Abstract
Primary angiosarcoma of the pleura is an extremely rare malignancy. Herein, we report the case of an elderly Chinese patient with primary left pleural epithelioid angiosarcoma. The 76-year-old man presented with a 4-month history of a cough with sputum expectoration and weight loss of 4 kg within one month. A chest scan showed a massive oval-shaped mass in the left pleural cavity. We then performed a left thoracotomy for tumor resection and surgical exploration. Histological examination of the resected specimen showed few viable tumor cells with significant atypia; tumor cells had large nuclei and prominent nucleoli and were arranged in a crack-like, sheeted pattern. Moreover, there was a significant amount of fibrinous exudates, hemorrhage, degeneration, and necrosis. With immunohistochemical analysis, tumor cells had strong expression of CD31, CD34, FLI-1, vimentin. Morphological and immunohistochemical findings supported the diagnosis of epithelioid angiosarcoma.
Keywords: Primary angiosarcoma, pleura, immunohistochemistry
Introduction
Angiosarcoma is an uncommon malignant tumor, accounting for only 1-2% of all soft tissue sarcomas. It arises from the endothelial cells of small blood vessels and may affect any organ, though it most commonly occurs in the skin, soft tissue, liver, spleen, heart, and breast [1]. Primary angiosarcoma of the pleura is an extremely rare malignancy; in addition to reviewing the relevant literature, herein we report the case of an elderly Chinese man diagnosed with primary epithelioid angiosarcoma of the pleura.
Case report
A 76-year old man presented with a 4-month history of a cough with sputum expectoration without evident etiologies and weight loss of 4 kg within one month. The patient did not display any fever, night sweats, chest pain, or wheezing. These symptoms worsened one week before admission. The patient had a 40-year history of smoking, though he had quit smoking 10 years previously.
A chest computed tomography scan revealed a large, oval-shaped mass (maximum size 12 × 17 cm on transverse section) in the left pleural cavity (Figure 1). The mass had an uneven density, with calcification located primarily on the periphery. Extensive thickening and calcification of the left pleura were also observed, accompanied by mediastinal shifting toward the right. Hybrid positron emission tomography only showed a large lesion occupying the left pleural cavity, with high 18F-fluorodeoxyglucose (FDG) uptake in the peripheral area and no 18F-FDG uptake in the central area. No abnormalities were observed during bronchoscopic examination. Fine-needle aspiration biopsy (FNAB) of the mass in the left pleural cavity was performed under B-ultrasound guidance, and subsequent microscopic examination demonstrated the presence of fibrinous exudates and the absence of tumor cells.
Figure 1.
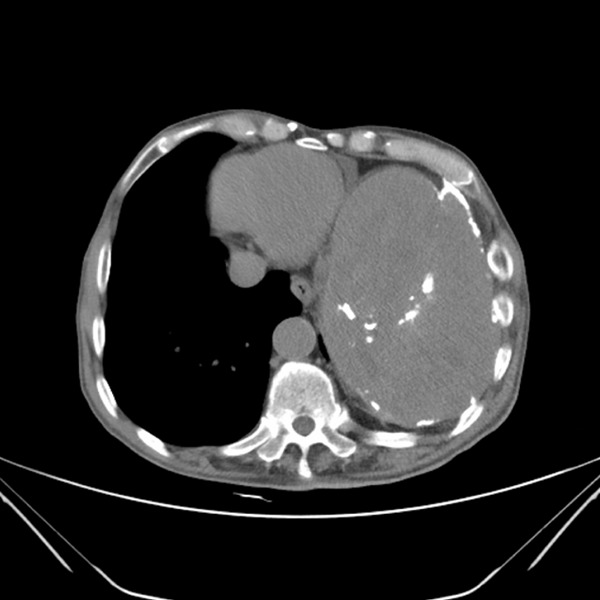
A chest computed tomography scan showed a massive oval mass in the left pleural cavity.
For the purposes of both diagnosis and treatment, a left thoracotomy under general anesthesia was performed for both surgical exploration and tumor resection in the left pleural cavity. By surgical exploration, a large mass sized 17 × 12 × 12 cm was observed in the left pleural cavity. The mass adhered to the inferior lobe of the left lung, which had developed atelectasis due to external compression. The surface of the mass exhibited calcification, and the tissue appeared hard and thickened. The mass was extensively adhered to the parietal pleura with obscure boundaries, and it was inseparable from the surrounding tissue. Hence, following maximal dissection of the tumor walls and the involved parietal pleura, the tumor was cut open and its contents were removed to the greatest extent possible. The adhesion at the inferior lobe of the left lung was freed to facilitate expansion of the lung. We then confirmed that the dorsal segment of the inferior lobe of the left lung was able to expand without restrictions.
Gross observation of the resected specimen demonstrated an irregular-shaped, gray-yellow to gray-red, multiple bits and pieces tissue mass with partly calcification and a diameter of 22 cm. Microscopic examination revealed that it mainly consisted of fibrinous exudates and hemorrhagic and degenerated necrotic tissue, in addition to a small amount of hyperplastic and degenerated fibrous connective tissue. Samples (n = 50) were collected from the dissected mass for further examination. A small proportion of the samples (12/50) in the periphery of the tissue block contained very scanty viable tumor cells with significant atypia (Figure 2A), arranged in a crack-like, sheeted pattern (Figure 2B, 2C) and accompanied by calcifications. Tumor cells had large nuclei with prominent nucleoli (Figure 2D), and mitotic figures were visible.
Figure 2.
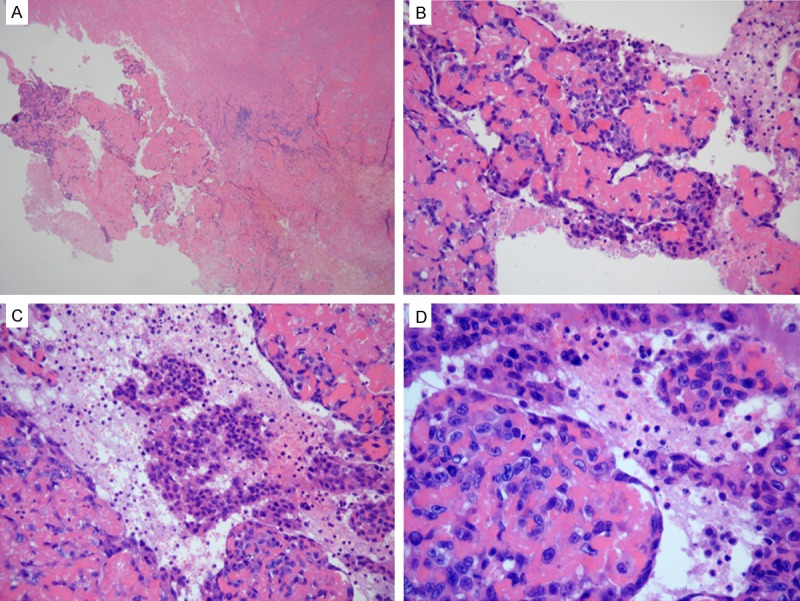
The angiosarcoma tissues were stained with hematoxylin and eosin. A: There were scanty viable tumor cells in the periphery of the tissue block (hematoxylin and eosin [H&E] stain, ×40 magnification). B, C: Tumor cells were arranged in a crack-like and sheeted pattern (H&E stain, ×200). D: Tumor cells had large nuclei with prominent nucleoli (H&E stain, ×400).
By immunohistochemistry, the tumor cells were strongly positive for CD31 (Figure 3A), CD34 (Figure 3B), FLI-1 (Figure 3C), and vimentin (Figure 3D). Tumor cells were also focally positive for Ki-67 (90% index; Figure 3E), focally weakly positive for cytokeratin (CK) (Figure 3F), and negative for D2-40, Glut-1, calretinin, CK5/6, Syn, and WT1.
Figure 3.
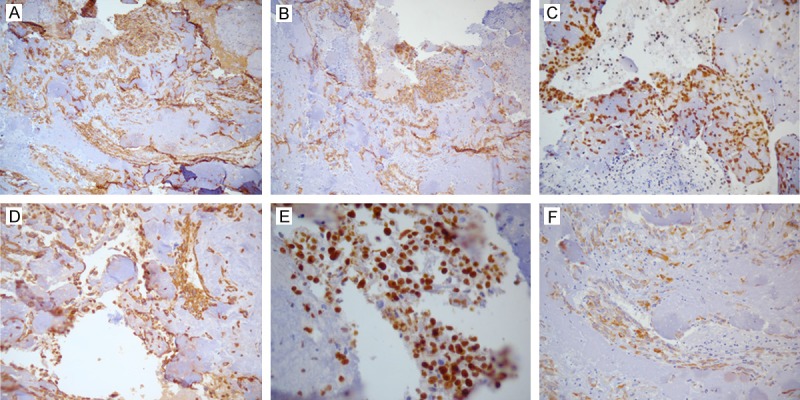
The angiosarcoma tissues were analyzed by immunohistochemistry. The tumor cells were strongly positive for CD31 (A), CD34 (B), FLI (C), vimentin (D ×200), and Ki-67 (F), and were weakly focally positive for cytokeratin (G).
Therefore, the diagnosis of primary epithelioid angiosarcoma was confirmed. Neither postoperative radiotherapy nor chemotherapy was performed in our patient due to his old age. Currently, seven months following surgery, the patient is alive and exhibits no evidence of disease recurrence.
Discussion
Primary angiosarcoma of the pleura is a very rare malignancy; we searched the English literature and found 18 previously reported cases of primary angiosarcoma of the pleura (excluding cases occurring in the pleuropulmonary and chest wall) [1-13]. Including our case, The 19 cases of primary pleural angiosarcoma are summarized in Table 1. The mean age of patients was 55 years (range, 34-77 years), and the male to female ratio was 15:4.
Table 1.
Reported cases of primary pleural angiosarcoma
| Case | Author | Age (year) | Sex | Clinical presentation | Past history | Metastasis at diagnosis | Treatment | Postoperative survival (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Alexious [1] | 57 | F | Massive recurrent hemothorax | None | No | S, RT | 10 |
| 2 | Zhang [2] | 53 | M | Pleural effusion | None | - | - | 6 |
| 3 | Zhang [2] | 62 | F | Pleural effusion, ascites | RT for ovarian cancer | - | S | D (shortly after surgery) |
| 4 | Zhang [2] | 66 | M | Pleural effusion | None | - | - | 6 |
| 5 | Zhang [2] | 45 | M | Recurrent pleural effusion | Hypertension | - | - | 6 |
| 6 | Zhang [2] | 60 | M | Bloody pleural effusion | None | - | - | 2 |
| 7 | Roh [3] | 34 | F | Dyspnea, chest tightness and pain | None | No | S, C | A (5 months after surgery) |
| 8 | Kimura [4] | 57 | M | Intermittent episodes of memory loss, headache | Tuberculous pyothorax | Brain | No | 2 |
| 9 | Pramesh [5] | 55 | M | Chest pain, cough, hemoptysis | None | - | S | - |
| 10 | Chen [6] | 39 | M | Movement-dependent, right-sided chest pain | None | No | S, C | 8 |
| 11 | Kurtz [7] | 61 | M | Recurrent massive bilateral hemothoraxes | None | Skin, oral mucosa | - | 2 |
| 12 | Dainese [8] | 62 | M | Progessive dyspnea, bilateral massive hemothoraxes | None | No | No | D (3 days after diagnosis) |
| 14 | Miyazaki [9] | 68 | M | Hoarseness | Left pneumonectomy for pulmonary squamous cell carcinoma | Right lung | - | 10 |
| 15 | Baisi [10] | 75 | M | Left-sided chest pain | - | - | S, C | 10 |
| 16 | Kao [11] | 49 | M | Intermittent right-sided chest pain | Asthma | - | S, R, C | A (9 months after surgery) |
| 17 | Lorentziadis [12] | 77 | M | Overweight | None | - | S | D (7 days after surgery) |
| 18 | Quesada [13] | 58 | F | Refractory dyspnea, fever | - | - | C | 4 |
| 19 | Present | 76 | M | Cough | None | No | S | Alive 7 months after the surgery |
F, female; M, male, S, surgery, RT, radiotherapy; CT, chemotherapy; D, dead; A, alive.
The clinical presentation of this malignancy is usually nonspecific, though the most frequent symptoms are pleural effusion and recurrent hemothorax. Distinct mass formation, which was observed in our case, is very unusual. Generally, radiological findings are also nonspecific and do not allow for differentiation between pleural angiosarcoma and other primary or metastatic pleural tumors. A positron emission tomography scan can only be used to determine the extent of the disease [14].
FNAB is usually unhelpful in the diagnosis of this disease [7,9,10,12]. As shown in this case, only fibrinous exudates were observed by FNAB. Definitive diagnosis of pleural angiosarcoma typically requires histological and immunohistochemical examination of sufficient samples collected during surgery. The common cellular morphology of angiosarcoma ranges from spindle-shaped to epithelioid. Epithelioid angiosarcoma is a subtype of angiosarcoma and is primarily comprised of large and round “epithelioid” endothelial cells with abundant cytoplasm and large vacuolated nuclei. In this case, the tumor displayed characteristics of epithelioid angiosarcoma. We also observed high 18F-FDG uptake in the peripheral area of the mass, which corresponded to an area of few viable tumor cells with a unique morphology. A possible mechanism for this phenomenon may be that the large size of the tumor resulted in ischemia at its center. Our experience with this case suggests that comprehensive and sufficient sample collection and meticulous histological examination are required for accurate diagnosis. Immunohistochemical analysis plays an important role in the diagnosis and differential diagnosis of angiosarcoma. Positive expression of at least one endothelial cell marker (including CD31, CD34, and factor VIII) is required to confirm the diagnosis of angiosarcoma, though CD31 is considered the most specific and most sensitive marker. Recently, Folpe et al. [15] reported that FLI-1 was expressed by 50 of 53 vascular tumors (94%), including 20 of 22 angiosarcoma cases. These results suggest a role for FLI-1 as a novel nuclear marker of vascular tumors. Angiosarcomas may also express epithelial markers, such as CK, especially epithelioid variants.
Most pleural angiosarcomas are epithelioid [2] and may be easily misdiagnosed as malignant mesothelioma or poorly differentiated carcinoma. However, mesothelioma tumor cells are characterized by less varied morphology and less significant atypia. Positive expression of immunohistochemical markers (CK5/6, calretinin, and D2-40) provides evidence for the diagnosis of malignant mesothelioma. However, expression of epithelial markers can also be present in poorly differentiated carcinoma and may thus lead to misdiagnosis. Therefore, for differential diagnosis, it is important to consider CK expression, which is either strongly or weakly focally expressed in epithelioid vascular tumors but more strongly diffusely positive in carcinomas and mesotheliomas [11]. In this case, CK was locally and weakly expressed in tumor cells.
Epithelioid hemangioendothelioma (EHE) is a low- to medium-grade malignant tumor. Compared with angiosarcoma, EHE tumor cells are characterized by bland nuclei with limited polymorphism, necrosis, and scant mitoses [6]. However, pleural EHE has been reported to have the same aggressive behavior as pleural angiosarcoma, suggesting that pleural EHE might only represent a morphological subtype of angiosarcoma [8]. Zhang et al. [2] reported that if a diagnosis of pleural EHE is ever made based on classic histological features, a clear statement of the potential highly malignant behavior should be communicated to the clinician.
The pathogenesis and etiology of primary pleural angiosarcoma are still unclear. Some Japanese researchers reported that this disease is associated with a history of chronic tuberculous pyothorax [4,16]. However, Hattori [17] suggested that angiosarcoma associated with pyothorax is not similar to primary pleural angiosarcoma and shows the rather ordinary feature of the tumor arising in the soft tissue. In addition, other western researchers have reported that pleural angiosarcoma is associated with a history of radiation and asbestos exposure [2]. Moreover, one study reported that pleural angiosarcoma arose from chronically expanding hematoma after pneumonectomy [9]. Our patient did not have any history of tuberculosis, asbestos exposure, or radiotherapy. When unrelated to these factors, this case may be described as a de novo tumor [7].
Treatment options for pleural angiosarcoma include surgery, chemotherapy, and radiation. Surgery is the best treatment for patients with localized lesions. Vascular embolization can decrease the size of the tumor and control pleural bleeding, and it may be performed before surgery [7,12]. While the effects of chemotherapy are limited, radiation therapy may be used in the adjuvant setting for angiosarcoma. In conclusion, primary pleural angiosarcoma is a highly malignant neoplasm. Despite the available therapeutic modalities, the prognosis of patients is usually poor, and most patients die of the disease within months. Our experience with this case suggests that comprehensive and sufficient sample collection and meticulous histological examination are required for accurate diagnosis of this malignancy.
Disclosure of conflict of interest
None.
References
- 1.Alexiou C, Clelland CA, Robinson D, Morgan WE. Primary angiosarcomas of the chest wall and pleura. Eur J Cardiothorac Surg. 1998;14:523–526. doi: 10.1016/s1010-7940(98)00211-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang PJ, Livolsi VA, Brooks JJ. Malignant epithelioid vascular tumors of the pleura: report of a series and literature review. Hum Pathol. 2000;31:29–34. doi: 10.1016/s0046-8177(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 3.Roh MS, Seo JY, Hong SH. Epithelioid angiosarcoma of the pleura: a case report. J Korean Med Sci. 2001;16:792–795. doi: 10.3346/jkms.2001.16.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura M, Ito H, Furuta T, Tsumoto T, Hayashi S. Pyothorax-associated angiosarcoma of the pleura with metastasis to the brain. Pathol Int. 2003;53:547–551. doi: 10.1046/j.1440-1827.2003.01510.x. [DOI] [PubMed] [Google Scholar]
- 5.Pramesh CS, Madur BP, Raina S, Desai SB, Mistry RC. Angiosarcoma of the pleura. Ann Thorac Cardiovasc Surg. 2004;10:187–190. [PubMed] [Google Scholar]
- 6.Chen L, Shih HJ, Seguerra E Jr, Lin JH. Pathologic quiz case: a 39-year-old man with diffuse pleural thickening and massive hemothorax. Epithelioid angiosarcoma of pleura. Arch Pathol Lab Med. 2004;128:1299–1300. doi: 10.5858/2004-128-1299-PQCAYM. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz JE, Serra S, Duclos B, Brolly F, Dufour P, Bergerat JP. Diffuse primary angiosarcoma of the pleura: a case report and review of the literature. Sarcoma. 2004;8:103–106. doi: 10.1155/2004/794907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dainese E, Pozzi B, Milani M, Rossi G, Pezzotta MG, Vertemati G, Tricomi P, Sessa F. Primary pleural epithelioid angiosarcoma. a case report and review of the literature. Pathol Res Pract. 2010;206:415–419. doi: 10.1016/j.prp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki H, Goto A, Hino R, Ota S, Okudaira R, Murakawa T, Nakajima J, Fukayama M. Pleural cavity angiosarcoma arising in chronic expanding hematoma after pneumonectomy. Hum Pathol. 2011;42:1576–1569. doi: 10.1016/j.humpath.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Baisi A, Raveglia F, De Simone M, Cioffi U. Primary multifocal angiosarcoma of the pleura. Interact Cardiovasc Thorac Surg. 2011;12:1069–1070. doi: 10.1510/icvts.2011.267708. [DOI] [PubMed] [Google Scholar]
- 11.Kao YC, Chow JM, Wang KM, Fang CL, Chu JS, Chen CL. Primary pleural angiosarcoma as a mimicker of mesothelioma: a case report **VS**. Diagn Pathol. 2011;6:130. doi: 10.1186/1746-1596-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorentziadis M, Sourlas A. Primary de novo angiosarcoma of the pleura. Ann Thorac Surg. 2012;93:996–998. doi: 10.1016/j.athoracsur.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Quesada A, Quesada J, Khalil K, Ferguson EC, Brown RE. Morphoproteomic study of primary pleural angiosarcoma of lymphangioendothelial lineage: a case report. Ann Clin Lab Sci. 2013;43:317–322. [PubMed] [Google Scholar]
- 14.Chen CY, Wu YC, Chou TY, Yang KY. Pleural angiosarcoma mimicking pleural haematoma. Interact Cardiovasc Thorac Surg. 2013;17:886–888. doi: 10.1093/icvts/ivt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25:1061–1066. doi: 10.1097/00000478-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Aozasa K, Naka N, Tomita Y, Ohsawa M, Kanno H, Uchida A, Ono K. Angiosarcoma developing from chronic pyothorax. Mod Pathol. 1994;7:906–911. [PubMed] [Google Scholar]
- 17.Hattori H. Epithelioid angiosarcoma arising in the tuberculous pyothorax: report of an autopsy case. Arch Pathol Lab Med. 2001;125:1477–1479. doi: 10.5858/2001-125-1477-EAAITT. [DOI] [PubMed] [Google Scholar]


