Abstract
This study aimed to evaluate the receptor activator of nuclear factor-κB (RANK) expression statuses of esophageal squamous cell carcinoma (ESCC) patients, high-grade intraepithelial neoplasia (HGIN), low-grade intraepithelial neoplasia (LGIN), and normal esophageal tissues (NETs) in Chinese Han and Kazakh ethnic, as well as the correlation of RANK expression with clinicopathological characteristics. RANK immunohistochemical analysis was conducted to investigate the expression of RANK in 113 ESCC, 36 HGIN, 63 LGIN, and 98 NETs from Han ethnic patients and in 196 ESCC and 76 NETs from Kazakh ethnic patients. The associations of RANK expression with ethnic and clinicopathological characteristics were examined using χ2-test. Upregulated RANK expression was detected in both Han and Kazakh ethnic ESCC tissues, compared with NETs (P = 1.11×10-5, 0.001, respectively). RANK expression was significantly increased during malignant transformation from normal epithelium into LGIN (P = 2.84×10-7) and HGIN (P = 7.83×10-6) tissues in Han ethnic patients. The increased expression of RANK also correlated with lymph node metastasis in Kazakh ethnic ESCC patients (P = 0.019). By contrast, no significant correlation existed between RANK expression and clinicopathological characteristics of Han ethnic ESCC patients. Furthermore, Kaplan-Meier survival analysis showed that ESCC patients with higher expression of RANK protein had significantly worse prognosis than ESCC patients with low or no expression (P = 0.001). In conclusion, this study is the first to identify RANK overexpression as a novel esophageal cancer marker in both Kazakh and Han ethnic ESCC patients. The results support the association of RANK with ESCC across ethnicities. In summary, RANK could be a therapeutic target in ESCC patients.
Keywords: RANK, esophageal squamous cell carcinoma, metastasis, Han and Kazakh ethnic
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors and the sixth leading cause of cancer-related deaths [1]. On histopathological classification, ESCC is the predominant histological subtype of esophageal cancer in East Asian countries [2]. Despite significant advances achieved in various therapeutic strategies for ESCC, including surgery, chemotherapy, radiotherapy, and combination therapy, ESCC prognosis remains unfavorable. The overall five-year survival rate of ESCC patients is < 30% depending on clinical stage at the time of diagnosis [3]. Moreover, almost all patients with advanced ESCC eventually develop primary tumor metastases to lymph nodes, which subsequently metastasize to secondary organs, ultimately leading to death. However, mechanisms by which ESCC cells metastasize are unclear.
Normal esophageal squamous epithelia undergo both genetic and histological changes during ESCC evolution, which involves a multistage process from noninvasive precursor lesions. These lesions initially contain low-grade intraepithelial neoplasia (LGIN), then high-grade intraepithelial neoplasia (HGIN), and finally invasive carcinoma [4]. Although some studies have reported the occurrence of this process [5-8], mechanisms regulating the malignancy and progression of ESCC remain under investigation [9]. Thus, the underlying molecular mechanisms of ESCC need further understanding, and the abilities of ESCC cells to invade and metastasize as well as to eradicate or control recurrent and disseminated malignancies should be determined.
Recent studies have introduced a new receptor-ligand system belonging to the tumor necrosis factor super family, which involves the receptor activator of nuclear factor-κB (RANK), RANK ligand (RANKL), and the protein osteoprotegerin (OPG) [10,11]. The RANK/RANKL/OPG signaling pathway is discovered as a major regulatory system for osteoclast formation and action [12,13]. RANKL is a membrane-bound protein primarily expressed on the surface of osteoblasts and bone marrow stromal cells. RANKL binds to RANK, which is present on the surface of osteoclast precursors, to induce osteoclastogenesis and activate mature osteoclasts in the presence of M-CSF [14,15]. OPG, a decoy receptor of RANKL, is also produced by osteoblast/stromal cells. OPG can prevent bone destruction by blocking the binding between RANK and RANKL, thereby inhibiting osteoclast differentiation and activation [16]. Although the RANK/RANKL/OPG system is the key regulator of bone metabolism, some reports have detected dysregulation of this system in various solid tumors. Sandra Casimiro [17] inadvertently expressed by breast and prostate cancer cells and that the activation of RANK/RANKL pathway correlates with an increased invasive phenotype. Similarly, differential expression of RANK, RANKL, and OPG was associated with the metastatic potential of cells in prostate cancer [18], non-small-cell lung cancer [16], oral squamous-cell carcinoma [19], giant-cell tumor of bone [20], and chondroblastoma [21]. However, characterizations of these proteins in ESCC patients remain unclear.
Accordingly, we hypothesize that RANK expression may correlate with ESCC progression. In our study, immunohistochemical analysis was conducted to assess the expression of RANK in primary ESCC tissues from 113 Han and 196 Kazakh ethnic patients, as well as in precursor lesions, including 36 HGIN and 63 LGIN tissues. We also analyzed the correlation between RANK expression and clinicopathological parameters of ESCC, including age, gender, differentiation, invasion depth, lymph node metastasis, tumor-node-metastasis (TNM) stage, and prognostic outcomes. We further analyzed the difference of RANK expression between Han and Kazakh ethnic ESCC tissues.
Materials and methods
Patients and tumor samples
We investigated surgical biopsy samples of 309 ESCC patients who underwent radical resection at the Cancer Hospital Cancer Hospital, including 113 cases of Han, 36 HGIN, 63 LGIN, and 196 cases of Kazakh. A total of 309 ESCC tissue samples and adjacent normal tissues excised were selected from the First University Hospital, Shihezi University School of Medicine, Xinjiang Yili Prefecture Friendship Hospital, and the People’s Hospital of Xinjiang Uyghur Autonomous Region from 1984 to 2013. All the patients did not receive chemotherapy, radiotherapy, or immunotherapyprior to surgery. We also obtained detailed clinical data of all the cases, including age, gender, differentiation, invasion depth, lymph node metastasis, and UICC stage (TNM stage) (Table 1). The ESCC patients were staged according to the Cancer Staging Manual of the American Joint Committee on Cancer. Written informed consent under the approval of the ethics committees was obtained from each study participant. All procedures were performed in accordance with the hospital’s ethical guidelines. Follow-ups were conducted on 98 Han patients, with a follow-up deadline of 10 July 2014.
Table 1.
Clinicopathological demographics for the 113 patients of Han ethnic and 196 patients of Kazakh ethnic with ESCC
| Characteristic | Kazakh ethnic | Han ethnic | P value |
|---|---|---|---|
|
| |||
| N (%) | N (%) | ||
| Age | |||
| < 65 | 135 (68.9) | 58 (51.3) | 0.002 |
| > 65 | 61 (31.1) | 55 (48.7) | |
| Gender | |||
| Male | 130 (66.3) | 82 (72.6) | 0.255 |
| Female | 66 (33.7) | 31 (27.4) | |
| Differentiation | |||
| Well-Moderate | 173 (88.3) | 100 (88.5) | 0.952 |
| Poor | 23 (11.7) | 13 (11.5) | |
| Invation depth | |||
| T1-T2 | 154 (78.6) | 58 (51.3) | 6.69×10-7 |
| T3-T4 | 42 (21.4) | 55 (48.7) | |
| Lymphatic invasion | |||
| N0 | 90 (45.9) | 75 (66.4) | 0.001 |
| N1-N3 | 106 (54.1) | 38 (33.6) | |
| UICC staging (TNM) | |||
| I/II | 126 (64.3) | 80 (70.8) | 0.242 |
| III/IV | 70 (35.7) | 33 (29.2) | |
P < 0.05 indicates a significant association among the variables.
Tissue microarray and immunohistochemical assay
Tissue microarrays containing ESCC tumor and adjacent normal mucosa were constructed. Sections (5 mm) were obtained from patients with precursor lesions. Formalin-fixed and paraffin-embedded tissue sections made from TMAs were immunostained for RANK expression analysis using Envision methods. The 4 μm thick sections of each specimen were dewaxed and dehydrated with graded xylene and ethanol. Antigen retrieval was performed by boilingin sodium citrate buffer (pH 6.0) for 10 minutes, minusing asteam cooker, and endogenous peroxidase activity was subsequently quenched. RANK mouse anti-human monoclonal antibody (Abcam, Hong Kong, China) was applied at 1:100 dilution at 4°C overnight. Tissue sections were then washed in PBS and incubated with Envision secondary antibodies for 30 min at 37°C. Subsequently, immunoreactivity was detected using DAB, and tissue structures were visualized by counterstaining with hematoxylin.
Expression level of RANK was scored semi-quantitatively according to the percentage of positive cells and the cytoplasmic staining intensity. Scores were given as follows: 0 (0% to 5% positive cells), 1 (6% to 25% positive cells), 2 (26% to 50% positive cells), 3 (51% to 75% positive cells), or 4 (75% positive cells). Immunohistochemical staining intensities of tumor cells were scored on a scale from 0 to 3 as follows: 0 (negative), 1 (buff), 2 (yellow), and 3 (brown) [22]. Scores from 0 to 4 were defined as “low expression of RANK” and those from 4 to 12 as “high expression of RANK.” Immunohistochemical staining was performed independently by two pathologists without any clinicopathological information.
Statistical analysis
Statistical comparisons were performed using SPSS 17.0 and GraphPad Prism 5.01. The associations between RANK expression and clinicopathological variables were analyzed using χ2-test. The results were expressed as mean ± standard deviation. Correlations between prognostic outcomes and RANK expression were investigated using Kaplan-Meier analysis. A two-sided P < 0.05 was considered statistically significant.
Results
Increased expression of RANK in precancerous lesions and ESCC tissues from Han and Kazakh ethnic patients
Immunostaining was performed to examine the expression of RANK in 113 Han ESCC tissues, 36 HGIN, 63 LGIN, and 98 normal esophageal tissues (NETs). The RANK protein was mainly localized in the cytoplasm of cancer cells. In Han ESCC tissues, 79 of 113 cases (69.9%) exhibited high expression of RANK; diffuse and strong immunostaining for RANK (6.457±0.2587) was observed in carcinoma cells. By contrast, 39 of 98 cases (39.8%) exhibited high expression of RANK in Han NETs, and 59 of 98 cases (60.2%) showed no or low expression with low average immunostaining for RANK (4.571±0.2097) (Figure 1A-C). Comparison of RANK expression between ESCC and NETs indicated that RANK proteins exhibited higher immunostaining in the tumor group than in the NETs (P = 1.11×10-5). Interestingly, with advanced degree of dysplasia from normal epithelium to LGIN or HGIN, RANK expression significantly increased (P = 2.84×10-7, 7.83×10-6, respectively) (Table 2; Figure 1D).
Figure 1.
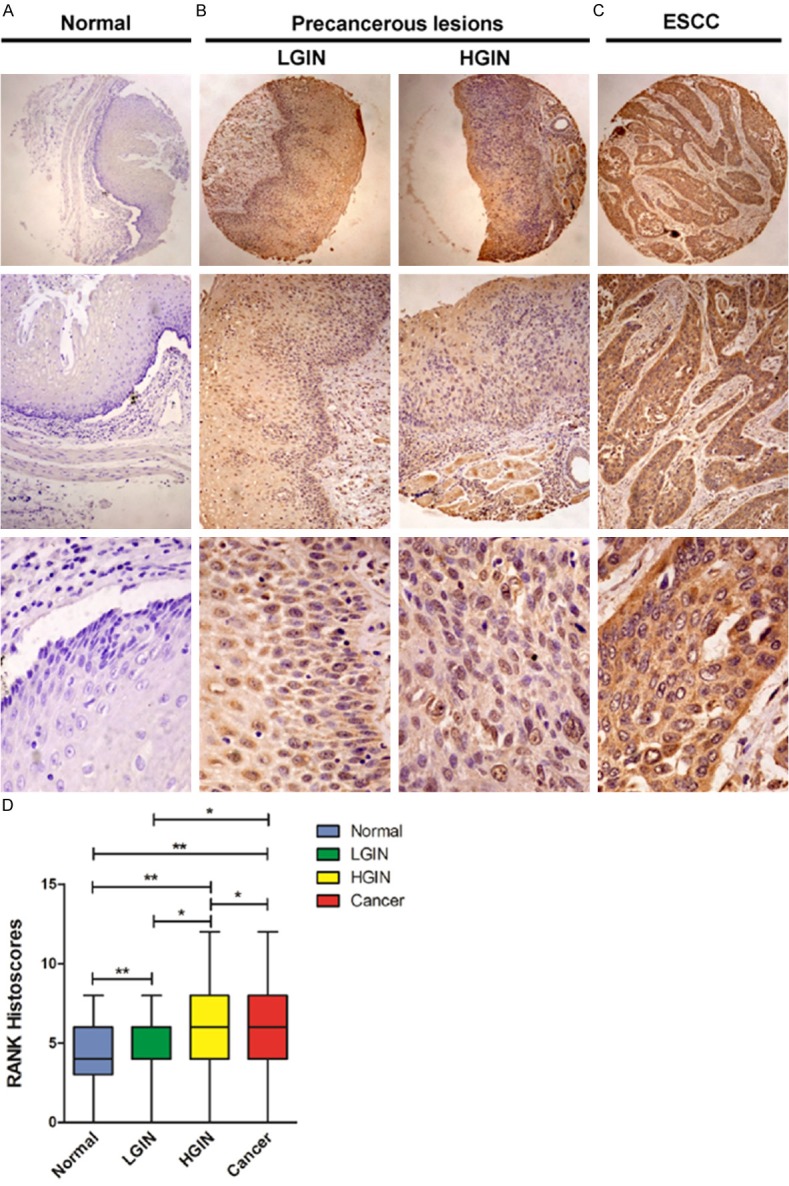
Immunohistochemical analysis of RANK staining in Han ethnic. A. Normal esophageal tissues. B. LGIN and HGIN tissues. C. ESCC tissues, intense immunostaining for RANK was presented in the cytoplasm of most of the cancer cells, compared with adjacent normal tissues. D. Boxplot showing significantly higher expression of RANK in ESCC tissues and HGIN and LGIN tissues than that in normal esophageal tissues (**P < 0.05, *P > 0.05).
Table 2.
Summary of immunohistochemical differences in different tissues in Han ethnic
| Immunostaining | Normal (%) | Precursor lesions (%) | ESCC (%) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| LGIN | HGIN | P 1 | P 2 | P 3 | P 4 | P 5 | P 6 | |||
| Low | 59 (60.2) | 12 (19.0) | 6 (16.7) | 34 (30.2) | 0.110 | 0.114 | 1.11×10-5 | 2.84×10-7 | 7.83×10-6 | 0.768 |
| High | 39 (39.8) | 51 (81.0) | 30 (83.3) | 79 (69.8) | ||||||
P 1: ESCC vs. LGIN; P 2: ESCC vs. HGIN; P 3: ESCC vs. Normal; P 4: LGIN vs. Normal; P 5: HGIN vs. Normal; P 6: LGIN vs. HGIN. P < 0.05 was considered statistically significant.
For Kazakh tissues, the RANK expression status was detected in 196 ESCC tumor tissues and 76 NETs. RANK overexpression was examined in 107 of 196 (54.6%, IS = 5.548±0.2134) tumor cases and 23 of 76 (30.3%, IS = 3.882 ± 0.3366) normal tissues (Figure 2A). Similar to Han ESCC tissues, RANK was significantly overexpressed in ESCC tumor specimens compared with normal samples (P = 0.001) (Figure 2B).
Figure 2.
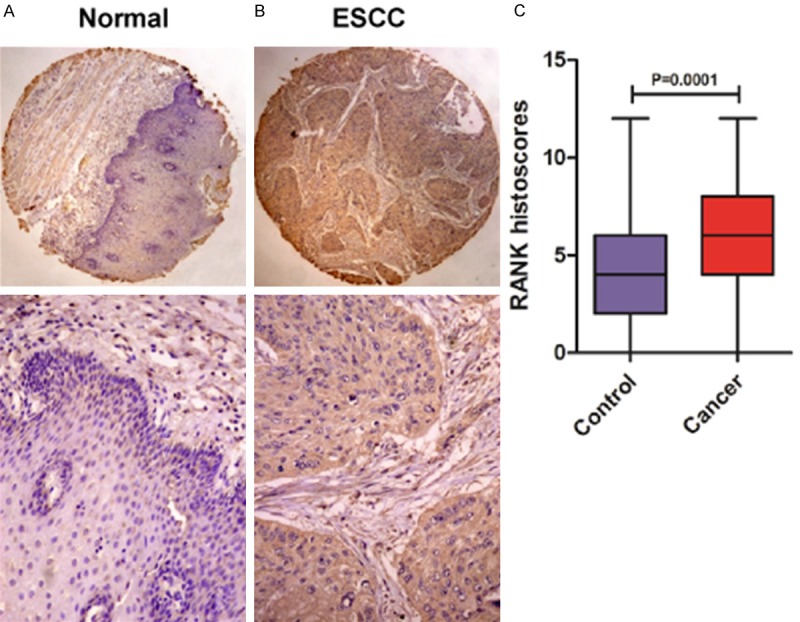
Representative immunohistochemical staining of RANK in Kazakh ethnic. (A) Normal esophageal epithelia, (B) esophageal carcinoma, (C) Boxplot showing significantly higher expression of RANK in ESCC tissues than that in normal esophageal tissues (P = 0.0001).
Correlation of RANK expression with clinicopathological characteristics
To evaluate the clinicopathologic impacts of RANK protein expression on ESCC, we analyzed the correlation of various clinicopathologic variables with expression patterns of the RANK in the Han and Kazakh ethnics (Table 3). Overexpression of RANK was remarkably correlated with lymph node metastasis in Kazakh ESCC tissues. RANK overexpression was observed in 61.7% (66 of 107) of cases with lymph node metastasis compared with low expression of RANK in 55.1% (49 of 89) of cases without lymph nodes metastasis (P = 0.019). However, no significant correlation existed in Han ESCC tissues (Table 3).
Table 3.
Correlations between RANK expression of squamous cell carcinoma and clinicopathological factors in Han and Kazakh ethnic
| Parameters | Total | RANK expression in Han ethnic | Total | RANK expression in Kazakh ethnic | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Low (%) | High (%) | P value | Low (%) | High (%) | P value | |||
| Gender | ||||||||
| Male | 82 | 25 (73.5) | 57 (72.2) | 0.880 | 130 | 64 (71.9) | 66 (61.7) | 0.131 |
| Female | 31 | 9 (26.5) | 22 (27.8) | 66 | 25 (28.1) | 41 (38.3) | ||
| Age (year) | ||||||||
| < 65 | 58 | 18 (52.9) | 40 (50.6) | 0.822 | 135 | 60 (67.4) | 75 (70.1) | 0.687 |
| > 65 | 55 | 16 (47.1) | 39 (49.4) | 61 | 29 (32.6) | 32 (29.9) | ||
| Differentiation | ||||||||
| Well-Moderate | 100 | 30 (88.2) | 70 (88.6) | 0.955 | 173 | 75 (84.3) | 98 (91.6) | 0.113 |
| Poor | 13 | 4 (11.8) | 9 (11.4) | 23 | 14 (15.7) | 9 (8.4) | ||
| Invasion depth | ||||||||
| T1-T2 | 58 | 17 (50.0) | 41 (51.9) | 0.853 | 154 | 73 (82.0) | 81 (75.7) | 0.283 |
| T3-T4 | 55 | 17 (50.0) | 38 (48.1) | 42 | 16 (18.0) | 26 (24.3) | ||
| Lymphatic invasion | ||||||||
| N0 | 75 | 24 (70.6) | 51 (64.6) | 0.534 | 90 | 49 (55.1) | 41 (38.3) | 0.019 |
| N1-N3 | 38 | 10 (29.4) | 28 (35.4) | 106 | 40 (44.9) | 66 (61.7) | ||
| TNM Staging | ||||||||
| I/II | 80 | 28 (82.4) | 52 (65.8) | 0.076 | 126 | 53 (59.6) | 73 (68.2) | 0.207 |
| III/IV | 33 | 6 (17.6) | 27 (34.2) | 70 | 36 (40.4) | 34 (31.8) | ||
Different expression patterns of RANK in Han and Kazakh ethnic ESCC tissues
Subsequently, we analyzed the difference of RANK expression between Han and Kazakh ethnic ESCC tissues and NETs (Table 4). We found a significant difference in the RANK expression between Han and Kazakh ethnic ESCC tissues (P = 0.007). However, no significant difference existed for NETs (P = 0.193). RANK expression in Han ethnic patients without lymph node metastasis was also remarkably increased compared with that in Kazakh ethnic patients (P = 0.008). Similarly, in patients with lymph node metastasis, the frequency of increased RANK expression in Han was higher than that in Kazakh ethnic ESCC tissues; however, the results were not statistically different (P = 0.093).
Table 4.
The different expression pattern of RANK in Han and Kazakh ethnic ESCC tissue
| Characteristic | Total | RANK expression | P value | |
|---|---|---|---|---|
|
|
||||
| Low (%) | High (%) | |||
| ESCC | ||||
| Kazakh ethnic | 196 | 89 (72.4) | 107 (57.5) | 0.007* |
| Han ethnic | 113 | 34 (27.6) | 79 (42.5) | |
| NETs | ||||
| Kazakh ethnic | 76 | 53 (47.3) | 23 (37.1) | 0.193 |
| Han ethnic | 98 | 59 (52.7) | 39 (62.9) | |
| No lymph node metastasis | ||||
| Kazakh ethnic | 90 | 47 (66.2) | 43 (45.7) | 0.008* |
| Han ethnic | 75 | 24 (33.8) | 51 (54.3) | |
| lymph node metastasis | ||||
| Kazakh ethnic | 106 | 40 (80.0) | 66 (70.2) | 0.205 |
| Han ethnic | 38 | 10 (20.0) | 28 (29.8) | |
NETs: Normal esophageal mucosa.
P < 0.05 was considered to indicate a statistically significant difference.
Prognosis of ESCC patients according to RANK protein expression
The association between RANK protein expression and overall survival (OS) of ESCC was estimated using log-rank test and multivariable Cox proportional hazard regression analysis. Univariate analysis of 98 enrolled patients revealed that patients with higher RANK staining scores had poorer outcomes than those with lower scores. The median survival time of patients with lower RANK expression was 33.24 months (range, 1-90 months), whereas that of patients with RANK overexpression was only 18.57 months (range, 1-96 months). As shown in Figure 3, Kaplan-Meier survival analysis showed that ESCC patients with higher expression of RANK protein had significantly worse prognosis than ESCC patients with low or no expression (log-rank test, x2 = 11.743, P = 0.001).
Figure 3.
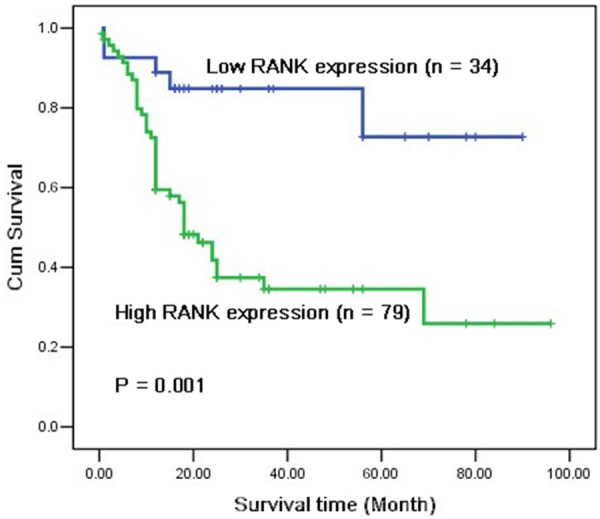
Comparison of survival time of low and high RANK protein expression in esophageal squamous cell carcinoma patients. Based on the results of immunohistochemical staining, the Han ethnic patients with ESCC (n = 113) were divided into high RANK expression (n = 79) and low RANK expression (n = 34) after resection. Log-rank test revealed that patients in the low-expression group exhibited significantly better survival than those in the high-expression group (P = 0.001).
Discussion
This study is the first to explore the expression of RANK in Han and Kazakh ESCC tissues, LGIN, HGIN, and NETs to further analyze the correlation between RANK expression and clinicopathological characteristics. We also investigated the differentiation of RANK levels between Han and Kazakh ethnic ESCC patients. Our immunohistochemical results found that up-regulated RANK expression was detected in both Han and Kazakh ethnic ESCC tissues compared with NETs. RANK expression was remarkably increased during malignant transformation from normal epithelium into LGIN and HGIN tissues in Han ethnic patients. Furthermore, the increased expression of RANK correlated with lymph node metastasis in Kazakh ethnic ESCC patients, and patients with higher levels of RANK had short overall survival. In summary, RANK could be a therapeutic target in ESCC patients.
Many tumor patients will ultimately suffer from primary tumor metastases to secondary organs. Solid tumor (lung, prostate, and breast cancers) metastasis to bone remains a significant cause of morbidity with few successful treatment options. In normal bone, osteoblast formation and osteoclast resorption are coordinately regulated and maintain dynamic balance. In the mid-1990s, RANK/RANKL/OPG pathway revealed a key molecular axis for osteoclast formation, function, and survival. This system provided crucial insights into the regulation of normal physiologic bone remodeling [23,24]. However, imbalance occurs in the course of bone metastasis of tumor cells. Notably, the RANKL/RANK/OPG pathway is dysregulated in several tumors. A recent study reported that high RANK expression in primary breast tumors is associated not only with a high risk of metastases to the bone or other organs but also with lymph node involvement [25], such as in human non-small-cell lung [16], prostate [18], and renal cell carcinomas [26]. Moreover, recent studies have examined functional RANK expression in cancer cell lines from human origin (non-small-cell lung cancer [16], breast and prostate carcinomas [17], and osteosarcoma [27]). In clinical pathology, esophageal precancerous lesions may consist of LGIN, HGIN, and carcinoma in situ [28]. These findings strongly suggest the importance of RANK protein in ESCC metastasis, which encouraged us to examine the relationship between the RANK expression and various clinical features of ESCC to evaluate the potential of RANK as a therapeutic target in ESCC metastasis. We also deduced that some biological systems account for the malignancy and development of ESCC and that some molecules could be identified as prognostic biomarkers in precursor lesions.
Thus, ESCC cells can express RANK and activate the RANK/RANKL pathway, leading to lymph node and distant metastases. The high RANK expression of ESCC cells would then be associated with tumor progression. To verify this hypothesis, we selected ESCC patients from two ethnic groups (Han and Kazakh) in China. Immunohistochemical analysis showed that RANK expression was detected in ESCC tissues, HGIN, LGIN, and NETs, which exhibited different metastatic potentials. Our results showed that RANK expression was significantly higher in both Han and Kazakh ethnic ESCC tissues than in normal epithelium. Notably, in Han ethnic ESCC tissues, although no correlation existed between the high expression of RANK in LGIN and HGIN or between the LGIN or HGIN and ESCC, the overexpression of RANK gradually increased in the transformation from normal epithelium into LGIN, HGIN, and invasive ESCC. Thus, in Han ethnic ESCC tissues, RANK correlated with the progression of ESCC, and the overexpression of RANK might represent a late event in the multistep pathogenesis of ESCC. Importantly, we also found that the Han ethnic ESCC patients with higher levels of RANK had short overall survival. These data might indicate that upregulation of RANK plays an important role in the activation and progression of precancerous lesions in ESCC, suggesting that RANK could be a potential biomarker for ESCC.
In China, ESCC is one of the most common malignancies of the digestive system. This cancer also ranks sixth in mortality and seventh in incidence in China with significant variations in geography, ethnicity, and socio-culture [29]. Han and Kazakh are the two major ethnic groups in China. ESCC of Kazakhs is characterized by a strong tendency toward familial aggregation, high incidence and mortality, and age-standardized rates of 90 to 150/100,000 [30], compared with other ethnic populations residing within Xinjiang, northwest of China. Thus, the pathogenesis of esophageal cancer in Han and Kazakh ethnic groups of China should be compared. Bone and lymph node metastases are common complications in ESCC patients, similar to human non-small-cell lung [16], prostate [18,31], and breast cancer patients [31-33]. Our current results indicated that the overexpression of RANK is closely correlated with lymph node metastases in Kazakh ethnic ESCC patients. However, in Han ethnic ESCC patients, no significant correlation existed between the expression level of RANK and clinicopathological characteristics. The statistical results of the two ethnic groups were inconsistent, which could be attributed to their differences in RANK levels and/or clinicopathological information. Further immunostaining analyses demonstrated that RANK expression is strongly significant in Han and Kazakh ethnic ESCC patients, as well as in those without lymph node metastasis. Thus, we deduce the difference in RANK expression among various ethnic groups. Further studies are necessary to clarify the relationship of RANK expression and the different ethnic ESCC patients in China.
In conclusions, this study is the first to identify RANK overexpression as a novel esophageal cancer marker in both Kazakh and Han ethnic ESCC patients. The results support the association between RANK and ESCC across ethnicities. In summary, RANK could be a therapeutic target in ESCC patients. Additional studies are warranted to confirm that the metastatic potential of ESCC cells was enhanced because of the disruption of RANK/RANKL interaction. Therefore, a further prospective study should investigate the expression levels of RANKL and OPG, as well as their correlation with the metastatic potential of ESCC in vitro and in vivo.
Acknowledgements
This work was supported by grants from The National Natural Science Foundation of China (No. 81160301, 81360358, 81460362, 81260301), the Ministry of Science and Technology of China (2012AA02A503), the jointly foundation for nurturing the outstanding young scientists of Shihezi University (No. 2013ZRKXYQ-YD19). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Rohatgi PR, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R, Walsh G, Vaporciyan A, Lynch PM, Rice DC, Roth JA, Ajani JA. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer. 2005;104:1349–1355. doi: 10.1002/cncr.21346. [DOI] [PubMed] [Google Scholar]
- 4.Shirakawa YNY, Kimura M, Kawashima R, Yamatsuji T, Tamaki T, Hamada M, Haisa M, Tanaka N. Topological analysis of p21WAF1/CIP1expression in esophageal squamous dysplasia. Clin Cancer Res. 2000;6:541–550. [PubMed] [Google Scholar]
- 5.Peng J, Hu Q, Liu W, He X, Cui L, Chen X, Yang M, Liu H, Wei W, Liu S, Wang H. USP9X expression correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Diagn Pathol. 2013;8:177. doi: 10.1186/1746-1596-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing T, Dong L, Tang E, Yang H. Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res Treat. 2007;104:21–30. doi: 10.1007/s10549-006-9393-7. [DOI] [PubMed] [Google Scholar]
- 7.Luo A, Yu X, Li G, Ma G, Chen H, Ding F, Li Y, Liu Z. Differentiation-Associated Genes Regulated by c-Jun and Decreased in the Progression of Esophageal Squamous Cell Carcinoma. PLoS One. 2014;9:e96610. doi: 10.1371/journal.pone.0096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase Inhibition by Small-Molecule WP1130 Triggers Aggresome Formation and Tumor Cell Apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 9.Hiyoshi Y, Kamohara H, Karashima R, Sato N, Imamura Y, Nagai Y, Yoshida N, Toyama E, Hayashi N, Watanabe M, Baba H. MicroRNA-21 Regulates the Proliferation and Invasion in Esophageal Squamous Cell Carcinoma. Clin Cancer Res. 2009;15:1915–1922. doi: 10.1158/1078-0432.CCR-08-2545. [DOI] [PubMed] [Google Scholar]
- 10.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 11.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tat SK, Pelletier JP, Velasco CR, Padrines M, Martel-Pelletier J. New Perspective in Osteoarthritis: The OPG and RANKL System as a Potential Therapeutic Target? Keio J Med. 2009;58:29–40. doi: 10.2302/kjm.58.29. [DOI] [PubMed] [Google Scholar]
- 13.Whyte MP, Mumm S. Heritable disorders of the RANKL/OPG/RANK signaling pathway. J Musculoskelet Neuronal Interact. 2004;4:254–267. [PubMed] [Google Scholar]
- 14.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 16.Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang S, Lu Q, Sun Y. Differential Expression of the RANKL/RANK/OPG System Is Associated with Bone Metastasis in Human Non-Small Cell Lung Cancer. PLoS One. 2013;8:e58361. doi: 10.1371/journal.pone.0058361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casimiro S, Mohammad KS, Pires R, Tato-Costa J, Alho I, Teixeira R, Carvalho A, Ribeiro S, Lipton A, Guise TA, Costa L. RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS One. 2013;8:e63153. doi: 10.1371/journal.pone.0063153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 19.Tada T, Jimi E, Okamoto M, Ozeki S, Okabe K. Oral squamous cell carcinoma cells induce osteoclast differentiation by suppression of osteoprotegerin expression in osteoblasts. Int J Cancer. 2005;116:253–262. doi: 10.1002/ijc.21008. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156:761–767. doi: 10.1016/s0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Cheng YY, Chow LT, Zheng MH, Kumta SM. Receptor activator of NF-kappaB ligand (RANKL) is expressed in chondroblastoma: possible involvement in osteoclastic giant cell recruitment. Mol Pathol. 2003;56:116–120. doi: 10.1136/mp.56.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui XB, Pang XL, Li S, Jin J, Hu JM, Yang L, Liu CX, Li L, Wen SJ, Liang WH, Chen YZ, Li F. Elevated expression patterns and tight correlation of the PLCE1 and NF-κB signaling in Kazakh patients with esophageal carcinoma. Med Oncol. 2013;31:791. doi: 10.1007/s12032-013-0791-5. [DOI] [PubMed] [Google Scholar]
- 23.Dougall WC. Molecular Pathways: Osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2011;18:326–335. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 25.Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A, Ortega C, Porta C, Galluzzo S, Armento G, La Verde N, Caroti C, Treilleux I, Ruggiero A, Perrone G, Addeo R, Clezardin P, Muda AO, Tonini G. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One. 2011;6:e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikami S, Katsube K, Oya M, Ishida M, Kosaka T, Mizuno R, Mochizuki S, Ikeda T, Mukai M, Okada Y. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J Pathol. 2009;218:530–539. doi: 10.1002/path.2567. [DOI] [PubMed] [Google Scholar]
- 27.Wittrant Y, Lamoureux F, Mori K, Riet A, Kamijo A, Heymann D, Redini F. RANKL directly induces bone morphogenetic protein-2 expression in RANK-expressing POS-1 osteosarcoma cells. Int J Oncol. 2006;28:261–269. [PubMed] [Google Scholar]
- 28.Xue LY, Hu N, Song YM, Zou SM, Shou JZ, Qian LX, Ren LQ, Lin DM, Tong T, He ZG, Zhan QM, Taylor PR, Lu N. Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer. 2006;6:296. doi: 10.1186/1471-2407-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 30.Chen YZ, Cui XB, Hu JM, Zhang WJ, Li SG, Yang L, Shen XH, Liu CX, Pan QF, Yu SY, Yuan XL, Yang L, Gu WY, Chen JZ, Wang LD, Li F. Overexpression of PLCE1 in Kazakh esophageal squamous cell carcinoma: implications in cancer metastasis and aggressiveness. Apmis. 2013;121:908–918. doi: 10.1111/apm.12095. [DOI] [PubMed] [Google Scholar]
- 31.Casimiro S, Mohammad KS, Pires R, Tato-Costa J, Alho I, Teixeira R, Carvalho A, Ribeiro S, Lipton A, Guise TA, Costa L. RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS One. 2013;8:e63153. doi: 10.1371/journal.pone.0063153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsubaki M, Komai M, Fujimoto S, Itoh T, Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D, Mukai J, Sakaguchi K, Satou T, Nishida S. Activation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and nduces epithelial-to-mesenchymal transition in mammary tumor cell lines. J Exp Clin Cancer Res. 2013;32:62. doi: 10.1186/1756-9966-32-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia P, Sanders MM, Hansen MF. Expression of Receptor Activator of Nuclear Factor-KB ligand is inversely correlated with metastatic phenotype in breast carcinoma. Clin Cancer Res. 2005;11:162–165. [PubMed] [Google Scholar]


