Abstract
Objective: To explore the expression of A-kinase anchor protein 95 (AKAP95), Cyclin D1, Cyclin E1, and Connexin43 (Cx43) in rectal cancer tissues and assess the associations between each of the proteins and pathological parameters, as well as their inter-relationships. Methods: AKAP95, Cyclin D1, Cyclin E1, and Cx43 protein expression rates were evaluated by immunohistochemistry in 50 rectal cancer specimens and 16 pericarcinoma tissues. Results: The positive rates of AKAP95, Cyclin E1, and Cyclin D1 proteins were 54.00 vs. 18.75%, 62.00 vs. 6.25%, and 72.00 vs. 31.25% in rectal cancer specimens and pericarcinoma tissues, respectively, representing statistically significant differences (P < 0.05). The positive rate of Cx43 protein expression in rectal cancer tissues was 44.00% and 62.50% in pericarcinoma tissues, and the difference between them was not significant (P > 0.05). No significant associations were found between protein expression of AKAP95, Cyclin E1, Cyclin D1, and Cx43, and the degree of differentiation, histological type, and lymph node metastasis of rectal cancer (P > 0.05). However, significant correlations were obtained between the expression rates of AKAP95 and Cyclin E1, Cyclin E1 and Cyclin D1, Cyclin E1 and Cx43 protein, and Cyclin D1 and Cx43, respectively (P < 0.05). Conclusion: AKAP95, Cyclin E1, and Cyclin D1 protein expression rates were significantly higher in rectal cancer tissues compared with pericarcinoma samples, suggesting an association between these proteins and the development and progression of rectal cancer. In addition, the significant correlations between the proteins (AKAP95 and Cyclin E1, Cyclin E1 and Cyclin D1, Cyclin E1 and Cx43 protein, and Cyclin D1 and Cx43) indicate the possible synergistic effects of these factors in the development and progression of rectal cancer.
Keywords: Rectal cancer, AKAP95, Cyclin E1, Cyclin D1, Cx43
Introduction
Cell cycle signaling pathway assessment has provided new insights for understanding the mechanisms involved in the development and progression of rectal cancer, a common digestive tract malignancy. AKAP95 was shown to anchor to the RII subunit of PKA; the anchored PKA catalyzes the phosphorylation of target proteins, thus ensuring signal transduction in the cAMP pathway [1,2]. Cyclin D and Cyclin E are known to promote mitosis of mammalian cells in the G1 phase. Previous studies have demonstrated that the Cyclin D1 protein is over-expressed in tissues from rectal and esophageal cancers [3,4]; meanwhile, Cyclin E1 is also over-expressed in liver cancer and serous cystadenocarcinoma of the ovary [5,6]. The Cx43 protein was shown to affect cell growth in the absence of gap junction [7]; in addition, Cx43 protein levels decreased in nasopharynx, gastric, and colorectal cancer cells [8-10]. AKAP95 has been shown to mediate the reaction of Cyclin D/E and the PKA’s RII subunit to form Cyclin D/E-AKAP95- PKA complex [11]; in turn, the PKA protein is able to phosphorylate Cx43 [12]. In a study performed by Yun et al., the cAMP/PKA pathway was found to regulate the gap junctional intracellular communication (GJIC) in mouse embryonic stem cells [13]; Cx43 was also shown to participate in cell cycle regulation [14,15]. We previously demonstrated that AKAP95 is associated with the expression of Cyclin E1, Cyclin D1, and Cx43 proteins in lung cancer tissues. In the present study, we assessed the expression of these 4 proteins in rectal cancer tissues and their associations with pathological parameters, as well as their inter-relationships.
Materials and methods
Tissue collection
Fifty rectal cancer tissues were obtained from rectal cancer patients that received surgical resection in the First Affiliated Hospital of Liaoning Medical University. In all patients, diagnosis was confirmed by pathological examination. The patients were 64±10 (ranging from 39 to 83) years old, including 32 males and 18 females. Forty-four patients had tubular or papillary adenocarcinoma, while 4 presented with mucinous adenocarcinoma and 2 had signet-ring cell carcinoma. Cancer cells were highly differentiated in 4 patients, moderately differentiated in 42 individuals, and poorly differentiated in 4 subjects. Twenty-six patients had lymph node metastasis, 18 were free from lymph node metastasis, and lymph node metastasis status was unclear for the remaining 6 individuals. In addition, pericarcinoma tissues were obtained in normal rectal tissues at least 3 cm away from cancerous tissues in 16 of the 50 patients. Pathological examination was also performed with pericarcinoma tissues to confirm the absence of cancer cells.
Reagents and methods
All specimens were fixed with 10% neutral formalin, embedded with paraffin, and sectioned at 4 μm. The S-P immunohistochemical method was performed to evaluate the expression of various proteins. In brief, high pressure antigen retrieval was carried out with citrate buffer (pH6.0) for 1.5 min, followed by DAB staining; cell nuclei were stained with hematoxylin. The SP kit and DAB substrate were purchased from Maixin biotechnology co., LTD (Fuzhou, Fujian, China); mouse anti-human AKAP95, Cyclin D1, and Cx43 monoclonal antibodies were purchased from Santa Cruz (Dallas, Texas, USA); mouse anti-human Cyclin E1 antibody was purchased from Epitomics (Burlingame, California, USA). PBS was used instead of primary antibody in negative controls.
Data assessment
Brown-yellow staining was considered as positive protein expression. For each slice, 10 different visual fields were randomly chosen under microscope (BA310 Digital, Motic China Group Co., Ltd), and 200 rectal cancer cells were counted in each visual field. The percentage of positive cells in the field was used for result determination. “-” indicated no brown-yellow staining to <10%; “+-” indicated ≥10% but <25%; “+” indicated ≥25% but <50%; “++” indicated ≥50% but <75%; and “+++” indicated ≥75%. “-” and “+-” were considered as negative protein expression, while “+”, “++”, and “+++” were considered to be positive protein expression [17].
Statistical analysis
The SPSS13.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Chi-square test was used for ratio comparisons. Spearman rank correlation was used to assess the associations among the proteins expression. P < 0.05 was considered statistically significant.
Results
AKAP95, Cyclin E1, Cyclin D1, and Cx43 protein expression in rectal cancer and pericarcinoma tissues
The positive rates of AKAP95 expression in rectal cancer tissues and pericarcinoma specimens were 54.00 and 18.75%, respectively (Table 1). The AKAP95 protein in pericarcinoma and rectal cancer tissues was mainly located in the cell nucleus, while a marginal portion was located in the cytoplasm (Figure 1). The positive rate of Cyclin E1 expression was 62.00% in rectal cancer tissues (31/50), but only 6.25% in pericarcinoma specimens (1/16). The Cyclin E1 protein in rectal cancer tissues was mainly in the cytoplasm and less represented in the cell nucleus (Figure 2). The positive rates of Cyclin D1 expression were 72.00% (36/50) and 31.25% (5/16) in rectal cancer tissues and pericarcinoma tissues, respectively. The Cyclin D1 protein in rectal cancer and pericarcinoma tissues was mainly confined to the cytoplasm (Figure 3). The positive rates of AKAP95, Cyclin E1, and Cyclin D1 protein expression were all significantly higher in cancer tissues compared with pericarcinoma specimens (P < 0.01 or 0.05); in contrast, Cx43 protein expression was lower in cancer tissues compared with pericarcinoma samples, although the difference was not statistically significant (P > 0.05); the Cx43 protein was also mainly located in the cytoplasm of rectal cancer cells (Figure 4).
Table 1.
AKAP95, Cyclin E1, Cyclin D1, and Cx43 protein expression in rectal cancer tissues
| Protein | Result | Rectal cancer tissues | Pericarcinoma tissues | χ2 | P |
|---|---|---|---|---|---|
| AKAP95 | Positive | 27 | 3 | 6.075 | 0.014 |
| Negative | 23 | 13 | |||
| Cyclin E1 | Positive | 31 | 1 | 15.083 | 0.0001 |
| Negative | 19 | 15 | |||
| Cyclin D1 | Positive | 36 | 5 | 8.554 | 0.003 |
| Negative | 14 | 11 | |||
| Cx43 | Positive | 22 | 10 | 1.661 | 0.197 |
| Negative | 28 | 6 |
The positive rates for AKAP95, Cyclin E1, and Cyclin D1 protein expression were significantly higher in rectal cancer tissues compared with pericarcinoma specimens; the positive rate of Cx43 protein expression was not significantly different between rectal cancer and pericarcinoma tissues.
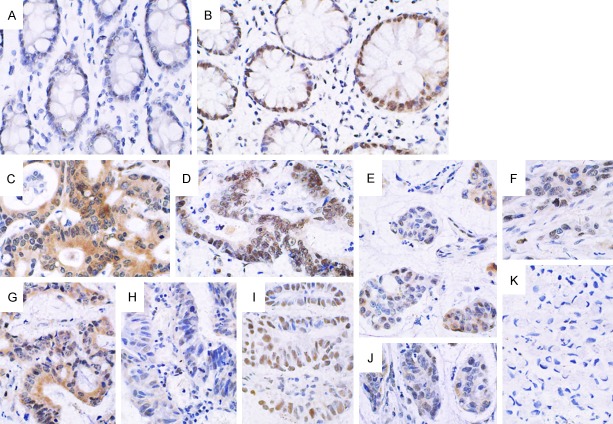
Figure 1.
Expression of the AKAP95 protein in pericarcinoma specimens and rectal cancer tissues (×400). A. No expression of AKAP95 in pericarcinoma rectal tissues; B. Positive expression of AKAP95 in rectal cancer tissues. The protein was mainly expressed in the nucleus, and a marginal proportion was found in the cytoplasm; C. Positive expression in cytoplasm and cell nuclei in poorly differentiated rectal adenocarcinoma; D. High expression in cell nuclei of moderately differentiated rectal adenocarcinoma tissues; E and J. Low expression in cell nuclei of poorly differentiated rectal mucinous adenocarcinoma tissues; F. Low expression in the cell nuclei in poorly differentiated rectum adenocarcinoma tissues; G. Low expression in the cell nuclei and cytoplasm in moderately differentiated rectal mucinous adenocarcinoma tissue; H. Negative expression in highly differentiated rectal adenocarcinoma tissues; I. High expression in cell nuclei in highly differentiated rectal adenocarcinoma tissues; K. Negative expression in rectal signet-ring cell carcinoma tissues.
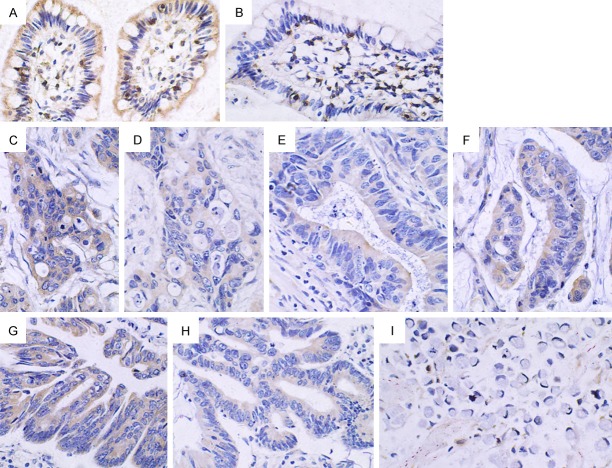
Figure 2.
Expression of Cyclin E1 in pericarcinoma and rectal cancer tissues (× 400). A. Low expression of Cyclin E1 in the cytoplasm in pericarcinoma rectal tissues; B. Negative expression in pericarcinoma rectal tissues; C and D. Positive expression in the cytoplasm in poorly and moderately differentiated rectal adenocarcinoma tissues, respectively; E. Positive expression in highly differentiated rectal adenocarcinoma tissue; the protein was mainly expressed in the cytoplasm; a marginal proportion was found in the cell nucleus; F. Low expression the cytoplasm in highly differentiated rectal adenocarcinoma tissues; G. Positive expression in the cytoplasm in poorly differentiated rectal mucinous adenocarcinoma tissues; H. Negative expression in rectal signet-ring cell carcinoma tissues.
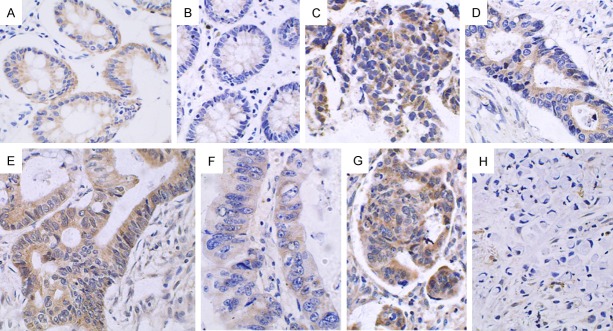
Figure 3.
Expression of the Cyclin D1 protein in pericarcinoma and rectal cancer tissues (× 400). A and B. Positive and low cytoplasmic expression of Cyclin D1 in pericarcinoma rectal tissues; C. Negative expression in pericarcinoma rectal tissues; D. High expression in the cytoplasm in poorly differentiated rectal adenocarcinoma tissues; E. Positive expression in the cytoplasm in poorly differentiated rectal mucinous adenocarcinoma tissues; F. Positive expression in the cytoplasm in moderately differentiated rectal adenocarcinoma tissues; G and H. Negative expression in moderately differentiated rectal adenocarcinoma and signet-ring cell carcinoma tissues.
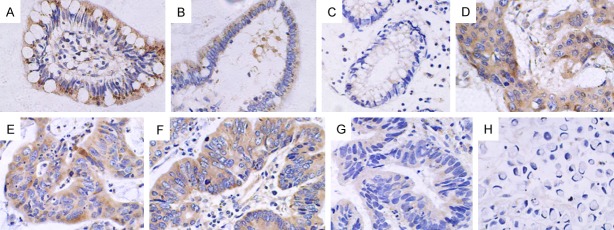
Figure 4.
Expression of the Cx43 protein in pericarcinoma and rectal cancer tissues (× 400). A. Positive cytoplasmic expression of Cx43 in pericarcinoma rectal tissues; B. Negative expression in pericarcinoma rectal tissues; C. Low expression in the cytoplasm in poorly differentiated rectal adenocarcinoma tissues; D and E. Negative expression in poorly and moderately differentiated rectal adenocarcinoma tissues, respectively; F. Low expression in the cytoplasm in poorly differentiated rectal mucinous adenocarcinoma tissues; G. Positive expression in the cytoplasm in moderately differentiated rectal adenocarcinoma tissues; H and I. Negative expression in moderately differentiated rectal adenocarcinoma and signet-ring cell carcinoma tissues.
Correlations among the expression of the AKAP95, Cyclin E1, Cyclin D1, and Cx43 proteins in rectal cancer tissues
As shown in Tables 2, 3, 4, 5, 6 and 7, significant correlations were obtained between the protein expression rates of AKAP95 and Cyclin E1 (Table 2), Cyclin E1 and Cyclin D1 (Table 3), Cyclin E1 and Cx43 (Table 4), and Cyclin D1 and Cx43 (Table 5) (P < 0.05) in rectal cancer specimens; no significant correlation was found between the expression rates of AKAP95 and Cyclin D1 (Table 6) as well as AKAP95 and Cx43 (Table 7) (P > 0.05).
Table 2.
Correlation between the protein expression rates of AKAP95 and Cyclin E1 in rectal cancer tissues
| AKAP95 | Cyclin E1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 7 | 2 | 2 | 2 | 1 | 0.353 | 0.012 |
| +- | 0 | 2 | 4 | 3 | 0 | ||
| + | 2 | 3 | 4 | 4 | 4 | ||
| ++ | 1 | 1 | 1 | 2 | 3 | ||
| +++ | 0 | 1 | 0 | 1 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 3.
Correlation between the protein expression rates of Cyclin E1 and Cyclin D1 in rectal cancer tissues
| Cyclin E1 | Cyclin D1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 3 | 2 | 2 | 3 | 0 | 0.421 | 0.002 |
| +- | 0 | 3 | 4 | 2 | 0 | ||
| + | 1 | 1 | 3 | 4 | 2 | ||
| ++ | 1 | 3 | 2 | 4 | 2 | ||
| +++ | 0 | 0 | 1 | 4 | 3 | ||
rs: Spearman’s rank correlation coefficient.
Table 4.
Correlation between the protein expression rates of Cyclin E1 and Cx43 in rectal cancer tissues
| Cyclin E1 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 8 | 0 | 1 | 1 | 0 | 0.288 | 0.043 |
| +- | 3 | 1 | 5 | 0 | 0 | ||
| + | 2 | 2 | 5 | 2 | 0 | ||
| ++ | 4 | 4 | 4 | 0 | 0 | ||
| +++ | 0 | 4 | 2 | 1 | 1 | ||
rs: Spearman’s rank correlation coefficient.
Table 5.
Correlation between the protein expression rates of Cyclin D1 and Cx43 in rectal cancer tissues
| Cyclin D1 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 4 | 0 | 1 | 0 | 0 | 0.481 | 0.0004 |
| +- | 7 | 2 | 0 | 0 | 0 | ||
| + | 3 | 2 | 7 | 0 | 0 | ||
| ++ | 2 | 4 | 7 | 3 | 1 | ||
| +++ | 1 | 3 | 2 | 1 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 6.
Correlation between the protein expression rates of AKAP95 and Cyclin D1 in rectal cancer tissues
| AKAP95 | Cyclin D1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 3 | 2 | 6 | 2 | 1 | 0.211 | 0.141 |
| +- | 1 | 2 | 1 | 3 | 2 | ||
| + | 1 | 2 | 4 | 7 | 3 | ||
| ++ | 0 | 1 | 1 | 5 | 1 | ||
| +++ | 0 | 2 | 0 | 0 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 7.
Correlation between the protein expression rates of AKAP95 and Cx43 in rectal cancer tissues
| AKAP95 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 7 | 2 | 5 | 0 | 0 | 0.071 | 0.623 |
| +- | 1 | 3 | 4 | 1 | 0 | ||
| + | 4 | 5 | 6 | 2 | 0 | ||
| ++ | 4 | 0 | 2 | 1 | 1 | ||
| +++ | 1 | 1 | 0 | 0 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Correlations between pathological parameters and protein expression rates of AKAP95, Cyclin E1, Cyclin D1, and Cx43 in rectal cancer tissues
No significant association was found between the protein expression rates of AKAP95, Cyclin E1, Cyclin D1, and Cx43 and the degree of differentiation, histological type, and lymph node metastasis in rectal cancer tissues (P > 0.05).
Discussion
The AKAP95 protein is mainly expressed in the cell nucleus; it participates in signal transduction, specifically in the cAMP pathway by anchoring the RII subunit of PKA, which phosphorylates target proteins [1]; in addition, AKAP59 also plays a role in chromosome condensation and DNA replication during mitosis, and cell apoptosis [16-18]. Cyclin D1 and Cyclin E1 are two proteins that regulate the G1 to S phase progression in mitotic cells; these two proteins are mainly over-expressed in cancer tissues [4-6]; in addition, over-expression of Cyclin D1 [19] and Cyclin E1 [6] was shown to predict poor prognosis. In a study performed by Arsenijevic [11], the investigators found that the AKAP95 protein could combine to Cyclin D and Cyclin E to form a complex in CHO cells; in addition, AKAP95 competitively combined with Cyclin D3 instead of CKD4, or with Cyclin E1 instead of CKD2, suggesting that the AKAP95 protein could participate in cell cycle regulation by affecting Cyclin D and Cyclin E. In the present study, we found a significant correlation between the expression of AKAP95 and Cyclin E1 but not between AKAP95 and Cyclin D1 expression rates in rectal cancer tissues, which was inconsistent with our previous findings in lung cancer tissues (in which AKAP95 and Cyclin D1 expression rates were significantly correlated, as well as those of AKAP95 and Cyclin E1 [20]), suggesting that the cell cycle regulatory role of AKAP95 could differ with tissue type. Lodén M et al. [21] found that in estrogen receptor (ER) positive breast cancer tissues, over-expressed Cyclin D1 increased the phosphorylation of the pRB protein, thus elevating cell proliferation; while in ER negative breast cancer tissues, over-expression of Cyclin E was accompanied with a down-regulation of the Cyclin D1 protein, which also increased cell proliferation in the absence of regulatory effects of pRB [21]. These findings suggested that Cyclin D1 and Cyclin E promote cell proliferation via different mechanisms or through different pathways, which could vary in different tissues. Harbour et al. [22] found that pRB phosphorylation by Cyclin D is the precondition for the transcription factor E2F to activate Cyclin E transcription. In the present study, the association between the expression rates of Cyclin D1 and Cyclin E1 suggested that these two proteins play synergistic roles in promoting tumor development, providing histological evidence to support Harbour’s findings.
Cx43 is known as a tumor suppressor; however, Cx43 protein expression is decreased in many tumors [8-10]. In our previous work, we found that AKAP95 and Cx43 protein expression rates were significantly associated in lung tissues [23]; cytological experiments in our laboratory also showed the dynamic combination of AKAP95 and Cx43 in the whole cell cycle (data not shown); however, no significant association between AKAP95 and Cx43 protein expression rates was found in rectal cancer tissues, suggesting that the mechanisms underlying the interactions between AKAP95 and Cx43 that regulate the cell cycle could be different in various tissues.
The PTEN protein is known to reduce Akt activity [24], thus regulating the phosphorylation of the Cx43 protein [25]; in addition, PTEN promotes p27kip1 to combine with CyclinE-CDK2 and form a complex [26]. MAPKs (p38, p42/44, and JNK) were shown to activate Cyclin D1 via transcription factors, including NF-κB and AP-1 [27]; meanwhile, phosphorylated MAPKs (p38, p42/44, and JNK) increased AP-1 and CREB levels, thus enhancing Cx43 protein synthesis [28]. Therefore, we speculated that Cx43 could regulate the cell cycle through the complex formed by AKAP95 and Cyclin D/E-CDKs; this was supported by the present findings that Cx43 expression is significantly associated with that of Cyclin D1 and Cyclin E1.
Of note, no significant correlations were obtained between the protein expression of AKAP95, Cyclin E1, Cyclin D1, and Cx43, and the degree of differentiation, histological type, and lymph node metastasis in rectal cancer tissues.
Acknowledgements
This work is supported by National Natural Science Foundation (No.81071927), Fujian Creative Project (No.2012-CXB-25), Graduate Creative Project (No.ZX11A1) and Xiamen University Creative Grant (No.CXB2013024).
Disclosure of conflict of interest
None.
References
- 1.Eide T, Coghlan V, Orstavik S, Holsve C, Solberg R, Skâlhegg BS, Lamb NJ, Langeberg L, Fernandez A, Scott JD, Jahnsen T, Taskén K. Molecular cloning, chromosomal localization, and cell cycle-dependent sub cellular distribution of the A-kinase anchoring protein, AKAP95. Exp Cell Res. 1998;238:305–316. doi: 10.1006/excr.1997.3855. [DOI] [PubMed] [Google Scholar]
- 2.Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwandner O, Bruch HP, Broll R. P21, p27, cyclin D1, and p53 in rectal cancer: immunohistology with prognostic significance. Int J Colorectal Dis. 2002;17:11–19. doi: 10.1007/s003840100333. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa S, Onda M, Sasajima K, Makino H, Yamashita K, Takubo K, Miyashita M. Cyclin D1 overexpression as a prognostic factor in patients with esophageal carcinoma. J Surg Oncol. 2001;78:208–214. doi: 10.1002/jso.1152. [DOI] [PubMed] [Google Scholar]
- 5.Farra R, Dapas B, Pozzato G, Scaggiante B, Agostini F, Zennaro C, Grassi M, Rosso N, Giansante C, Fiotti N, Grassi G. Effects of E2F1-cyclin E1-E2 circuit down regulation in hepatocellular carcinoma cells. Dig Liver Dis. 2011;43:1006–1014. doi: 10.1016/j.dld.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, Lu Y, Wang J, Liu X, Keller ET, Liu Q, Zhou Q, Zhang J. Targeting the Notch signaling pathway in cancer therapeutics. Thoracic Cancer. 2014;5:473–486. doi: 10.1111/1759-7714.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- 8.Yi ZC, Wang H, Zhang GY, Xia B. Downregulation of connexin 43 in nasopharyngeal carcinoma cells is related to promoter methylation. Oral Oncol. 2007;43:898–904. doi: 10.1016/j.oraloncology.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Tang B, Peng ZH, Yu PW, Yu G, Qian F. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med Oncol. 2011;28:502–508. doi: 10.1007/s12032-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 10.Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131:570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 11.Arsenijevic T, Degraef C, Dumont JE, Roger PP, Pirson I. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. doi: 10.4161/cc.5.11.2802. [DOI] [PubMed] [Google Scholar]
- 12.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun SP, Ryu JM, Park JH, Kim MO, Lee JH, Han HJ. Prostaglandin E2 maintains mouse ESC undifferentiated state through regulation of connexin31, connexin43 and connexin45 expression: Involvement of glycogen synthase kinase 3β/β-catenin. Biol Cell. 2012;104:378–396. doi: 10.1111/boc.201100032. [DOI] [PubMed] [Google Scholar]
- 14.Solan JL, Lampe PD. Connexin phosphorylation as a regulatoy event link to gap junction chanel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D’Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cellmodel. Cell Death Differ. 2009;16:151–163. doi: 10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 16.Eide T, Carlson C, Taskén KA, Hirano T, Taskén K, Collas P. Distinct but overlapping domains of AKAP95 are implicated in chromosome condensation and condensin targeting. EMBO Rep. 2002;3:426–432. doi: 10.1093/embo-reports/kvf089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eide T, Taskén KA, Carlson C, Williams G, Jahnsen T, Taskén K, Collas P. Protein kinase A-anchoring protein AKAP95 interacts with MCM2, a regulator of DNA replication. J Biol Chem. 2003;278:26750–26756. doi: 10.1074/jbc.M300765200. [DOI] [PubMed] [Google Scholar]
- 18.Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. A-kinase-anchoring protein 95 functions as a potential carrier for the nuclear translocation of active caspase 3 through an enzyme-substrate- like association. Mol Cell Biol. 2005;25:9469–9477. doi: 10.1128/MCB.25.21.9469-9477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 20.Hu SX, Kong XY, Yuan YY, Teng BG, Zhi XH, Zhuang WX, Yu XY, Liu WZ, Zhang YX. Relationship between AKAP95, cyclin E1, cyclin D1, and clinicopathological parameters in lung cancer tissue. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013;31:890–894. [PubMed] [Google Scholar]
- 21.Lodén M, Stighall M, Nielsen NH, Roos G, Emdin SO, Ostlund H, Landberg G. The cyclin D1 high and cyclinE high subgroups of breast cancer: separate pathways in tumorogenesis based on pattern of genetic aberrations and inactivation of the pRb node. Oncogene. 2002;21:4680–4690. doi: 10.1038/sj.onc.1205578. [DOI] [PubMed] [Google Scholar]
- 22.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen YD, Chen XX, Shen LN, Liang FC, Ding Y, Yu XY, Xue MQ, Zhang YX. Expression of A-kinase anchor protein 95, cyclinE2, and connexin43 in lung cancer tissue, clinical significance of their expression, and their expression correlation. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30:725–729. [PubMed] [Google Scholar]
- 24.Kenneth MY, Masaru A. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375–2382. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- 25.Dunn CA, Su V, Lau AF, Lampe PD. Activation of Akt, not connexin43 protein ubiquitination, regulates gap junction stability. J Biol Chem. 2012;287:2600–2607. doi: 10.1074/jbc.M111.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheney IW, Neuteboom ST, Vaillancourt MT, Ramachandra M, Bookstein R. Adenovirus-mediated gene transfer of MMAC1/PTEN to glioblastoma cells Inhibits S phase entry by the recruitment of p27Kip1 into Cyclin E/CDK2 complexes. Cancer Res. 1999;59:2318–2323. [PubMed] [Google Scholar]
- 27.Mishra S, Tripathi A, Chaudhari BP, Dwivedi PD, Pandey HP, Das M. Deoxynivalenol induced mouse skin cell proliferation and inflammation via MAPK pathway. Toxicol Appl Pharmacol. 2014;279:186–197. doi: 10.1016/j.taap.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Salameh A, Krautblatter S, Karl S, Blanke K, Gomez DR, Dhein S, Pfeiffer D, Janousek J. The signal transduction cascade regulating the expression of the gap junction protein connexin43 by β-adrenoceptors. Br J Pharmacol. 2009;158:198–208. doi: 10.1111/j.1476-5381.2009.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]