Abstract
CD317 was first identified as a multiple myeloma-associated antigen. Here we report the expression of CD317 in normal B cells and B-cell malignancies. In normal bone marrow, CD317 demonstrates a biphasic expression pattern, with higher expression on stage 1 and stage 3 hematogones, but not on stage 2 hematogones. CD317 is over-expressed in B-cell chronic lymphocytic leukemia, and appears associated with negative CD38 expression. Moreover, CD317 is barely detectable in B-cell acute lymphoblastic leukemia. Our results suggest that CD317 expression might be of prognostic significance for B-CLL, and CD317 could be used as a new marker for minimal residual disease detection in B-ALL.
Keywords: CD317, B lymphocytes, chronic lymphocytic leukemia, acute lymphoblastic leukemia
Introduction
CD317, or Tetherin/BST2/HA1.24, is a lipid raft-associated protein with a N-terminal transmembrane domain and a C-terminal glucosyl-phosphatidylinositol (GPI) anchor. The unique extracellular “loop” structure localizes CD317 on the outer surface of cell membrane. CD317 also has a short cytoplasmic tail on its N-terminus. Both the extracellular loop and cytoplamic tail may be important for its regulation and functions. [1]. Accordingly, CD317 appears to be a molecule with multiple activities. CD317 has been proved to regulate the release of several viruses from infected cells [2-5]. Another proposed function of CD317 is to bind Ig-like transcript-7 (ILT7), negatively regulating the Fcε RIγ signaling pathway, and inhibiting TLR-7/9 induced cytokine responses [6]. Importantly, CD317 has been reported to activate NFκB pathway [7,8].
CD317 was originally identified as a multiple myeloma antigen and is highly expressed on terminally differentiated human B cells [9-12]. CD317 has been proved to be an effective target for multiple myeloma immunotherapy [13-18]. CD317 can induce antibody dependent cell-mediated cytotoxicity (ADCC) and cytotoxic T cell responses. Anti-CD317 antibody has been shown to effectively inhibit multiple myeloma growth in vivo and in vitro. Additionally, CD317 was reported to be expressed on bone marrow stromal cells, and at least one study indicated that it is capable of supporting Pre-B cell growth [10]. CD317 over-expression was also detected on solid tumors derived from various organs, including breast, lung, and kidney [19-21]. Although widely recognized as a multiple myeloma antigen, the exact role of CD317 in the pathogenesis of multiple myeloma is still unknown. Similarly, the expression profiles of CD317 on hematopoietic cells, and its potential diagnostic value on human lymphoid malignancies, have not been adequately characterized.
Some authors reported that CD317 expression was restricted on terminally differentiated B cells [9], whereas others suggested relatively broad expression, although the pattern is not well-defined [22]. We are especially interested in the expression of CD317 in B-lymphocytes of various maturing stages, and whether CD317 has potential diagnostic, prognostic, or therapeutic values on common lymphoid malignancies, like B-cell chronic lymphocytic leukemia (B-CLL) and B-cell acute lymphoblastic leukemia (B-ALL). Of note, B-CLL is the most common adult leukemia in the western world, and therapies are largely based on several prognostic factors, including CD38, ZAP70, IgH mutation status, and cytogenetic abnormalities; B-ALL is a leukemia predominantly affecting children and minimal residual disease (MRD) detection remains a challenging issue for the management of B-ALL.
In this paper, we studied the expression of CD317 on normal bone marrow hematogones and mature B cells, and investigated its potential values on the management of common lymphoid malignancies. Our results demonstrated that human stage 1 and stage 3 hematogones express higher level of CD317, but stage 2 hematogones do not; CD317 is over-expressed by majority of CLL cases and associated with negative CD38, thus may be of prognostic significance; CD317 is barely detectable in B-ALL and could be useful for MRD detection.
Materials and methods
Patients
29 B-CLL patients and 8 B-ALL patients seen at the University Hospitals Case Medical Center, Cleveland, Ohio, from November 2010 to September 2012, were studied. The clinical and laboratory information were retrieved from the electronic medical record. For B-CLL cases, we included the age, sex, cytogenetics, absolute lymphocyte count, as well as CD38 and ZAP-70 expression in our analysis (Figure 3; Tables 1, 2). While in Table 3, we reported the age, sex, cytogenetics, and immunophenotype for B-ALL cases. Bone marrow or peripheral blood specimens were selected based on morphologic and phenotypic analysis and procured after all clinical evaluation has been completed. Discarded “normal” bone marrow (negative lymphoma staging or metastatic carcinoma screening) was procured. All studies with human material involved were carried out following an approved Internal Review Board protocol (04-95-91).
Figure 3.
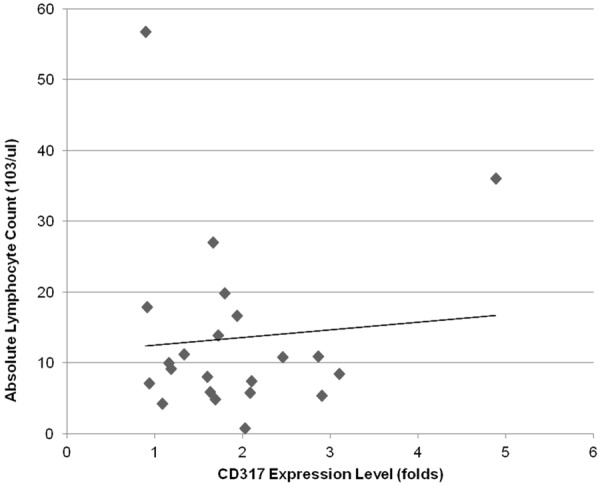
Linear regression analysis of absolute lymphocyte count and CD317 expression on B-CLL cells. Absolute lymphocyte counts (ANC) of 29 CLL patients (103/ul) were plotted against CD317 expression levels (folds). The correlation of ANC and CD317 over-expression was analyzed by linear regression.
Table 1.
Correlation of CD317 and CD38 expression in CLL
| CD317+ | CD317- | Total | |
|---|---|---|---|
| CD38+ | 5 (56%) | 4 (44%) | 9 |
| CD38- | 10 (100%) | 0 (0%) | 10 |
| Total | 15 | 4 | 19 |
Fisher’s exact test: P = 0.0325.
Table 2.
Correlation of CD317 and ZAP-70 expression in CLL
| CD317+ | CD317- | Total | |
|---|---|---|---|
| ZAP70+ | 5 (71%) | 2 (29%) | 7 |
| ZAP70- | 10 (67%) | 5 (33%) | 15 |
| Total | 15 | 7 | 22 |
Fisher’s exact test: P = 1.0000.
Table 3.
Sex, age, cytogenetics and immunophenotype of 8 all cases
| Case | Sex | Age | Cytogenetics | Phenotype |
|---|---|---|---|---|
| 1 | M | 5 | Amplification of AML1 (RUNX1) | CD10+, CD19+, CD20-, CD34+ (partial) |
| 2 | F | 4 | Amplification of AML1 (RUNX1) | CD10+, CD19+, CD20-, CD34+ |
| 3 | M | 48 | t(4;11)(q21;q23),add(9)(q34) | CD10-, CD19+, CD20-, CD34+ (partial) |
| 4 | M | 5 | t(12;21)(p13;q22) | CD10+, CD19+, CD20-, CD34+ (partial) |
| 5 | M | 1.8 | Normal | CD10+, D19+, CD20-, CD34- |
| 6 | M | 12 | Hypodiploid | CD10+, CD19+, CD20+ (partial), CD34+ (partial) |
| 7 | F | 54 | t(9;22)(q34;q11.2) | CD10+, CD19+, CD20 dim to negative, CD34+ |
| 8 | F | 3 | t(1;17)(q42;q12) | CD10+, CD19+, CD20+ (partial), CD34+ |
Reagents
Anti-CD317 (BST2/PDCA-1), clone 26F8, is a well-characterized mouse anti-human monoclonal antibody [6,23-25]. PE-conjugated anti-CD317 (26F8) and mouse IgG1κ isotype control were purchased from eBioscience (San Diego, CA). Fluorescent-labeled human CD10, CD19, CD20, CD34, CD45, and CD5 antibodies were purchased from BD Pharmingen.
Flow cytometric analysis
200 ul of bone marrow or peripheral blood sample was stained with anti-CD317 in combination with other antibodies for 20 min at room temperature. After RBC lysis and washing with wash buffer (PBS with 2% BSA), the cells were analyzed on a FACSCanto flow cytometer. At least 1 × 106 events were collected. The data analysis was performed using FCS Express software. For both mouse and human specimens, the specific CD317 staining was determined based on comparison to the isotype control.
Statistical analysis
The differences in mean fluorescence intensities (MFI) of CD317 in B-CLL cells and B-ALL cells, compared to those of normal B cells, were analyzed by Student’s t test. The correlation of ALC and CD317 expression was analyzed by linear regression. The association of CD317 and CD38 or ZAP-70 was analyzed by Fisher’s exact test.
Results
CD317 shows biphasic expression during B cell maturation
CD317 was reported to be expressed on bone marrow stromal cells and terminally diffe-rentiated B cells [9,10], but the expression status on developing B lymphocytes is unclear. To investigate the expression of CD317 on human precursor and mature B cells, we procured healthy human bone marrow, and flow cytometry was used to determine the mean fluorescence intensity (MFI) of CD317 on various subsets of developing B cells. Three “healthy” bone marrows, from negative staging marrow for neuroblastoma, rhabdomyesarcoma, and lymphoma patients, were studied. All three patients demonstrated similar staining patterns of CD317 expression. The flow cytometry dot plots from one patient were shown in Figure 1. Stage 2 hematogones (CD34- CD10+CD19+) express very low level of CD317 (MFI: 2.4), while stage 1 hematogones (CD34+ CD10+CD19+) and stage 3 hematogones (CD34-CD10-CD19+) have higher levels of CD317 (MFI: 15 and 14, respectively). The findings are consistent with our earlier observations that murine CD317 was highly expressed by pro-B cells, then down-regulated to very low level, and markedly up-regulated again in terminally differentiated B cells (unpublished data).
Figure 1.
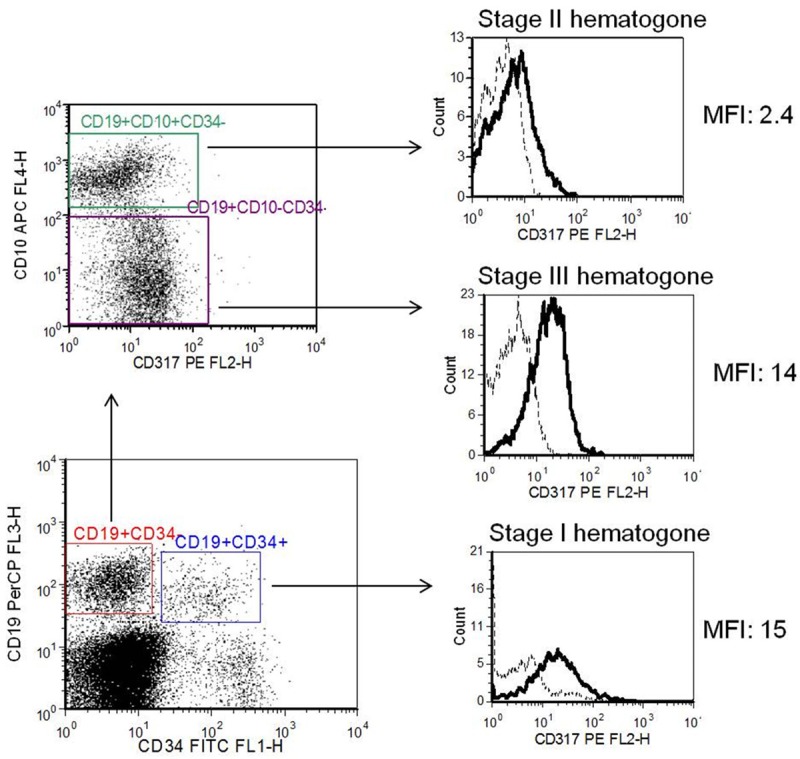
Expression of CD317 on human normal bone marrow hematogones. Bone marrow specimens from 3 healthy individuals were stained with anti-CD317-PE or isotype-PE, and anti- CD34-FITC, anti-CD19-PerCP, anti-CD10-APC. The cells were analysed by flow cytometry. CD317 expression was analyzed by gating on: stage 1 hematogones (CD34+CD10+CD19+), stage 2 hematogones (CD34-CD10+CD19+), and stage 3 hematogones (CD34-CD10-CD19+). The dotted line and solid line in the histograms represent isotype control and CD317 staining, respectively. Results from one representative patient were shown.
Over-expression of CD317 in B-cell chronic lymphocytic leukemia
Because CD317 is significantly up-regulated on multiple myeloma compared to normal plasma cells [11], and several solid tumors also over-express CD317 [19-21], we asked whether tumor cells in B-CLL also over-express CD317. Compared to the normal peripheral blood internal control B cells, B-CLL cells demonstrate increased expression of CD317 (MFI: 54.3±13.7 vs. MFI of normal B cells: 29.8±9.4, n = 29). To decide whether CD317 over-expression is a common feature of B-CLL cells, 29 B-CLL patients were evaluated. Twenty-one of them (72%) had detectable over-expression of CD317 (MFI increased 50% or higher, compared to that of normal B cells). The over-expression of CD317 in B-CLL was statistically significant. The MFI increase in B-CLL was 2.1±1.2 fold (P < 0.001, Student’s t-test), compared to normal B cells (Figure 2).
Figure 2.
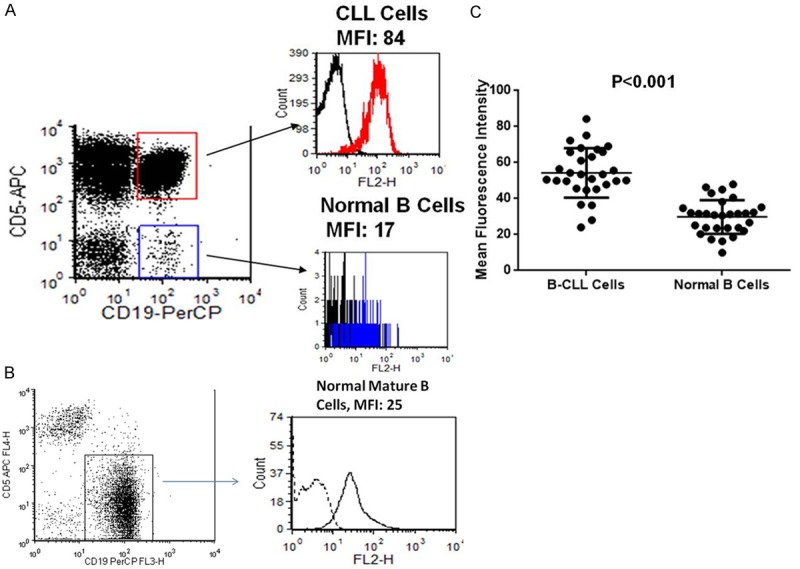
CD317 is over-expressed on B-CLL cells. A. Peripheral blood specimens from 29 B-CLL patients were stained with anti-CD317-PE or isotype-PE, and anti-CD19-PerCP, anti-CD5-APC. The cells were analysed by flow cytometry. CD317 expression was analyzed by gating on: B-CLL cells (CD19+CD5+) and internal control normal B cells (CD19+CD5-). The black line and red/blue lines in the histograms represent isotype control and CD317 staining, respectively. Results from one representative patient were shown. B. Peripheral blood of a healthy individual was stained and analyzed as in A. C. The mean fluorescence intensities (MFIs) of CD317 on B-CLL cells and normal B cells from 29 B-CLL patients were plotted and shown. Student’s t-test was used to determine the statistical significance of CD317 over-expression on B-CLL cells, in comparison with normal B cells.
Next, we analyzed whether CD317 over-expression is associated with absolute lymphocyte count (ALC), CD38 and ZAP-70 expression. CD38 or ZAP-70 positivity was defined as MFI ≥ 25 or ≥ 20, respectively. As shown in Figure 3, CD317 over-expression is not related to ALC by linear regression analysis (r = 0.0795, P = 0.72). Impressively, CD317 over-expression is significantly associated with negative CD38 expression (Table 1, P < 0.05 by Fisher’s exact test). Among 19 patients who had both CD38 and CD317 tested, all 4 patients without CD317 over-expression were positive for CD38, but only 33% (5/15) of patients with CD317 over-expression were positive for CD38. On the other hand, all 10 CD38 negative patients were positive for CD317, but only 56% (5 of 9) of CD38 positive patients were positive for CD317. As shown in Table 2, CD317 over-expression is not related to ZAP-70 expression status (P = 1.0000 by Fisher’s exact test).
Reduced CD317 expression in B-cell acute lymphoblastic leukemia
As CD317 is expressed on human precursor B cells at variable levels, we asked whether it has an altered pattern in B-cell acute lymphoblastic leukemia. Eight B-ALL patients were evaluated in this study. Surprisingly, compared to normal B-precursors, the leukemia blasts of B-ALL had reduced expression of CD317 (MFI: 2.7±2.1 vs. MFI of normal B precursors: 22.0±12.8, n = 8). Compared to normal B cell precursors, the average MFI change of CD317 expression in leukemic blasts was -11.4±6.2 fold (P < 0.01, Student’s t-test, Figure 4). The down-regulation of CD317 appears universal, as all 8 ALL patients we tested, irrelevant of age, sex, cytogenetics and immunophenotype of ALL cells, showed reduction of CD317 expression levels (Table 3).
Figure 4.
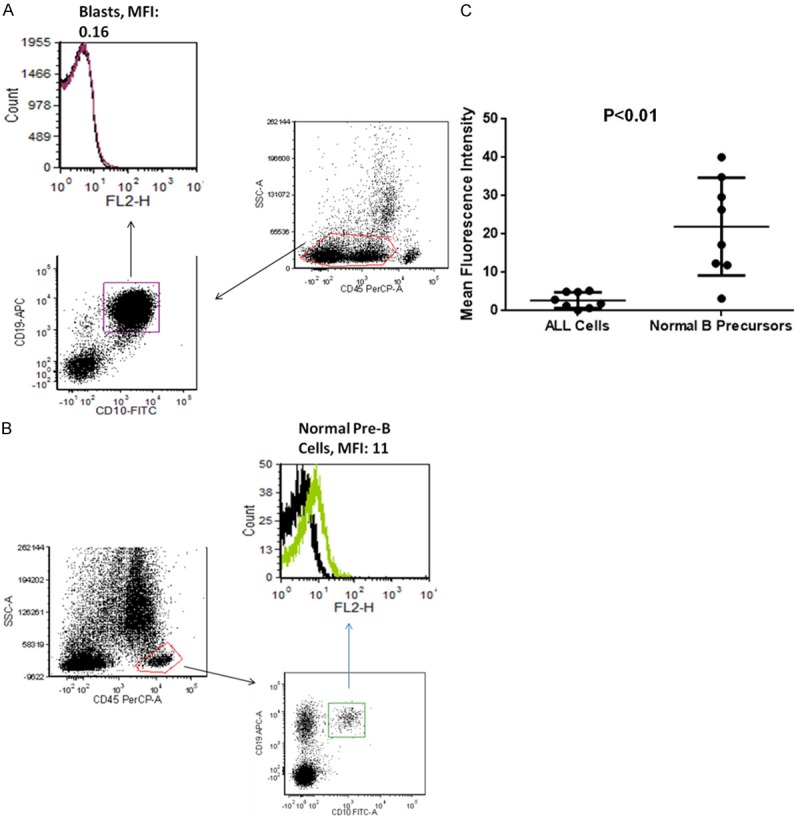
Reduced expression of CD317 in B-ALL. A. Bone marrow specimens from 8 B-ALL patients and 8 healthy individuals were stained with anti-CD317-PE or isotype-PE, and anti-CD10-FITC, anti-CD45-PerCP, anti-CD19-APC. The cells were analysed by flow cytometry. CD317 expression was analyzed by gating on: leukemic blasts (CD45loCD10+CD19+), normal B cells (CD45hiCD19+ CD10+ or -). The black line and red/green lines in the histograms represent isotype control and CD317 staining, respectively. Results from one representative patient were shown. B. Bone marrow from a healthy individual was stained and analyzed as in A. C. The mean fluorescence intensities (MFIs) of CD317 on B-ALL cells from 8 B-ALL patients and normal B cell counterparts (with the same immunophenotype as B-ALL cells) from 8 healthy individuals were plotted and shown. Student’s t-test was used to determine the statistical significance of reduced CD317 expression on B-ALL cells, in comparison with normal B cell counterparts.
Discussion
CD317 is reportedly expressed constitutively at high level on terminally differentiated B cells [9] and Type I IFN-producing cells (plasmacytoid dendritic cells) [26]. Multiple cell lines dramatically up-regulate CD317 following viral infection [3,27]. However, CD317 is only moderately expressed on other immune cells, including T cells, B cells, NK cells and NK T cells [22]. The expression status of CD317 on bone marrow developing B cells is unclear. In this paper, we found, unexpectedly, that CD317 had higher expression on stage 1 hematogones, but barely detectable expression on stage 2 hematogones, and the expression level went up again on stage 3 hematogones. The expression profile is similar to our earlier observation on murine bone marrow B cells. The higher level expression of CD317 on stage 1 hematogones suggests that it may be involved in lymphopoiesis. However, only very limited data supports such a role [10]. CD317 knockout mice appear to have largely normal development of immune system [28].
B cell chronic lymphocytic leukemia (B-CLL) is the most common adult leukemia in the western world. Although indolent in most cases, B-CLL is incurable. Many patients progress to higher grade lymphomas which are often fatal [29]. The prognosis of B-CLL is based on cytogenetic features and expression of several markers like CD38 [30-33] and ZAP-70 [34,35]. The patients with indolent disease and relatively good prognosis are usually put on watchful waiting. CLL is only treated when presenting with advanced diseases [36]. Currently, the standard treatments of CLL include chemotherapeutic drugs like cyclophosphamide, and monoclonal antibodies against common B-cell markers, like Rituximab (anti-CD20) and Campath (anti-CD52) [37]. In this study, we found that most of CLL patients over-express CD317 on their leukemic cells. Importantly, CD317 over-expression is significantly associated with CD38 negativity. On the contrary, ZAP-70 appears not related to CD317 over-expression. Because negative CD38 is generally considered a better prognostic factor, it would be interesting to study whether CD317 over-expressed CLL patients have slower disease progression and better prognosis. To our best knowledge, neither the expression of CD317 in B-CLL, nor the biological significance of CD317 over-expression, has been reported.
B-cell acute lymphoblastic leukemia (B-ALL) is a neoplasm of B cell precursors (lymphoblasts). In the United States, around 3,000 new B-ALL cases are diagnosed each year. 75% of B-ALL cases occur in children under six years of age [29]. With modern chemotherapy, pediatric patients can easily achieve higher than 85% cure rate. For adults, the overall survival rate after chemotherapy is about 40% [38]. B-ALL tumor cells generally express marker molecules similar to their normal progenitor lymphocyte counterparts. Minimal residual disease (MRD) is of great prognostic significance in B-ALL and currently only a limited number of antigenic aberrations have been described to aid in MRD detection [29]. In this paper, we demonstrated that CD317 expression is reduced in the leukemic blasts from all 8 B-ALL patients we tested. In another study, the authors studied a series of potential molecules that may be significant for B-ALL disease monitoring. They also found that CD317 appears significantly reduced in leukemic blasts, compared to their normal counterparts [39]. Therefore, there is great potential that CD317 could be used for minimal residual disease (MRD) testing in B-ALL.
Additionally, the over-expression of CD317 in B-CLL, but not B-ALL, suggests that CD317 may play different roles in the pathogenesis of these two forms of leukemia. Further investigation of the biology of CD317 and the mechanisms how CD317 is involved in lymphoid malignancy may add knowledge to our understanding of the immune system and provide new insights into the biology of B-cell CLL and ALL.
Acknowledgements
This work was supported by the Case Western Reserve University Department of Pathology intramural research fund (2010-04, to S.G.), and National Institutes of Health, USA (AI-050059 to YHC). Contributions: S.G. and H.M. designed the study; S.G., E.S.O. performed the experiments; D.K. and Y.H.C provided important conceptual contribution to the study; S.G. analyzed the data and drafted the manuscript; H.M. critically revised the manuscript; all authors reviewed the manuscript and approved the final version.
Disclosure of conflict of interest
None.
References
- 1.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 2.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 4.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol. 2012;87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto T, Kennel SJ, Abe M, Takishita M, Kosaka M, Solomon A, Saito S. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1994;84:1922–1930. [PubMed] [Google Scholar]
- 10.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazawa J, Oritani K, Itoh M, Ochi T, Ishihara K, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki S, Kosaka M, Wakatsuki S, Abe M, Koishihara Y, Matsumoto T. Immunotherapy of multiple myeloma with a monoclonal antibody directed against a plasma cell-specific antigen, HM1.24. Blood. 1997;90:3179–3186. [PubMed] [Google Scholar]
- 12.Ohtomo T, Sugamata Y, Ozaki Y, Ono K, Yoshimura Y, Kawai S, Koishihara Y, Ozaki S, Kosaka M, Hirano T, Tsuchiya M. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki S, Kosaka M, Wakahara Y, Ozaki Y, Tsuchiya M, Koishihara Y, Goto T, Matsumoto T. Humanized anti-HM1.24 antibody mediates myeloma cell cytotoxicity that is enhanced by cytokine stimulation of effector cells. Blood. 1999;93:3922–3930. [PubMed] [Google Scholar]
- 14.Ono K, Ohtomo T, Yoshida K, Yoshimura Y, Kawai S, Koishihara Y, Ozaki S, Kosaka M, Tsuchiya M. The humanized anti-HM1.24 antibody effectively kills multiple myeloma cells by human effector cell-mediated cytotoxicity. Mol Immunol. 1999;36:387–395. doi: 10.1016/s0161-5890(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 15.Becker M, Sommer A, Kratzschmar JR, Seidel H, Pohlenz HD, Fichtner I. Distinct gene expression patterns in a tamoxifen-sensitive human mammary carcinoma xenograft and its tamoxifen-resistant subline MaCa 3366/TAM. Mol Cancer Ther. 2005;4:151–168. [PubMed] [Google Scholar]
- 16.Ge Y, Dombkowski AA, LaFiura KM, Tatman D, Yedidi RS, Stout ML, Buck SA, Massey G, Becton DL, Weinstein HJ, Ravindranath Y, Matherly LH, Taub JW. Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006;107:1570–1581. doi: 10.1182/blood-2005-06-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rew SB, Peggs K, Sanjuan I, Pizzey AR, Koishihara Y, Kawai S, Kosaka M, Ozaki S, Chain B, Yong KL. Generation of potent antitumor CTL from patients with multiple myeloma directed against HM1.24. Clin Cancer Res. 2005;11:3377–3384. doi: 10.1158/1078-0432.CCR-04-0650. [DOI] [PubMed] [Google Scholar]
- 18.Jalili A, Ozaki S, Hara T, Shibata H, Hashimoto T, Abe M, Nishioka Y, Matsumoto T. Induction of HM1.24 peptide-specific cytotoxic T lymphocytes by using peripheral-blood stem-cell harvests in patients with multiple myeloma. Blood. 2005;106:3538–3545. doi: 10.1182/blood-2005-04-1438. [DOI] [PubMed] [Google Scholar]
- 19.Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W, Yuan Z. Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer. 2009;9:102. doi: 10.1186/1471-2407-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Nishioka Y, Ozaki S, Jalili A, Abe S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T, Sone S. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother. 2009;58:967–976. doi: 10.1007/s00262-008-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai S, Azuma Y, Fujii E, Furugaki K, Ozaki S, Matsumoto T, Kosaka M, Yamada-Okabe H. Interferon-alpha enhances CD317 expression and the antitumor activity of anti-CD317 monoclonal antibody in renal cell carcinoma xenograft models. Cancer Sci. 2008;99:2461–2466. doi: 10.1111/j.1349-7006.2008.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal-Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell Immunol. 2005;236:6–16. doi: 10.1016/j.cellimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Tavano B, Galao RP, Graham DR, Neil SJ, Aquino VN, Fuchs D, Boasso A. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes. J Immunol. 2013;190:2622–2630. doi: 10.4049/jimmunol.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staudinger M, Glorius P, Burger R, Kellner C, Klausz K, Gunther A, Repp R, Klapper W, Gramatzki M, Peipp M. The novel immunotoxin HM1.24-ETA’ induces apoptosis in multiple myeloma cells. Blood Cancer J. 2014;4:e219. doi: 10.1038/bcj.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci U S A. 2011;108:13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 27.Miyagi E, Andrew AJ, Kao S, Strebel K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci U S A. 2009;106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108:18097–18101. doi: 10.1073/pnas.1113694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bain BJ. Chichester, West Sussex. Hoboken, NJ: Wiley-Blackwell; 2010. Leukaemia diagnosis. [Google Scholar]
- 30.D’Arena G, Musto P, Cascavilla N, Dell'Olio M, Di Renzo N, Perla G, Savino L, Carotenuto M. CD38 expression correlates with adverse biological features and predicts poor clinical outcome in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2001;42:109–114. doi: 10.3109/10428190109097682. [DOI] [PubMed] [Google Scholar]
- 31.Durig J, Naschar M, Schmucker U, Renzing-Kohler K, Holter T, Huttmann A, Duhrsen U. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia. 2002;16:30–35. doi: 10.1038/sj.leu.2402339. [DOI] [PubMed] [Google Scholar]
- 32.Eisele L, Haddad T, Sellmann L, Duhrsen U, Durig J. Expression levels of CD38 on leukemic B cells but not on non-leukemic T cells are comparably stable over time and predict the course of disease in patients with chronic lymphocytic leukemia. Leuk Res. 2009;33:775–778. doi: 10.1016/j.leukres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Morabito F, Mangiola M, Oliva B, Stelitano C, Callea V, Deaglio S, Iacopino P, Brugiatelli M, Malavasi F. Peripheral blood CD38 expression predicts survival in B-cell chronic lymphocytic leukemia. Leuk Res. 2001;25:927–932. doi: 10.1016/s0145-2126(01)00049-2. [DOI] [PubMed] [Google Scholar]
- 34.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez-Guillermo A, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 35.Durig J, Nuckel H, Cremer M, Fuhrer A, Halfmeyer K, Fandrey J, Moroy T, Klein-Hitpass L, Duhrsen U. ZAP-70 expression is a prognostic factor in chronic lymphocytic leukemia. Leukemia. 2003;17:2426–2434. doi: 10.1038/sj.leu.2403147. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien S, Vose JM, Kantarjian H. Management of hematologic malignancies. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 37.Abrisqueta P, Villamor N, Terol MJ, Gonzalez-Barca E, Gonzalez M, Ferra C, Abella E, Delgado J, Garcia-Marco JA, Gonzalez Y, Carbonell F, Ferrer S, Monzo E, Jarque I, Muntanola A, Constants M, Escoda L, Calvo X, Bobillo S, Montoro JB, Montserrat E, Bosch F. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122:3951–9. doi: 10.1182/blood-2013-05-502773. [DOI] [PubMed] [Google Scholar]
- 38.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, Chopra R, Wiernik PH, Foroni L, Paietta E, Litzow MR, Marks DI, Durrant J, McMillan A, Franklin IM, Luger S, Ciobanu N, Rowe JM. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 39.Mirkowska P, Hofmann A, Sedek L, Slamova L, Mejstrikova E, Szczepanski T, Schmitz M, Cario G, Stanulla M, Schrappe M, van der Velden VH, Bornhauser BC, Wollscheid B, Bourquin JP. Leukemia surfaceome analysis reveals new disease-associated features. Blood. 2013;121:e149–159. doi: 10.1182/blood-2012-11-468702. [DOI] [PubMed] [Google Scholar]


