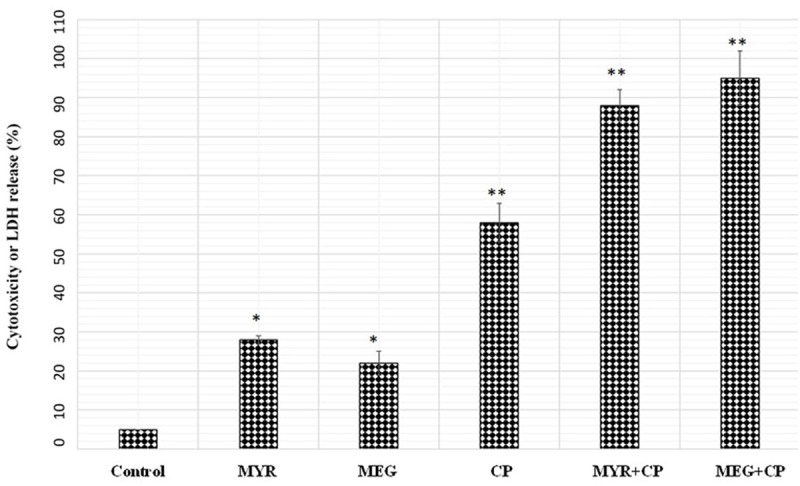
The Effects of myricetin, methyl eugenol and cisplatin alone or in Combination on Lactate Dehydrogenase (LDH) Leakage (% of total) in cervical cancer Cells (HeLa). Cells were treated with myricetin (MYR), methyl eugenol (MEG) and cisplatin (CP) alone or in combination for 48 h. Control wells were treated with equivalent amount of media alone. Combination treatment of myricetin and methyl eugenol with cisplatin significantly increased the LDH leakage compared with myricetin, methyl eugenol or cisplatin alone. The results showed the mean ± SEM from triplicated experiments. (*P < 0.05 vs. control; **P < 0.01 vs. control).
Hoechst 33258/AO-ETBR staining to detect apoptosis in HeLa cells
Apoptosis is a highly organized biochemical process to eradicate injured or abnormal cells in multicellular organisms. In order to establish whether cell death induced by MYR, MEG, CP or their combination is mediated through apoptosis, HeLacells were treated with myricetin (MYR, 60 μM), methyl eugenol (MEG, 60 μM) or their combinations (MYR + CP and MEG + CP) for 48 hours, and the characteristic morphological features of apoptosis were examined under an inverted light fluorescence microscope. Using Hoechst 33258 to stain the nucleus, it was observed (Figure 5) that as compared to control cells, MYR, MEG, CP and their combination treatment resulted in the appearance of cell shrinkage along with membraneblebbing which are characteristic features of cell apoptosis. As compared to the separate treatments, the combinations of MYR (60 μM) + CP (1 μM) and MEG (60 μM) + CP (1 μM) exhibited remarkable apoptotic features.
Figure 5.
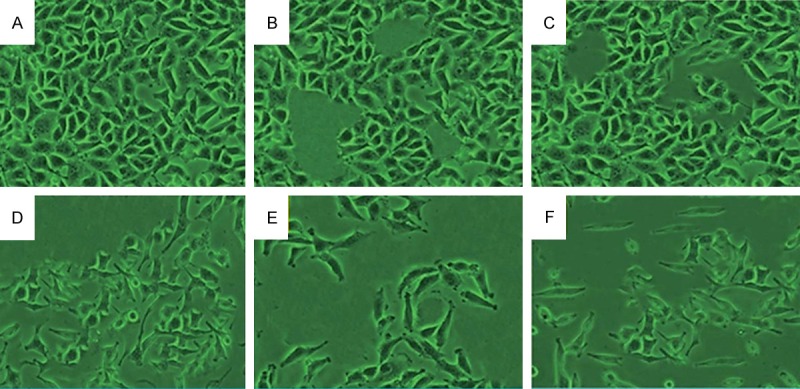
Cell apoptosis observed by Hoechst 33342 staining. HeLa cells were treated with (B) 60 μM myricetin (MYR), (C) 60 μM methyl eugenol (MEG), (D) 1 μM cisplatin (CP), (E) combination of MYR + CP and (F) combination of MEG + CP for 48 h. (A) Compared with the control group, treated cells exhibited chromatin condensation, nuclear fragmentation and apoptotic body formation.
Acridine orange and ethidium bromide (AO/ETBR) double staining of the HeLacells in order to observe cell apoptosis with the help of a fluorescence microscope after staining with a mixture of acridine orange and ethidium bromide, control cells (no drug treatment) (Figure 6) exhibited large green nuclei, which indicated the fact that the cell membranes were undamaged. However when treated with 60 μM each of MYR and MEG, the number of cells with large green nuclei reduced significantly (Figure 6). However, the effect of the combination of MYR (60 μM) and MEG (60 μM) with CP (1 μM) induced a significant reduction of cells with large green nuclei which points that the number of apoptotic cells grew significantly with combination treatment. Myricetin, methyl eugenol and cisplatin augment each other in inducing apoptosis in HeLa cancer cells as their combination is much more potent in inducing apoptosis as compared to the compounds alone.
Figure 6.
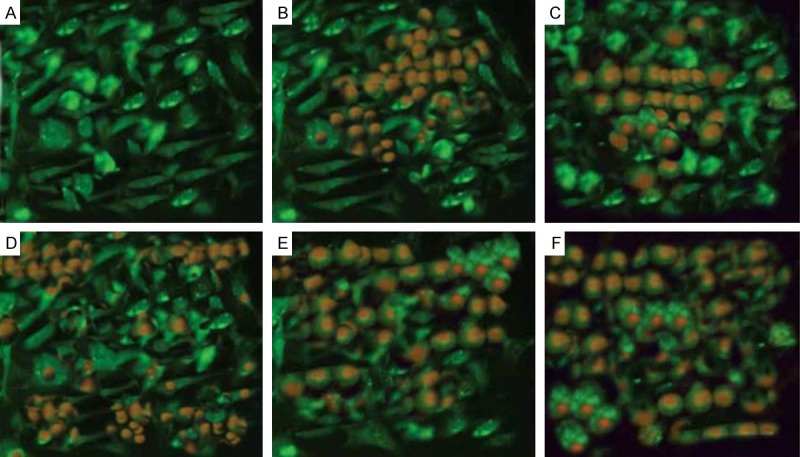
Cell apoptosis observed by AO-ETBR staining. HeLa cells were treated with (B) 60 μM myricetin (MYR), (C) 60 μM methyl eugenol (MEG), (D) 1 μM cisplatin (CP), (E) combination of MYR + CP and (F) combination of MEG + CP for 48 h. (A) Compared with the control group, treated cells exhibited chromatin condensation, nuclear fragmentation and apoptotic body formation.
Effect of MYR and MEG combinations with CP on cell cycle
In order to establish whether combination treatment of MYR, MEG with CP induces cell cycle disturbances in cervical cancer (HeLa) cells, flow cytometric analysis using propidium iodide (PI) as a staining agent was performed after MYR, MEG and their combination treatment with CP for 48 hours. The results revealed that the proportions of the treated cells at G0/G1 were increased in a dose-dependent manner (Figure 7). Control treated cells showed 4% cells in G0/G1 phase which got increased to 9% and 15% on treatment with MYR and MEG respectively. The increase in the G0/G1 cells was slight in case of MYR or MEG treatment alone. However, the combination treatment MYR (60 μM) + CP (1 μM) or MEG (60 μM) + CP (1 μM) produced a much more potent effect because the number of G0/G1 cells. MYR + CP and MEG + CP showed 45% and 49% of cells in the G0/G1 phase which was quite higher as compared to separate treatments by any of the compounds or the drug (cisplatin).
Figure 7.
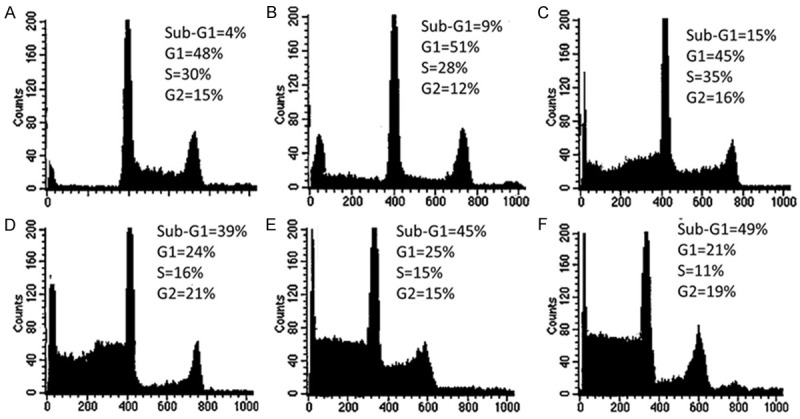
Effect of myricetin, methyl eugenol, cisplatin and their combinations on the cell cycle phase distribution in cervical (HeLa) cancer cells. A. Represents control cells. B-F. Represent effect of 60 μM myricetin (MYR), 60 μM methyl eugenol (MEG), 1 μM cisplatin (CP), combination of MYR + CP and combination of MEG + CP respectively. Compared to control cells and MYR and MEG treated cells, combination of MYR + CP and MEG + CP indicate a significant increase in the sub-G1 population of cells which is a hallmark of apoptosis and DNA damage caused by the combination treatment.
Effect of the MYR, MEG and their combination with CP on cell apoptosis
To verify the synergistic effects of MYR and MEG with CP on HeLa cells, the apoptotic effects of MYR + CP and MEG + CP were tested using Annexin V-FITC and PI apoptosis kit. Annexin V staining can detect phosphatidyl serine and as such can be used for its analysis. After cells are stained with annexin V in tandem with propidium iodide (PI), this reagent enters the cell only when the plasma cell membrane is deteriorated. The representative dot-plots illustrating apoptotic status are shown in Figure 8. The percentage of apoptotic cells (early apoptotic plus late apoptotic cells) treated with MYR (60 μM) + CP (1 μM) and MEG (60 μM) + CP (1 μM) combination solutions was 63.85% ± 4.12% and 85.47 ± 6.42% respectively, which was significantly higher in comparison with MYR (60 μM) (17.82% ± 2.16%, P < 0.01), MEG (60 μM) (48.93 ± 3.26%, P < 0.01) or CP (1 μM) (58.34% ± 5.32%, P < 0.01) treatment alone. These results indicated the potential synergistic enhancement of cancer therapy using MYR + CP and MEG + CP combinations.
Figure 8.
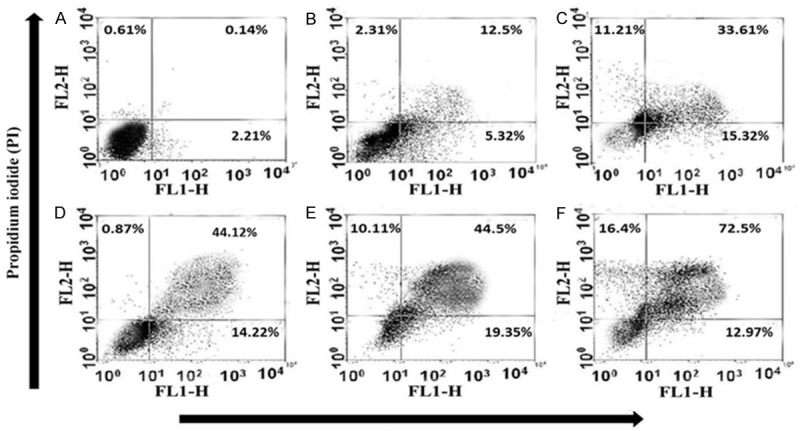
Induction of apoptosis caused by myricetin (MYR) (B), methyl eugenol (MEG) (C), cisplatin (CP) (D), and their combinations in cervical (HeLa) cancer cell lines. Cells were treated with either MYR or MEG (60 μM), CP (1 μM), or MYR + CP (60 μM MYR + 1 μM CP) (E) and MEG + CP (60 μM MEG + 1 μM CP) (F) for 48 h incubation, while 0.5% DMSO-PBS served as control (A). Data summary and analysis of the proportion of HeLa cells in different periods was according to the results of flow cytometric analysis.
Effect of the MYR and MEG combinations with CP on mitochondrial membrane potential loss (ΛΨm) in HeLa cervical cancer cells
The fluorescent dye, rhodamine-123 (Rh-123) is a specific probe for the detection of alterations in mitochondrial membrane potential in living cells. Our results revealed that the combination treatment of MYR and MEG with CP induced a significant reduction in the number of cells with intact membrane potential and increased the number of cells with low ΛΨm after 48 h. As can be seen from Figure 9, as compared to the control cells (no drug treatment), the MYR (60 μM), MEG (60 μM) and MYR (60 μM) + CP (1 μM) and MEG (60 μM) + CP (1 μM) treated cells indicated a much higher loss of mitochondrial membrane potential.
Figure 9.
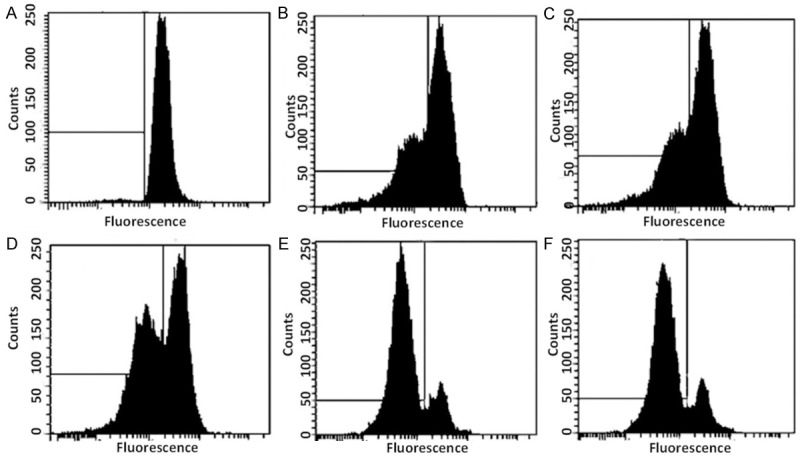
Effect of myricetin, methyl eugenol, cisplatin and their combinations on the mitochondrial membrane potential loss in cervical (HeLa) cancer cells. A. Represents control cells. B-F. Represent effect of 60 μM myricetin (MYR), 60 μM methyl eugenol (MEG), 1 μM cisplatin (CP), combination of MYR + CP and combination of MEG + CP respectively. Compared to control cells and MYR and MEG treated cells, combination of MYR + CP and MEG + CP indicate a significant loss of mitochondrial membrane potential.
Activation of Caspase-3 HeLa cells by MYR + CP and MEG + CP combination therapy
Caspases-3 is one of the vital mediators of apoptosis, being critical for certain processes associated with the dismantling of the cell and the formation of apoptotic bodies [13]. To confirm the probable pathways of synergistic apoptotic effect of MYR + CP and MEG + CP, Caspase-3 activities of cervical cancer cells were evaluated by a Caspase-3 Activity Assay Kit. As shown in Figure 10, significantly higher Caspase-3 activity of HeLa cells was observed in cells treated with MYR + CP (60 μM MYR + 1 μM CP) and MEG + CP (60 μM MEG + 1 μM CP) comparing with that of cells treated with MYR (60 μM), MEG (60 μM) or CP (1 μM), alone (P < 0.01). These results were in agreement with the study of apoptosis tests mentioned in other assays here. Taking these results together, it was obvious to believe that the synergistic enhancement of apoptosis caused by MYR + CP and MEG + CP was probably mediated by the significant activation of Caspase-3.
Figure 10.
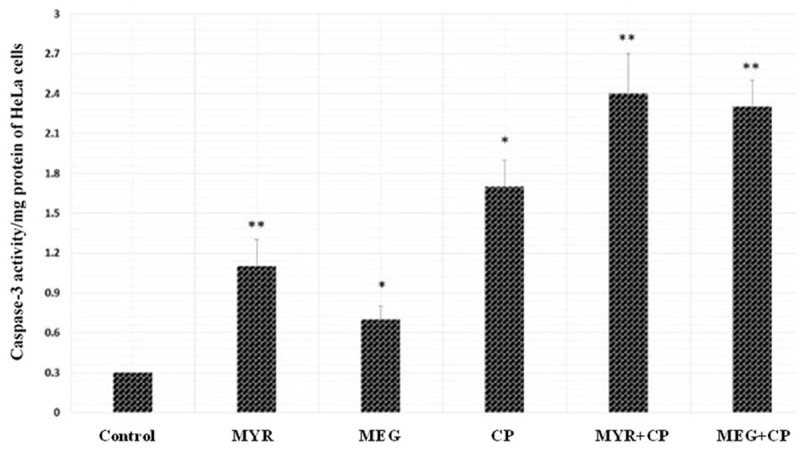
Significant enhancement of Caspase-3 activity caused by MYR + CP or MEG + CP combinations on HeLa cancer cells (n = 3). Cells were treated with either (myricetin) MYR (60 μM), (methyl eugenol) MEG (60 μM), (cisplatin) CP (1 μM ) or MYR + CP (60 μM MYR + 1 μM CP) and MEG + CP (60 μM MYR + 1 μM CP) for 48 h incubation, while 0.5% DMSO-PBS served as control. The Caspase-3 activity of cells with different treatments was evaluated by assessing the capacity to catalyze the cleavage of Caspase-3 substrate (Ac-DEVD-pNA) and release the pNA fluorochrome. *P < 0.05, **P < 0.01, statistically significant difference between MYR and MYR + CP and MEG and MEG + CP.
Discussion
Combination therapy plays a more and more important role in the field of anti-cancer therapy. At present, cisplatin is the most active chemotherapeutic agent for the treatment of different cancers and is usually combined with other agents such as docetaxel, gemcitabine and paclitaxel [14]. However, its use is limited due to severe side effects such as anemia, neurotoxicity, nephrotoxicity and the attainment of drug resistance [15]. To address these problems, attention has been focused on identifying novel agents that can be combined with cisplatin to increase the therapeutic efficacy and decrease side effects. The toxic side effects of currently used anticancer drugs can be reduced by using non-toxic potentiating agent or synergistic compound in combination with anticancer drugs which may considerably potentiate their effectiveness while minimizing their toxicity by reducing the dose. Such non-toxic potentiating agents which can possibly solve many problems of the clinical cancer treatment include low molecular weight terpenoids present in essential oils [16,17]. Myricetin and methyl eugenol are also found naturally in many plants and essential oils. Myricetin is a naturally occurring flavonol found in many grapes, berries, fruits, vegetables, herbs etc. Walnuts are a rich dietary source. Trace amounts can be found as glycosides. It is one of the phenolic compounds present in red wine [18,19]. Myricetin has been reported to possess antioxidant properties. A study correlated high myricetin consumption with lowered rates of prostate cancer [20]. Another clinical study revealed that intake of three flavonoids (kaempferol, quercetin, and myricetin) were connected with a reduced occurrence of pancreatic cancer in people [21]. Methyleugenol (is a phenylpropene, a type of phenylpropanoid compound,) is a natural constituent of a large number of essential oils of plant origin including plants like in Artemisia dracunculus French type (tarragon), Syzygium aromaticum (clove), Daucus carota (carrot), Myrstica fragrans (nutmeg) and Rosmarinus officinalis (rosemary) [22,23].
In the present study, we evaluated these two naturally occurring compounds for their anticancer activity and their effect in enhancing the anticancer activity of cisplatin against HeLa cervical cancer cells. The results indicated that these compounds individually exhibit cancer growth inhibitory activity and when combined with cisplatin in specific dose form produce synergistic enhancement of the anticancer effect of cisplatin against these cells. Their combination with cisplatin was found always to be more active as compared to separate treatment. We further evaluated the effect of the combination of MYR and MEG with CP on apoptosis, cell cycle phase distribution, mitochondrial membrane potential as well as Caspase-3 activity. All these experiments indicated that the combination of MYR and MEG with CP was more effective as compared to the compound or drug alone.
The mechanism of action of cisplatin includes the inhibition of cell proliferation and induction of cell apoptosis [24]. To determine whether MYR and MEG enhance the anticancer effect of cisplatin, HeLa cells were treated with MYR or MEG alone or in combination with cisplatin and cell growth and apoptosis were evaluated using MTT assay, flow cytometry assay and fluorescence microscopy. We found that in comparison with single agent treatment, the combination of MYR or MEG with cisplatin produced greater effectiveness in growth inhibition and apoptosis induction, suggesting that MYR and MEG play a combination role in cisplatin-induced apoptosis and growth inhibition in cervical cancer cells.
In conclusion, this study made a reasonably comprehensive investigation on the potential application of MYR, MEG in combination with anti-cancer drugs like cisplatin (CP), making up for the deficiency of the previous research in the field of combination therapy. Our results indicated that MYR and MEG synergistic combinations with cisplatin are more active than separate treatment and could be developed further for effective cancer treatments against cervical cancers. Further in vivo studies will establish whether these combinations hold true for animal studies as well. The severe side effects associated with cisplatin therapy could be reduced by such combinations because it leads to dose-reduction which has a direct relation with side effects.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bellone S, Pecorelli S, Cannon MJ, Santin AD. Advances in dendritic-cell-based therapeutic vaccines for cervical cancer. Expert Rev Anticancer Ther. 2007;7:1473–1486. doi: 10.1586/14737140.7.10.1473. [DOI] [PubMed] [Google Scholar]
- 3.Leitao MM Jr, Chi DS. Recurrent cervical cancer. Curr Treat Options Oncol. 2002;3:105–111. doi: 10.1007/s11864-002-0056-6. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander M. Guidelines for the treatment of recurrent and metastatic cervical cancer. Oncologist. 2002;7:342–347. [PubMed] [Google Scholar]
- 5.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. Int J Gynecol Obstet. 2006;95:43–103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Abbas AK, Fausto N, Mitchell RN. Robbins Basic Pathology. 8th edition. Saunders Elsevier; 2007. [Google Scholar]
- 7.Holland-Frei cancer medicine. 8th edition. New York: McGraw-Hill Medical; 2009. [Google Scholar]
- 8.Dunne EF, Park IU. HPV and HPV-associated diseases. Infect Dis Clin North Am. 2013;27:765–78. doi: 10.1016/j.idc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Dornhöfer N, Höckel M. New developments in the surgical therapy of cervical carcinoma. Ann NY Acad Sci. 2008;1138:233–252. doi: 10.1196/annals.1414.029. [DOI] [PubMed] [Google Scholar]
- 10.Chao A, Lin CT, Lai CH. Updates in systemic treatment for metastatic cervical cancer. Curr Treat Options Oncol. 2014;15:1–13. doi: 10.1007/s11864-013-0273-1. [DOI] [PubMed] [Google Scholar]
- 11.Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26:314–21. doi: 10.1097/GCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 12.FDA Approves First Drug Treatment for Late-Stage Cervical Cancer. U. S. Food and Drug Administration. 2006-06-15. Retrieved 2007-12-02.
- 13.Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 15.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Legault J, Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha- humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59:1643–7. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Park EJ, Lee CR, Chun JN, Cho NH, Kim IG, Lee S, Kim TW, Park HH, So I, Jeon JH. Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int J Oncol. 2012;40:1683–90. doi: 10.3892/ijo.2011.1318. [DOI] [PubMed] [Google Scholar]
- 18.Miean KH, Mohamed S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J Agric Food Chem. 2001;49:3106–12. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 19.Maggiolini M, Recchia AG, Bonofiglio D, Catalano S, Vivacqua A, Carpino A, Rago V, Rossi R, Andò S. The red wine phenolicspiceatannol and myricetin act as agonists for estrogen receptor in human breast cancer cells. J Mol Endocrinol. 2005;35:269–81. doi: 10.1677/jme.1.01783. [DOI] [PubMed] [Google Scholar]
- 20.Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 21.Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924–31. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 22.Tan KH, Nishida R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior andpollination. J Insect Sci. 2011;12:56. doi: 10.1673/031.012.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koezuka Y, Honda G, Tabata M. Genetic control of phenylpropanoids in Perillafrutescens. Phytochemistry. 1986;25:2085–2087. [Google Scholar]
- 24.Yoshizumi N, Fujiwara J, Yoshizaki A, Sato M, Sakai R, Nishiya I. Cytokinetic effects of carboplatin and cisplatin on a human ovarian cancer cell line. Hum Cell. 1988;1:301–307. [PubMed] [Google Scholar]


