Abstract
This study aimed to screen the potential diagnostic biomarkers for distinguishing the malignant pheochromocytoma (PCC) from benign PCC. A total of 59 patients with PCC (benign and malignant) were enrolled in this study. The expression level of miRNAs in patients with different kind PCCs (healthy control, benign, malignant, malignant with or without SDHD mutation, adrenal and extra-adrenal) was analyzed using the qRT-PCR analysis. Besides, the diagnosis accuracy of miRNA in PCC samples was analyzed using the ROC analysis. Moreover, level of miR-101 in serum was detected by qRT-PCR analysis and serum VEGF level in patients with PCC was detected using the ELISA kit. Compared with benign PCC, miR-101 level was higher in patients with malignant PCC (P < 0.05), while the level of miR-513-5p and miR-26b showed no difference between malignant PCC and benign PCC (P > 0.05). miR-101 expression was significantly increased in malignant tumor tissue with SDHD mutation (P < 0.05) and in extra-adrenal tissues (P < 0.05), respectively. Besides, AUCs for miR-101 in PCC samples was 0.79 and for which in PCC samples with non-SDHD mutation was 0.77. Besides, serum miR-101 in malignant PCC was high but showed no difference among groups (P > 0.05). Moreover, serum VEGF level in malignant tumors was significantly high compared with benign tumor, as well as that in malignant PCC with SDHD mutation (P < 0.05). Our study suggested that SDHD mutation may enhance the overexpression of miR-101 in malignant tumors and miR-101 may be a potential diagnostic biomarker for malignant PCC and benign PCC.
Keywords: Pheochromocytoma, miRNA, SDHD, diagnosis, biomarker
Introduction
Pheochromocytoma (PCC) is a neuroendocrine tumor that originates in adrenal medulla or at extra-adrenal sites and is responsible for production and secretion of catecholamine [1]. Statistics show that morbidity of PCC originate from adrenal is about 13% to 29% while that of PCC at extra-adrenal is about 43% [2]. Studies reveal that clinical pathological morphology of tumors between benign and malignant PCC is similar [3]. The 5-year survival rate for malignant PCC is approximately 40% due to the hard detection and less of diagnostic methods, which results in the difficulty for begin PCC diagnose [4]. Although recent studies prove that multiple genes mutations such as succinate dehydrogenase complex (SDHB and SDHD) and von Hippel-Lindau (VHL) are available for the understanding of increasing risk of malignant PCC [5,6], the molecular mechanisms between malignant and benign PCC have not yet been fully described. Therefore, exploring several key biomarkers for distinguishing begin PCC from malignant PCC will be of great significance.
miRNAs are some short (22 bp) noncoding RNAs that can regulate gene expression in posttranscriptional level by targeting specific genes [7]. Increasing evidences suggest that miRNAs can be useful in regulating biological processes such as cell growth and proliferation, apoptosis, and differentiation in many cancers, including lung cancer [8], prostate cancer [9], and PCC [10,11]. For instance, Erin reports that miR-483-5p has the defining characteristic of adrenocortical malignancies and can be used to accurately distinguish between benign and malignant adrenocortical tumors [12]. Meyer and his colleagues report that miR-15a and miR-16 inhibits the cell proliferation and induces cell death in malignant PCC [10]. Besides, studies reveal that genes mutation contributed to the risk of malignant PCC. For example, Tang refers that mutation of SDHB induces the overexpression of miR-210 in PCC and miR-210 may be different between benign and malignant PCC [13]. Yeap proves that tumor formation of PCC in SDHD mutation requires the loss of both wild-type SDHD allele and maternal 11p15, leading to the inheritance after SDHD transmission [14]. Also, Korpershoek refers that mutation of SDHA is available for the identification of SDHA-related tumors in sporadic PCC [15]. Despite many studies devote to the mechanism exploration of biomarkers for distinguishing benign and malignant PCC. However, role of miR-101 in benign and malignant PCC still remains largely unknown.
In this study, we focused on analyzing the expression levels of three miRNAs in patients with benign and malignant PCCs. Comprehensive exprimental methods were used to detect the expression of miRNA in malignant PCC with SDHD mutation, the level of serum miRNA, and the level of vascular endothelial growth factor (VEGF) in malignant PCC. This study aimed to investigate several potential biomarkers for distinguishing benign from malignant PCC.
Materials and methods
Definition
The definition of PCC tumors is shown as described previously [16]. PCCs (adrenal or extra adrenal) are some catecholamine-producing tumors of chromaffin cell origin; benign PCCs are the solitary chromaffin tumors with no evidence of metastasis with at least 2 years of follow-up after surgery resection; malignant PCCs are the adrenal chromaffin tumor cells with metastasis to a region where adrenal tissue would not be expected wither at initial presentation or during follow-up [17].
Patients and samples
A total of 53 samples of patients that suffered from PCC were enrolled in this study. Thereinto, 29 patients with benign PCC, including 27 with PCC from adrenal and 2 with PCC from extra-adrenal; 24 patients with malignant PCC, including 14 with PCC from adrenal and 10 with PCC from extra-adrenal; and 16 malignant PCC patients associated with SDHD mutation, 1 benign PCC patient with VHL mutation, and 1 benign PCC patient with HF1 mutation. The characteristics of the total patients are summarized in Table 1. The informed consent and approval of patients were obtained from Binzhou People’s Hospital. This study was approved by the Ethics Committee of General Hospital of Binzhou People’s District, in accordance with the Declaration of Helsinki.
Table 1.
Clinical characteristics for the patients used in this study
| Information | total | benign | malignant | P value |
|---|---|---|---|---|
| Number of patients | 53 | 29 | 24 | |
| Age (years)* | 43.8 ± 14.7 (16-75) | 45.3 ± 15.2 (21-75) | 41.6 ± 13.9 (16-69) | P > 0.05 |
| Gender (male/female) | 31/22 | 17/12 | 14/10 | P > 0.05 |
| Greatest tumor diameter (cm)# | 4.5 ± 2.7 (1.1-15.0) | 4.2 ± 2.1 (1.1-13.0) | 4.9 ± 3.4 (1.7-15.0) | P > 0.05 |
| Site | ||||
| Adrenal | 41 | 27 | 14 | |
| Extra-adrenal | 12 | 2 | 10 | |
| Positive mutation | ||||
| SDHD | 16 | 0 | 16 | |
| VHL | 1 | 1 | 0 | |
| NF1 | 1 | 1 | 0 | |
Stands for the Mean ± S.E.M (range);
stands for Mean ± S.D (range).
The PCC tissue samples were collected at the time of surgery, snap frozen in liquid nitrogen, and then stored at -80°C. Besides, serum samples from all patients were frozen at -20°C immediately after collection.
The diagnosis of patients with PCC was conducted when there was clinical evidence of tumors at distant sites from the primary tumor location where chromaffin cells are not found or when there was gross local invasion.
Normal adrenal tissues were obtained from the healthy organ donors through an IRB-approved protocol. Laser capture microdissection was used to separate tissue from the adrenal medulla [18].
RNA extraction
The fresh PCC tissues were grinded in the liquid nitrogen and then washed with the PBS buffer (PH 7.4). Total RNA was extracted from the collected fresh-frozen PCC tissues (50 mg) of normal adrenal medulla, benign PCC and malignant PCC using the Trizol (Tiangen, China) and then treated with RNAse-free Dnase I (Sigma, USA) according to the manufacturer’s protocol [19]. The extracted RNA was further purified by two subsequent precipitations with NaCI-ethanol and stored at -80°C. The concentration and purity of extracted RNA was detected using SMA4000 UV-VIS (Merinton, Shanghai, China). The quality of total RNA was determined at an absorbance ratio of 260/280 using a UV-spectrophotometer (UVIKON 860 spectrophotometer, Kontron Instruments, Switzerland).
Quantitative real-time PCR analysis
To detect the expression levels of miRNAs (miR-101, miR-513-5p, and miR-26b) in PCC tissues, quantitative real-time PCR (qRT-PCR) was carried out in an Eppendorf Mastercycler (Eppendorf, Westbury, NY) using the SYBR ExScript qRT-PCR Kit (Takara, China) as previously described [20]. The total essential miRNAs data was shown in Table 2. Each reaction system was performed in a final volume of 10 μL containing 2 μL cDNA, 5 μL 2 × SYBR Green master mix, 1 μL each of the primers, and 2 μL ddH2O with the following reaction conditions: template denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, combined primer annealing/elongation at 60°C for 1 min, and 72°C for 30 s. Each sample was run in triplicate for analysis. The expression level of U6 was tested as possible control for data normalization.
Table 2.
Information of the detected miRNAs
| Name | type | Exqion ID |
|---|---|---|
| miR-101-5p | Mature miRNA | 204379 |
| miR-513-5p | Mature miRNA | 205620 |
| miR-26b-3p | Mature miRNA | 204117 |
| U6 snRNA | Control miRNA | 203907 |
Serum miRNA measurement
In order to analyze the level of miR-101 in serum of patients with PCC, the total miRNA was extracted from 500 μL of serum from patients with PCC using the Trizol (Tiangen, China) and then treated with RNAse-free Dnase I (Sigma, USA) according to the manufacturer’s instructions. The final elution volume was 100 μL. The concentration of RNA samples was quantified by NanoDrop 1000 (Wilmington, DE, USA). Besides, a fixed volume of 2 μL RNA was added to synthesis the cDNA using the reverse cDNA synthesis Kit (Exiqon, catalog 203300, Vedbaek, Denmark) for the miRNA quantification [20]. The reaction system and reaction conditions were performed as described above. Reactions without RNA template were used as a control.
Quantification of serum VEGF production
The level of serum VEGF was measured using a VEGF ELISA kit (Amsterdam, Netherlands) according to the manufacturer’s instructions. Three-fold dilutions of serum and standards were measured in duplicate. Absorbance was measured with a Molecular Devices Emax plate reader, and the results analyzed with SoftMax Pro 4.8 processing software.
Statistical analysis
All the statistic analysis was performed using SPSS 16.0 (Chicago, IL, USA). As for the qRT-PCR data, Mann-Whitney U test was used to determine the statistical significance for comparisons between groups. Differences of other data in different tissue were performed using one-way ANOVA plus LSD pos hoc multiple comparisons. Also, one-way ANOVA was applied to compare the RNA concentration between different groups. All statistical test were two sided and the P-value < 0.05 was considered as statistically significant. Receiver operation characteristic (ROC) curve and under the ROC curve (AUC) were used to determine thresholds of miR-101 levels. Sensitivity, specificity, and diagnostic values were calculated for miR-101 cut-off.
Results
qRT-PCR analysis of the miRNAs in PCC
In order to investigate whether miRNAs expression is related to PCC, qRT-PCR was used to detect the expression levels of miRNAs in patients with benign and malignant PCC (Figure 1). The expression level of miR-101 in malignant PCC patients was significantly increased compared with the benign PCC patients (P = 0.0152), and there were significant differences of miR-101 level in malignant PCC (P = 0.0027) and benign PCC compared with the healthy controls (P = 0.0421) (Figure 1A). However, the expression levels of miR-513-5p and miR-26b in PCC patients displayed no significant difference between benign and malignant PCC (miR-513-5p, P = 0.5621; miR-26b, P = 0.4782), and there were no significant difference of miR-513-5p and miR-26b in malignant PCC compared with the healthy controls (miR-513-5p, P = 0.1756; miR-26b, P = 0.1021), and no significant difference was found of miR-513-5p and miR-26b in benign PCC compared to the healthy controls (miR-513-5p, P = 0.4863; miR-26b, P = 0.3791) (Figure 1B, 1C).
Figure 1.
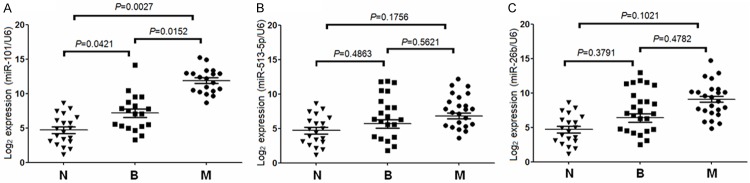
Expressions of three miRNAs (miR-101, miR-513-5p, and miR-26b) in pheochromocytoma patients. N: healthy controls, B: benign, M: malignant. A: Quantitative real-time RT-PCR (qRT-PCR) analysis of miR-101 in three different groups. B: qRT-PCR analysis of miR-513-5p in three different groups. C: qRT-PCR analysis of miR-26b in three different groups.
Besides, we examined the relationship between miRNA expression and specific clinical and pathologic factors to understand the basis for the differences in PCC. However, there was no significant difference between miRNA expression and the comparison factors such as the age, gender, or tumor size. In addition, our data showed that miR-101 level was increased in malignant PCC with SDHD mutations compared with benign PCC (P = 0.0023, Figure 2B).
Figure 2.
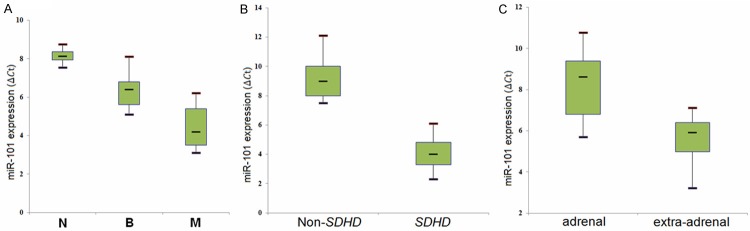
Box plot of miR-101 expression in different groups. A lower ICt stands for a higher miRNA expression level. N: healthy controls, B: benign, M: malignant. A: miR-101 expression in benign and malignant tumors. B: miR-101 expression in adrenal and extra-adrenal tumors. C: miR-101 expression in malignant tumors with SDHD mutation and non-SDHD mutation.
In this study, although the majority of PCCs samples are collected from adrenal, there were 10% of extra-adrenal PCCs samples. Studies revealed that extra-adrenal tumors have a higher likelihood of being malignant. The results showed that there was a significant difference in the expression of the malignancy-associated miR-101 (P = 0.0121, Figure 2C) between adrenal and extra-adrenal tumors.
Diagnostic accuracy of differentially expressed miRNAs
The receiver operating characteristic (ROC) curve was used to analyze the accurate diagnostic marker of miR-101 based on the qRT-PCR values (Figure 3A). The area under the curve (AUC) for miR-101 was 0.79 (95% confidence interval (CI), 0.65-0.83), suggesting the usefulness in molecular diagnostics between benign and malignant PCC. Besides, since previous study have demonstrated that PCC patients with SDHD mutations are likely to have malignant PCC [21], the ability of miR-101 to be a marker of malignancy in non-SDHD tumors was assessed in this study. The AUC for miR-101 was 0.77 (95% CI, 0.63-0.86) (P < 0.01, Figure 3B).
Figure 3.
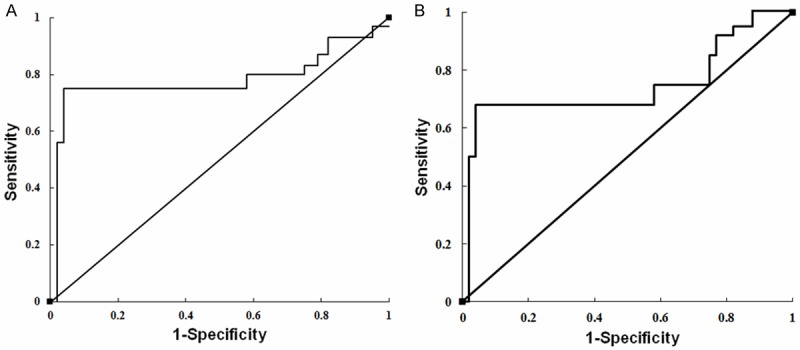
Receiver operating characteristic (ROC) curves for miR-101. Curves were generated based on the. A: ROC curve for miR-101 in all tumor samples. B: ROC curve for miR-101 in tumor samples with non-SDHD.
Measurement of miRNA expression in serum
A number of studies have referred that tumor-derived miRNAs are stable in serum and plasma and are correlated with the levels of miRNAs in tumor tissues, miRNA expression levels can be used to predict tumor type in clinical [22]. We focused on analyzing the expression level of miR-101 in PCC patient serum. A total of 20 serum samples including 12 from patients with benign PCC and 8 from patients with malignant PCC were enrolled in this study. qRT-PCR measurement of miR-101 in serum showed that the level of miR-101 was higher in malignant PCC compared with benign PCC. However, there was no difference in serum miR-101 in patients with benign PCC compared to malignant PCC (P = 0.4513, Figure 4A).
Figure 4.
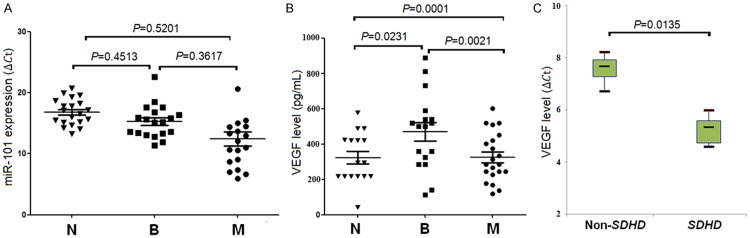
Expressions of miR-101 and VEGF in serum. N: healthy controls; B: benign; M: malignant. A: The serum miR-101 level in three different groups. B: The serum VEGF level in three different groups. C: The serum VEGF level in malignant PCC with SDHD mutation and non-SDHD mutation.
Quantification of VEGF in PCC
Previous study revealed that VEGF level is higher in malignant PCC compared with benign, and VEGF may be a potential index for diagnosing patients in malignant PCC. We analyzed the serum level of VEGF in malignant tumors and benign tumors compared with healthy controls, the results showed that there was significant difference of serum VEGF level in malignant tumors compared with benign tumors (P = 0.0021, Figure 4B), and the significant difference of VEGF level in malignant tumors serum compared with that in healthy controls (P = 0.0231, P = 0.0001, Figure 4B). Besides, qRT-PCR measurement of VEGF in serum showed that the level of VEGF in malignant PCC with SDHD mutation was higher than that in malignant PCC with non-SDHD mutation (P = 0.0135, Figure 4C).
Discussion
Pheochromocytoma (PCC) is a neuroendocrine tumor that originates in the adrenal medulla or at extra-adrenal sites and is responsible for production and secretion of catecholamine with a poor 5-year survival rate and a high morbidity due to the difficulties to distinguish benign PCC from malignant PCC [1,4]. Exploring several key biomarkers for distinguishing the begin PCC from malignant PCC will be of great significance. In this study, we reviewed the relevant literatures and selected three miRNAs (miR-101, miR-513-5p, and miR-26b) that have been proved to be involved in the development of malignant PCC and then analyzed the potential miRNAs that may be correlated with PCC development. Our data showed that miR-101 level in malignant tumor tissue was high compared with benign tumor (P < 0.05). Also, the level of miR-101 in malignant PCC with SDHD mutation and in extra-adrenal tumors were significantly high (P < 0.05). Moreover, level of serum VEGF in patients with malignant PCC was significantly increased compared with benign (P < 0.05), as well as that in malignant PCC with SDHD mutation (P < 0.05).
miR-101 is a short non-coding RNA that is involved in post-transcriptional regulation of many genes expression in multiple cancers by affecting both the stability and translation of mRNAs [23]. Smits proved that downregulation of miR-101 resulted in the EZH2-induced cell proliferation and migration in glioblastoma [24], while Hao said that overexpression of miR-101 inhibited the cell growth in prostate cancer through modulating the COX-2 pathway, suggesting its important role in cancer development. However, role of miR-101 in PCC has not been fully discussed. Patterson proved the miR-101 level in malignant PCC tissues was higher than that in benign PCC [25]. Coincidence with the previous studies, our data showed that level of miR-101 in patients with malignant PCC was significantly increased compared with benign PCC (P < 0.05, Figure 1A). Meanwhile, Gill. analyzed that PCC patients with SDHD gene mutation was likely to enhance the risk of malignant PCC [26]. Tsang proved that miR-210 was overexpressed in PCC with SDHD mutation [27]. Also, in Patterson’s research, the level of miR-101 was higher in patients with SDHB mutation malignant PCC [25]. Coincidence with the previous studies, the level of miR-101 in malignant PCC with SDHD mutation was significantly high compared with the non-SDHD mutation in this study (P < 0.05, Figure 2B), indicating that SDHD mutation may contribute to the expression of miR-101 in malignant PCC. Besides, our data displayed that miR-101 level was higher in extra-adrenal tissues than that in adrenal tissues (P < 0.05, Figure 2C), suggesting the role of miR-101 in different tissues. Wang demonstrated that level of miR-101 in extra-adrenal PCC was high [28]. Also, Patterson and his colleagues reported that miR-101 level in extra-adrenal was higher [25]. Besides, the AUCs for miR-101 in tumor samples and in samples with non-SDHD mutation were 0.79 and 0.77, respectively (Figure 3), indicating the high diagnosis accurate of miR-101 in PCC. Based on our results, we speculated that miR-101 level may significantly increased in malignant PCC compared with benign PCC and miR-101 may be useful for distinguishing malignant and benign PCC.
VEGF is the most stronger vessel formation contributor which has various effects including mediating increased vascular permeability, inducing angiogenesis and endothelial cell growth [29]. Previous studies revealed that VEGF level in malignant PCC tissues was higher and VEGF may be a biomarker for malignant PCC diagnosis [30]. Jyung proved that the positive staining of VEGF were mainly in cytoplasm of paraganglioma cells [31]. Also, VEGF level in serum from patients with adrenal PCC was high before surgery and then decreased after surgery [32]. Therefore, VEGF level may be useful for malignant PCC diagnosis. Our study showed that VEGF level in malignant tumors was higher than that in benign tumors (P < 0.05, Figure 4B). On the other hand, this study found that VEGF level in malignant PCC with SDHD mutation was significantly increased compared with non-SDHD mutation (P < 0.05, Figure 4C). Horbet said that the SDHD mutation induced the production of succinic acid which can inhibit the HIF-1 prolyl hydroylase, resulting in the activity of HIF-1 [33]. The activated HIF-1 may activated the overexpression of VEGF in hypoxia sensing [34]. Thus, SDHD mutation was related to the overexpression of VEGF. Based on our study, we speculated that SDHD may enhance the expression of VEGF in malignant PCC.
Meanwhile, our data showed that level of miR-101 in serum from patients with PCC was high compared with healthy controls, but there was no significant difference among three groups (P > 0.05, Figure 4A). Mitchell have proved that expression level of tumor-specific circulating miRNA in serum or in plasma has moderated correlation with tumor tissues [22]. The role of serum miRNAs in patients with PCC has not been fully discussed. However, Patterson reported the level of miR-183 in serum from patients with PCC was low while that of miR-483-5p and miR-101 were high but showed no significant difference between benign and malignant PCC [25]. Further study will be needed to investigate the mechanism of serum miR-101 in PCC.
In conclusion, this study demonstrated that miR-101 expression was significantly increased in malignant tumor tissues compared with benign tumor and its level is high in malignant tumors with SDHD mutation. VEGF level is high in malignant tumors with SDHD mutation. Our study suggested that SDHD mutation may enhance the overexpression of miR-101 in malignant tumors and miR-101 may be a potential diagnostic biomarker for distinguishing malignant PCC and benign PCC. This study may provide certain basis for future researches on exploring the potential diagnostic role of miR-101 in malignant PCC. However, further experimental studies will be still needed to delve into the diagnostic mechanism of miR-101 in malignant PCC.
Disclosure of conflict of interest
None.
References
- 1.Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr pathol. 2012;23:4–14. doi: 10.1007/s12022-011-9188-1. [DOI] [PubMed] [Google Scholar]
- 2.Kaloostian PE, Zadnik PL, Kim JE, Groves ML, Wolinsky JP, Gokaslan ZL, Witham TF, Bydon A, Sciubba DM. High incidence of morbidity following resection of metastatic pheochromocytoma in the spine: Report of 5 cases. J Neurosurg Spine. 2014:1–8. doi: 10.3171/2014.3.SPINE13535. [DOI] [PubMed] [Google Scholar]
- 3.Welander J, Söderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocrine-related Cancer. 2011;18:R253–R276. doi: 10.1530/ERC-11-0170. [DOI] [PubMed] [Google Scholar]
- 4.Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, de la Fouchardiere C, Libé R, Do Cao C, Niccoli P. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:4006–4012. doi: 10.1210/jc.2013-1907. [DOI] [PubMed] [Google Scholar]
- 5.van Nederveen FH, Korpershoek E, Lenders JW, de Krijger RR, Dinjens WN. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med. 2007;357:306–308. doi: 10.1056/NEJMc070010. [DOI] [PubMed] [Google Scholar]
- 6.McCall PR. The phenotypic effects of expressing mutant VHL in a VHL-knockdown PC12 line. Adelphi University; 2012. [Google Scholar]
- 7.Zhao Y, He S, Liu C, Ru S, Zhao H, Yang Z, Yang P, Yuan X, Sun S, Bu D. MicroRNA regulation of messenger-like noncoding RNAs: a network of mutual microRNA control. Trends Genet. 2008;24:323–327. doi: 10.1016/j.tig.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhao ZG, Jin JY, Zhang AM, Zhang LP, Wang XX, Sun JG, Chen ZT. MicroRNA profile of tumorigenic cells during carcinogenesis of lung adenocarcinoma. J Cell Biochem. 2015;116:458–66. doi: 10.1002/jcb.24999. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer-Rochow GY, Jackson NE, Conaglen JV, Whittle DE, Kunnimalaiyaan M, Chen H, Westin G, Sandgren J, Stålberg P, Khanafshar E. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer. 2010;17:835–846. doi: 10.1677/ERC-10-0142. [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic M, Hengartner M. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 12.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang V, Dwight T, Benn DE, Meyer-Rochow GY, Gill A, Sywak M, Sidhu S, Veivers D, Sue C, Robinson BG. Overexpression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr Relat Cancer. 2014;21:415–426. doi: 10.1530/ERC-13-0519. [DOI] [PubMed] [Google Scholar]
- 14.Yeap PM, Tobias ES, Mavraki E, Fletcher A, Bradshaw N, Freel EM, Cooke A, Murday VA, Davidson HR, Perry CG. Molecular analysis of pheochromocytoma after maternal transmission of SDHD mutation elucidates mechanism of parent-of-origin effect. J Clin Endocrinol Metab. 2011;96:E2009–E2013. doi: 10.1210/jc.2011-1244. [DOI] [PubMed] [Google Scholar]
- 15.Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:E1472–E1476. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- 16.Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Rochow GY, Jackson NE, Conaglen JV, Whittle DE, Kunnimalaiyaan M, Chen H, Westin G, Sandgren J, Stålberg P, Khanafshar E. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer. 2010;17:835–846. doi: 10.1677/ERC-10-0142. [DOI] [PubMed] [Google Scholar]
- 18.Curran S, McKay J, McLeod H, Murray G. Laser capture microscopy. Mol Pathol. 2000;53:64. doi: 10.1136/mp.53.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnichon N, Vescovo L, Amar L, Libé R, de Reynies A, Venisse A, Jouanno E, Laurendeau I, Parfait B, Bertherat J. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20:3974–3985. doi: 10.1093/hmg/ddr324. [DOI] [PubMed] [Google Scholar]
- 20.Denman SE, McSweeney CS. Methods in gut microbial ecology for ruminants. Springer; 2005. Quantitative (real-time) PCR; pp. 105–115. [Google Scholar]
- 21.Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med. 2009;266:19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strillacci A, Griffoni C, Sansone P, Paterini P, Piazzi G, Lazzarini G, Spisni E, Pantaleo MA, Biasco G, Tomasi V. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exper Cell Res. 2009;315:1439–1447. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J, Krichevsky AM. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710. doi: 10.18632/oncotarget.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson E, Webb R, Weisbrod A, Bian B, He M, Zhang L, Holloway A, Krishna R, Nilubol N, Pacak K. The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endocr Relat Cancer. 2012;19:157–166. doi: 10.1530/ERC-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JW, Corssmit E. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet. 2012;49:768–776. doi: 10.1136/jmedgenet-2012-101192. [DOI] [PubMed] [Google Scholar]
- 27.Tsang V, Dwight T, Benn DE, Meyer-Rochow GY, Gill A, Sywak M, Sidhu S, Veivers D, Sue C, Robinson BG. Overexpression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr Relat Cancer. 2014;21:415–426. doi: 10.1530/ERC-13-0519. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liang J, Di C, Zhao G, Zhao Y. Identification of miRNAs as potential new biomarkers for nervous system cancer. Tumour Biol. 2014;35:11631–8. doi: 10.1007/s13277-014-2387-x. [DOI] [PubMed] [Google Scholar]
- 29.Senger DR, Van De Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez C, Rohren E, Habra MA, Rich T, Jimenez P, Ayala-Ramirez M, Baudin E. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–371. doi: 10.1007/s11912-013-0320-x. [DOI] [PubMed] [Google Scholar]
- 31.Jyung RW, LeClair EE, Bernat RA, Kang TS, Ung F, McKenna MJ, Tuan RS. Expression of angiogenic growth factors in paragangliomas. Laryngoscope. 2000;110:161–167. doi: 10.1097/00005537-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Kolomecki K, Stepien H, Bartos M, Kuzdak K. Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr Regul. 2001;35:9–16. [PubMed] [Google Scholar]
- 33.Hobert JA, Mester JL, Moline J, Eng C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet Med. 2012;14:616–619. doi: 10.1038/gim.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]


