Abstract
Peripheral ameloblastoma (PA), a rare and unusual variant of odontogenic tumors, comprises about 1% of all ameloblastomas. PA is an exophytic growth localized to the soft tissues overlying the tooth-bearing areas of the jaws, and the initial diagnosis is often fibrous epulis. PA with histologically low-grade malignant features is extremely rare. We report a case of peripheral ameloblastoma with histologically low-grade malignant features in a 69-year-old woman that presented with a hemorrhage from a tumor on the right buccal mucosa. The tumor was surgically removed by blunt dissection, with no evidence of recurrence after two years and six months. After the case presentation, microscopic and genetic findings are discussed.
Keywords: Peripheral ameloblastoma, odontogenic tumor, buccal mucosa
Introduction
It is difficult to establish with certainty if the origin of the ameloblastoma is from the surface epithelium of the jaw. Numerous cases have reported direct communication between the ameloblastoma and the overlying mucosal epithelium. Nevertheless, these do not preclude the possibility that the neoplasm may grow peripherally until it reaches the surface epithelium, and then fuse with the tissue. Occasional cases have been demonstrated in which the ameloblastoma is situated entirely outside the bone, strengthening the case for surface epithelial origin; however, this still does not rule out a possible origin from the persistent remnants of the dental lamina. PA is a rare odontogenic tumor, which has been reported to account for approximately 1% to 5% of all ameloblastomas [1-3].
Case report
A 69-year-old Japanese woman was admitted to our hospital due to intratumoral hemorrhage in the right buccal mucosa, and was positive for hypertension. She stated that a nodule had been slowly growing for about 6 months; the clinical features revealed a lesion covered by normal mucosa with ulceration and firm consistency, measuring approximately 30 mm in diameter (Figure 1A). The tumor was a soft, fixed lesion hemorrhaging from ulceration, involving the buccal mucosa. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a well-demarcated circular mass anterior to the buccal space. The tumor was enhanced in contrast CT scan and showed a high signal in T2-weighted MRI images. CT and MRI did not demonstrate a direct relationship to the maxillary and mandibular bones (Figure 1B-D). There was no associated cervical lymphadenopathy or evidence of distant metastasis. The blood test parameters were all normal. The cytodiagnosis of the tumor was class II, and the lesion was excised and submitted for microscopic examination. The presumptive clinical diagnosis was suspected basal cell carcinoma, with a differential diagnosis of basal cell adenoma, basal cell carcinoma, and ameloblastoma (Figure 2A, 2B). The tumor was removed through an intraoral approach under general anesthesia. Although there was no attachment to the periosteum of the mandible, there was communication with the surface epithelium. The tumor was well encapsulated, and was totally removed by blunt dissection with relative ease. The tumor specimen was immersed in 10% formalin solution for histopathological study. The tumor epithelium was arranged as a network bounded by a layer of cuboidal to columnar cells, including cells resembling stellate reticulum. The tumor cells connected to the surface epithelium and showed squamous metaplasia. These findings closely resemble the histology of plexiform type ameloblastoma (Figure 3A, 3B). Mitoses and invasive growth of tumor nests were observed. Immunohistochemical examination indicated that the tumor cells were positive for Ki-67. These findings indicated low-grade malignancy. The tumor cells did not stain for smooth muscle actin, calponin, s-100 protein, or glial fibrillary acidic protein (Figure 4A, 4B). In addition, we compared gene expression signatures between the tumor and normal mucosa of the present case with the tissues from two other ameloblastoma cases using the Applied Biosystems Human Genome Survey Microarray systems. This patient was fully informed and had given written consent before surgery in compliance with the regulations of the ethical committees of Ehime University Hospital. Comparing the ameloblastoma tissues and normal mucosa, the microarray data identified 192 genes that were significant overexpressed. Among the genes identified, 120 genes were overexpressed in the present case’s tumor, including ameloblastin, also known as amelin, a gene- coding for a protein found in tooth enamel, and MMP-13, which is known to be associated with protease-associated tumor invasion and metastasis (Figure 5A, 5B). PA was diagnosed on the basis of clinical, histopathological, and genetic examination. The patient was followed up for 9 years and 11 months, and there has been no evidence of recurrence.
Figure 1.
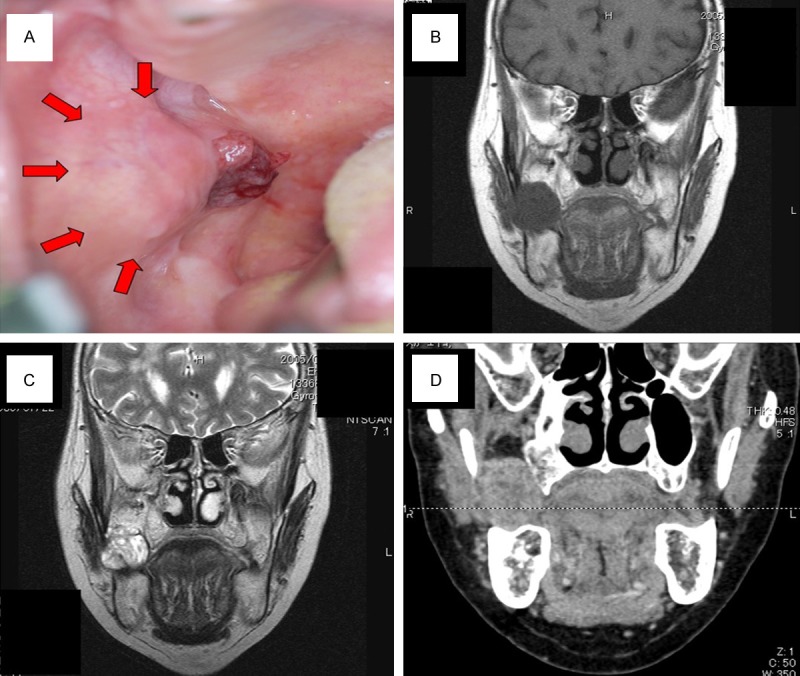
A: Pretreatment intra oral photograph. B: Horizontal T1-weighted magnetic resonance imaging (MRI) scan showing the extent of tumor. C: Horizontal T2-weited MRI scan. D: Computed tomography image and MRI did not demonstrate a direct relationship to the maxillary and mandibular bones.
Figure 2.
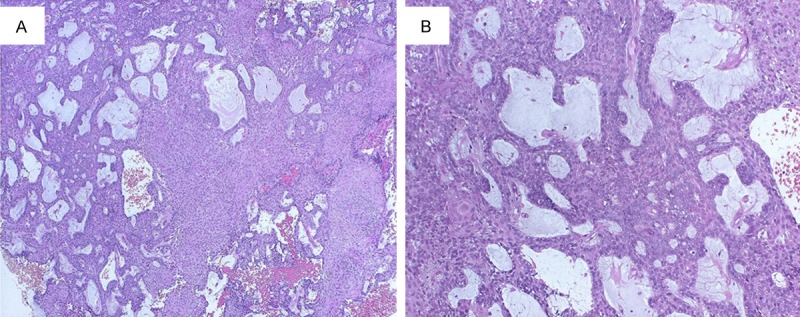
Histopathological examination of the biopsy material (haematoxylin and eosin; original magnification. A: ×40. B: ×100). The presumptive clinical diagnosis was suspected basal cell carcinoma. The differential diagnosis must be made with basal cell adenoma, basal cell carcinoma and ameloblastoma.
Figure 3.
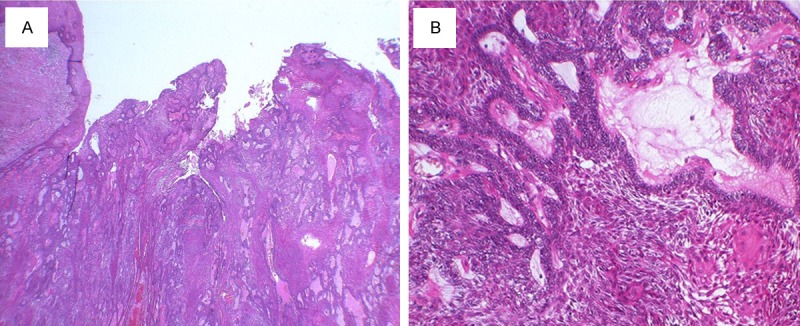
Histopathological examination of the tumor (haematoxylin and eosin; original magnification. A: ×20. B: ×100). The tumor epithelium was arranged as a network which is bounded by a layer of cuboidal to columnar cells and includes cells resembling stellate reticulum. Tumor cells connected to surface epithelium and showed squamous metaplasia.
Figure 4.
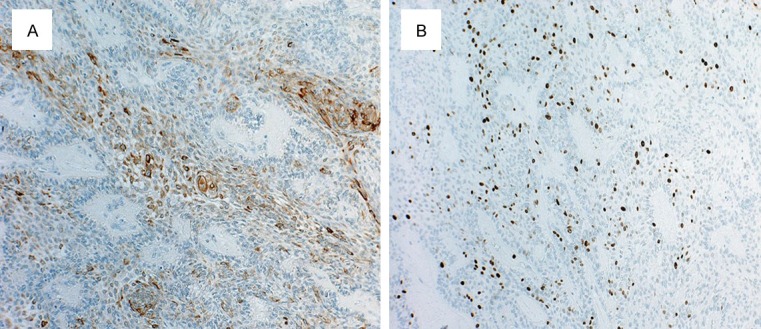
Immunohistochemiical staining of a specimen (A: Cytokeratin-19. B: Ki-67, original magnification ×100).
Figure 5.
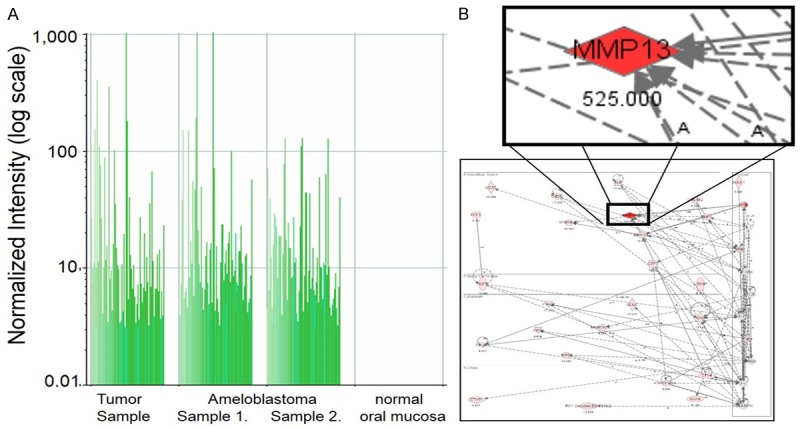
A: Gene expression analysis between tumor, ameloblastoma and normal mucosa using microarray analysis. B: This tumor had a very similar feature for ameloblastoma. The Ingenuity Pathway Analysis (IPA) tool was used to analyze the identified genes.
Discussion
Ameloblastoma is a locally aggressive epithelial odontogenic tumor, comprising 1.3%-10% of all ameloblastomas [4]. PA was first described by Kuru in 1911 since then, many cases of PA have been reported [5]. However, cases of PA in the extragingival area are extremely rare. The most frequent sites of PA are the upper and lower gingiva, with the buccal mucosa being an uncommon site. Only six cases of extragingival PA have been reported [6-11]. Histological examination of PA is often difficult, and the WHO classification of tumors (Pathology & Genetics, Head and Neck Tumours) does not contain a histological definition of PA. PA and basal cell carcinoma (BCC) exhibit similar growth patterns and share histological features. It is generally believed that PA and BCC represent the same neoplasm [12]. Nauta et al. reviewed the existing literature, totaling 53 cases of peripheral ameloblastoma, and concluded that there were no consistent histological features by which PA could be distinguished from BCC [13]. In our case, the tumor closely resembled the histology of plexiform type ameloblastoma. However, there were no histological features which distinguished it from BCC. We therefore examined the genetic features of the tumor using microarray analysis, and found that it shared very similar features with ameloblastoma. Additionally, ameloblastin, a gene coding for a protein found in tooth enamel, was overexpressed in this tumor. The current case demonstrated the ability to make a microarray-based diagnosis. Neoplasms are usually classified according to their histology and location, or the occurrence of specific molecular markers. Microarray analysis allowed a more efficient method of analysis, enabling a better diagnosis.
Acknowledgements
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Japanese Government Study Program).
Disclosure of conflict of interest
None.
References
- 1.Gurol M, Burkes EJ. Peripheral ameloblastoma. J Periodontal. 1995;66:1065–1068. doi: 10.1902/jop.1995.66.12.1065. [DOI] [PubMed] [Google Scholar]
- 2.El-Mofty SK, Gerard NO, Farish SE, Rodu B. Peripheral ameloblastoma: a clinical and histologic study of 11 cases. J Oral Maxillofac Surg. 1991;49:974–975. doi: 10.1016/0278-2391(91)90061-p. [DOI] [PubMed] [Google Scholar]
- 3.Lin SC, Lieu CM, Hahn LJ, Kwan HW. Peripheral ameloblastoma with metastasis. Int J Oral Maxillofac Surg. 1987;16:202–204. doi: 10.1016/s0901-5027(87)80131-5. [DOI] [PubMed] [Google Scholar]
- 4.Philipsen HP, Reichart PA, Nikai H, Takata T, Kudo Y. Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol. 2001;37:17–27. doi: 10.1016/s1368-8375(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuru H. Ueber das adamantinom. Zentrablatt fur allgemeine. Pathologie und Anatomie. 1911;22:291–295. [Google Scholar]
- 6.Braunstein E, Mass B. Case report of an extraosseous adamantoblastoma. Oral Surg Oral Med Oral Pathol. 1949;2:726–728. doi: 10.1016/0030-4220(49)90105-x. [DOI] [PubMed] [Google Scholar]
- 7.Klinar KL, McManis JC. Soft-tissue ameloblastoma. Report of a case. Oral Surg Oral Med Oral Pathol. 1969;28:266–272. doi: 10.1016/0030-4220(69)90296-5. [DOI] [PubMed] [Google Scholar]
- 8.Ramnarayan K, Nayak RG, Kavalam AG. Peripheral ameloblastoma. Int J Oral Surg. 1985;14:300–301. doi: 10.1016/s0300-9785(85)80044-2. [DOI] [PubMed] [Google Scholar]
- 9.Woo SB, Smith-Williams JE, Sciubba JJ, Lipper S. Peripheral ameloblastoma of the buccal mucosa: case report and review of the English literature. Oral Surg Oral Med Oral Pathol. 1987;63:78–84. doi: 10.1016/0030-4220(87)90344-6. [DOI] [PubMed] [Google Scholar]
- 10.Shibata T, Kaneko N, Hokazono K, Nishiwaki C, Kamiya M, Tajima Y, Utsumi N. An ameloblastoma-like neoplasm of the buccal mucosa. Report of a case. Int J Oral Maxillofac Surg. 1990;19:203–204. doi: 10.1016/s0901-5027(05)80390-x. [DOI] [PubMed] [Google Scholar]
- 11.Yamanishi T, Ando S, Aikawa T, Kishino M, Nakano Y, Sasai K, Isomura Tanaka E, Tsuji T, Koizumi H, Iida S, Kogo M. A case of extragingival peripheral ameloblastoma in the buccal mucosa. J Oral Pathol Med. 2007;36:184–186. doi: 10.1111/j.1600-0714.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DG. Peripheral ameloblastoma: a study of 21 cases, including 5 reported as basal cell carcinoma of the gingiva. Cancer. 1977;39:1625–1633. doi: 10.1002/1097-0142(197704)39:4<1625::aid-cncr2820390437>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Nauta JM, Panders AK, Schoots CJ, Vermey A, Roodenburg JL. Peripheral ameloblastoma. A case report and review of the literature. Int J Oral Maxillofac Surge. 1992;21:40–44. doi: 10.1016/s0901-5027(05)80451-5. [DOI] [PubMed] [Google Scholar]


