Abstract
Reported herein is a renal anastomosing hemangioma which developed slowly in the past four years. A 25-year-old woman was found a mass localized in the upper portion four years ago, and only slow progression in the past four years. She underwent a laparoscopic partial nephrectomy of right kidney and diagnosed as anastomosing hemangioma. On histology the vascular components of the tumor had an anastomosing pattern without well-definite margins. Immunohistochemically, only endothelial markers (CD31, CD34) were expressed on the vascular components of tumor cells. Smooth muscle actin (SMA), cytokeratin (CK), EMA and S-100 and so on were all negative in the epithelioid tumor cells. The patient was alive at 16 months after operation, without any evidence recurrence or metastasis. Anastomosing hemangioma is an extremely rare vascular neoplasm; only 23 cases were previously described until now. Our report of anastomosing hemangioma arising from the kidney with slow progression will improve the knowledge of primary vascular tumors arising in the kidney.
Keywords: Anastomosing hemangioma, rare tumors, progression, kidney
Introduction
Primary renal anastomosing hemangioma is a vascular neoplasm with a malignant mimicking pathological feature, which was included in the renal capillary haemangioma group [1,2]. Anastomosing hemangioma is an extremely rare neoplasm in kidney; only 23 cases have been previously described since the first report from Montgomery and Epstein in 2009 [2-10]. Microscopically, the typical pathological image of this tumor is sinusoidal anastomosing capillary-sized vessels with biological features of infiltrative growth. The malignancy-mimic lesions must be avoided to be over-diagnosed as a malignance of angiosarcoma [2,3,9,10]. The differential diagnosis is of great clinical importance for surgical procedure selection and maximal preservation of renal function. It was suggested that anastomosing hemangioma must be concerned in the capillary-sized vessel lesions with malignant mimicking features [3,7]. We h erein report a case of anastomosing hemangioma affecting the right kidney of a 29-year-old female. Slow progression was observed in the past four years, which could help us to understand the biological potential of anastomosing hemangioma.
Case presentation
A 29-year-old female with four-year history of an impalpable mass in the right kidney was admitted to our hospital for a fellow up examination. She was found with a 1.5 cm × 1.5 cm space occupying lesion in right kidney during a routine medical examination four years ago, but all laboratory results were within the normal levels. So no special treatment was conducted since then, but routine examination was performed every year. In this routine screen, ultrasound examination of the right kidney revealed a 2.0 cm × 1.7 cm hyperechoic mass occupying the upper portion of right kidney with a clear border. No rich blood flowing signal was detected by color Doppler flow imaging (CDFI). No abnormal echo was found in the left kidney. Additional physical investigations here were unremarkable. Specialist examination of the kidney region found no bulge in both kidneys, no tenderness in bilateral rib ridges, rib waist points or ureteral zones. All laboratory results, including routine blood chemistry, complete blood count, kidney and liver function tests and urine analysis, were still all within normal limits. Nothing abnormal was found in chest radiograph. A laparoscopic partial nephrectomy of the right kidney was performed, in case of the risk of neoplasm progression.
Pathologic findings
Grossly, the partial resected kidney specimen showed an uncapsulated round mass measuring 1.2 × 1.2 × 1.0 cm. The cut surface of tumor was fleshy, mahogany brown with a spongy texture, which abutting with invasion into the renal capsule. Microscopically, the tumor was located on the renal parenchyma. The borderline between the tumor and the adjacent kidney tissue was not well-defined. At higher power, the tumor cells were arranged into an anastomosing sinusoidal architecture with tightly packed capillary-sized blood vessels (Figure 1A). Various numbers of blood cells existed in the anastomosing vessels and different sized vascular compartments. Some vessels were lined by hobnail endothelial cells. The simple cuboidal epithelial cells were characterized with scarce cytoplasm, small and round nucleus and hyperchromatic nucleus (Figure 1B). The intensive short-spindled cells were also seen inside of the vessels. Similar morphology of tumor cells were shown without marked nuclear atypia, multi-layering or mitotic and apoptotic activity; only slightly enlarged nucleolus was observed. In particular, tumor cells showed an infiltrative growth pattern. Moderate amount of stromal hemorrhage and few vascular thrombi were distributed in tumor tissue. No indication of intracytoplasmic hyaline globules and extramedullary hematopoiesis was found from the histopathologic image. A few lymphocytes were scattered but no plasma cells or acute inflammation cells was found. A small amount of residual renal tubules was seen to distribute peripherally (Figure 1A).
Figure 1.
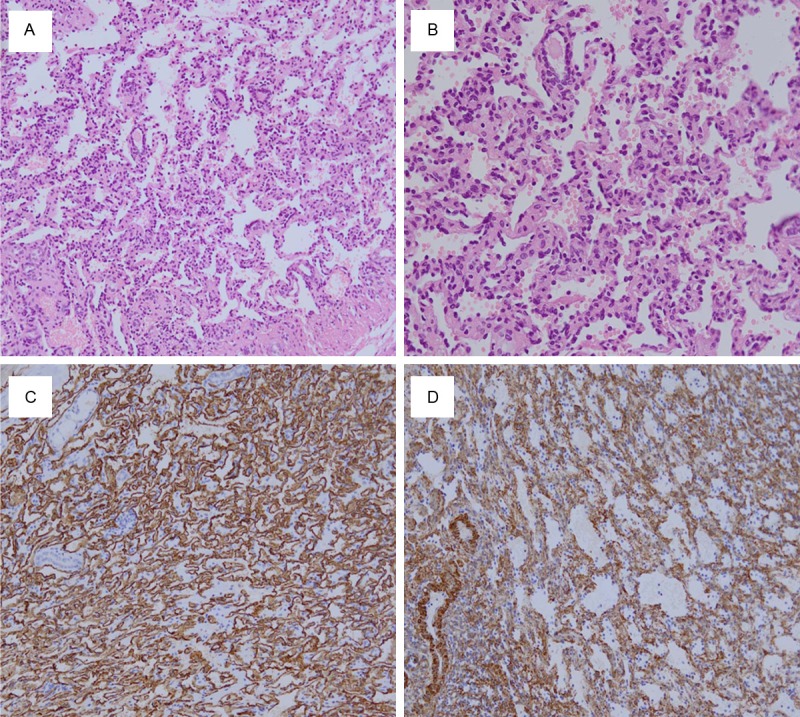
Histopathological observation of renal anastomosing hemangioma. A: Histological evaluation showed tumor tissues were composed of sinusoidal anastomosing capillaries, similar to the red pulp of spleen. Residual renal tubular can be seen in the periphery of the lesion; B: The endothelial cells lined in the vessels were simple cuboidal in shape. Some cells showed hobnail changes but without significant cellular atypia; C: Immunohistochemistry: tumor cells showed diffuse strong positive for CD34; D: Immunohistochemistry: intravascular stromal cells showed strongly positive for SMA.
Immunohistochemical analysis revealed that the endothelial cells stained diffusely positively with endothelial markers, including CD34 (Figure 1C), CD31 and vimentin, negative for SMA (Figure 1D), EMA, HMB45, desmin, Ckpan, CK7, CA9, CD10, D2-40, p504s or S100 protein. The supporting stromal cells were positive for SMA and negative for desmin, CD31 or CD34. EMA highlighted the residual renal tubules. The histopathologic appearance together with the immunophenotypic feature of this tumor was indicated of a diagnosis of anastomosing hemangioma of the kidney. Patient had followed up after surgery for 16 months and is still alive.
Discussion
Renal hemangioma is a rarely occurred benign vascular tumor, which is usually classified into two main types, capillary hemangioma and cavernous hemangioma. Anastomosing hemangioma fits into the capillary hemangioma with a special sinusoidal histological pattern reminiscent of splenic parenchyma. Totally 24 histologically confirmed renal anastomosing hemangiomas have been reported since the first report in 2009 [2], including our report here. The clinicopathologic characteristics of all published cases of this kind of lesion are summarized in Table 1.
Table 1.
Summary of the reported cases of renal anastomosing hemangioma
| Ref/year | No. | Age (year)/gender | Clinical Manifestation | Site/Lateral | Operation | Tumor Size (cm) | Fellow-up (months) |
|---|---|---|---|---|---|---|---|
| Montgomery, E. et al. 6/2009 | 1 | 74/M | Intermittent hematuria | NA | Nephrectomy | 1.5 | NED (36) |
| 2 | 75/M | Intermittent hematuria | Kidney in adipose tissue near ureter | Nephrectomy | 2.0 | NA | |
| 3 | 65/F | Vague abdominal pain | Perinephric adipose tissue | Excision of lesion | 2.0 | NED (8) | |
| 4 | 49/M | NA | Renal hilum | Nephrectomy | 1.3 | NED (12) | |
| Brown, J. G. et al. 3/2010 | 5 | 56/M | NA | Right | Partial nephrectomy | 1.3 | NA |
| 6 | 33/F | NA | Left, upper pole | Nephrectomy | 3.2 | NA | |
| 7 | 21/M | ESRD, post transplant, incidental | Right | Nephrectomy | 2.2 | NED (24) | |
| 8 | 44/F | Left | Nephrectomy | 2.0 | NED (72) | ||
| 9 | 83/F | Left | Nephrectomy | 3.5 | NED (24) | ||
| Kryvenko, O. N. et al. 7/2011 | 10 | 51/F | ESRD, post transplant, incidental | Right, hilum | Nephrectomy | 1.0 | NED (7) |
| 11 | 39/M | Chronic polycythemia, incidental | Right | Nephrectomy | 5.0 | NED (122) | |
| 12 | 67/F | Pulmonary embolism, previous knee replacement, incidental | Left, perinephric | Nephrectomy | 1.2 | NED (6) | |
| 13 | 54/F | ESRD, post transplant, incidental | Right | Nephrectomy | 1.2 | NED (3) | |
| 14 | Left | Nephrectomy | 0.6 | NED (3) | |||
| Mehta, V. et al. 4/2012 | 15 | 49/M | ESRD | NA | Nephrectomy | 2.0 | NED (3) |
| 16 | 55/M | ESRD, papillary adenoma | NA | Nephrectomy | 0.6 | NED (3) | |
| 17 | 45/M | ESRD | NA | Nephrectomy | 1.9 | NED (12) | |
| Zhao M. et al. 6/2013 | 18 | 48/M | TACE for HCC, incidental | Right, upper pole | Partial nephrectomy | 2.5 | NED (12) |
| Wetherell, D. R. et al. 8/2013 | 19 | 74/M | Lower urinary tract symptoms | Right, upper pole | Nephrectomy | 5.0 | DUD (1) |
| Chou S. et al 6/2013 | 20 | 50/F | ESRD, incidental | Left, renal sinus | Nephrectomy | 1.0 | NED (14) |
| 21 | 60/M | ESRD, post transplant, incidental | Left, mid to upper pole | Nephrectomy | 0.5-1.8 | NED (8) | |
| Heidegger I. et al. 8/2014 | 22 | 56/M | Febrile prostatitis | Right | Nephrectomy | 5.0 | NED (156) |
| Tao LL. et al. 8/2014 | 23 | 32/F | Routine screening | Left | Nephrectomy | 2.6 | NED (21) |
| Here | 24 | 29/F | Follew up examination | Right | Partial nephrectomy | 1.2 | NED (16) |
Anastomosing hemangioma most commonly affect middle aged adults, with an average age of 52.6 years old (ranging 21-83). Totally 23 patients are included because one with bilateral lesions [5]. There is no significant sex predilection (10 females, 13 males), as well as the location of lesions. The tumor size of the 24 cases ranges from 0.6-5.0 cm (median: 2.0 cm, average: 2.1 cm). Most lesions are asymptomatic and were illustrated incidentally [6,7,9,10]. The current case was detected at the routine screening. Remarkably, eight (34.8%) patients had preexisting end-stage renal failure [3-5,8]. Higher incidence of anastomosing hemangiomas was observed in end stage renal disease (ESRD) [4], which indicated a tendency of anastomosing hemangiomas to develop in ESRD. The symptomatic patients present with typical intermittent hematuria [2], flank pain [2], lower urinary tract symptoms [6]. However, it has not been reported associated with any known syndrome, including Klipple-Trenaunay syndrome, Sturge-Weber syndrome which is found in other systemic angiomatosis [11]. Good prognosis of anastomosing hemangioma was always observed after surgical resection [9]. The followed up information was ranged from 3 to 156 months (average: 26.8 months) and showed no recurrences, metastases or dead from anastomosing hemangioma in all cases, only one case were dead from an unrelated erebrovascular event within one month after operation [6]. Moreover, the clinical observation of the case reported here also supports the benign biological behavior of anastomosing hemangioma. Thus, conservative management such as a partial nephrectomy should be recommended for treatment and the maximal preservation of renal function.
Grossly, a defined borderline is usually found between renal anastomosing hemangioma and adjacent tissue, but majority does not have coating. Moreover, renal capsule abutting is less commonly observed as the case reported here. Histologically, the well-marinated lesions are usually manifested as logarithmic growth pattern. However, focally infiltration was reported in the adipose tissue of the renal pelvis [2] and adrenal gland [4]. Microscopically, the tumor cells were arranged in an irregular loosely lobulated pattern. They exhibited the typical histological features of haemangiomas, i.e, irregular blood-filled vascular spaces lined by a single layer of endothelial cells. It is a common feature of the unique sinusoidal architecture composed anastomosing small-caliber capillaries in the reported cases. Hemorrhage, thrombosis [2,5,6,10] and extra-medullary hemopoiesis [2,4,5] are usually observed in the lesions. The endothelial cells are hobnail-like in shape, which show an intravascular growth pattern [2,5,6,10]. Spindle cells are rich in mesenchymal tissues. They may show an infiltrative growth pattern in the borderline, but lack the mitosis and nuclear pleomorphism as seen in angiosarcomas. Tumor cells express CD31, CD34 and factor VIII-related antigen, and absent for other original antigen, which further confirmed its endothelial origin [2,3,6,10].
Differential diagnosis should be especially concerned on capillary angiosarcoma, which is a rare malignant vascular tumor in kidney [2,4,7]. Angiosarcoma is commonly exhibited as anastomosing capillary network and hobnail endothelial cells. However, the hobnail endothelial cells were observed significantly cellular atypia and mitotic phase, as well as significant infiltrative growth pattern [12-14]. Moreover, cytokeratin expression in some cases will be also helpful for the differential diagnosis [15]. Thus, carefully observation will avoid the misdiagnosis of angiosarcoma in these lesions.
We should also pay attention to the differential diagnosis of the following ones: 1) Capillary hemangioma: no typical anastomosing vessels are observed, lack of stroma but rich in spindle cells [16]. 2) Hemangioblastoma: tumor cells were of abundant cytoplasm. Stromal cells were rich in lipid vacuoles, as well as specifically expression of S100 proteins, NSE and α inhibin in stromal cells by immunohistochemistry will favor for differential diagnosis [17,18]. 3) Glomus tumors: net or oval-shaped tumor cells are patchily distributed around the expanded capillaries, but anastomosing capillaries and hobnail endothelial cells are not seen. Alpha smooth muscle actin (α-SMA) is positive in immunohistochemistry staining [19]. 4) Renal cell carcinoma rich in blood vessels: tumor tissue is consisted with a large number of branch-like capillaries, and small clear cells which are rich in cytoplasm scattered among the vessels. Immunohistochemical positive for Ckpan, EMA, CD10 can be helpful in identification [20]. Taken together, combining histological examination with immunochemical analysis could easily identify anastomosing hemangioma from other tumors.
In conclusion, we reported a case of renal anastomosing hemangioma, a rare occurred variant of a capillary hemangioma in the kidney. It exhibited a slow progression in the past four years. In addition, we summarized all 24 reported such lesions about the clinicopathologic features. These lesions are characterized by anastomosing vessel proliferation, which mimicking the infiltrative progresion of angiosarcoma. As pathologists, accurate diagnosis and distinction from malignant lesions must be aware of during diagnosis with these lesions.
Acknowledgements
This work was supported by research grants from Shandong province science and technology development plan item (2013GSF11866).
Disclosure of conflict of interest
None.
References
- 1.Mallet R, Game X, Lefi M, Mouzin M, Malavaud B, Otal P, Joffre F, Rischmann P. [Conservative management of renal haemangioma: value of a synergistic combination of flexible ureteroscopy and CT angiography] . Prog Urol. 2007;17:108–110. doi: 10.1016/s1166-7087(07)92237-x. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009;33:1364–1369. doi: 10.1097/PAS.0b013e3181ad30a7. [DOI] [PubMed] [Google Scholar]
- 3.Brown JG, Folpe AL, Rao P, Lazar AJ, Paner GP, Gupta R, Parakh R, Cheville JC, Amin MB. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. 2010;34:942–949. doi: 10.1097/PAS.0b013e3181e4f32a. [DOI] [PubMed] [Google Scholar]
- 4.Mehta V, Ananthanarayanan V, Antic T, Krausz T, Milner J, Venkataraman G, Picken MM. Primary benign vascular tumors and tumorlike lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch. 2012;461:669–676. doi: 10.1007/s00428-012-1333-9. [DOI] [PubMed] [Google Scholar]
- 5.Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol. 2011;136:450–457. doi: 10.1309/AJCPJPW34QCQYTMT. [DOI] [PubMed] [Google Scholar]
- 6.Wetherell DR, Skene A, Manya K, Manecksha RP, Chan Y, Bolton DM. Anastomosing haemangioma of the kidney: a rare morphological variant of haemangioma characteristic of genitourinary tract location. Pathology. 2013;45:193–196. doi: 10.1097/PAT.0b013e32835c782b. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Li C, Zheng J, Sun K. Anastomosing hemangioma of the kidney: a case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int J Clin Exp Pathol. 2013;6:757–765. [PMC free article] [PubMed] [Google Scholar]
- 8.Chou S, Subramanian V, Lau HM, Achan A. Renal Anastomosing Hemangiomas With a Diverse Morphologic Spectrum: Report of Two Cases and Review of Literature. Int J Surg Pathol. 2013;22:369–373. doi: 10.1177/1066896913492850. [DOI] [PubMed] [Google Scholar]
- 9.Heidegger I, Pichler R, Schafer G, Zelger B, Zelger B, Aigner F, Bektic J, Horninger W. Long-term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int J Urol. 2014;21:836–838. doi: 10.1111/iju.12433. [DOI] [PubMed] [Google Scholar]
- 10.Tao LL, Dai Y, Yin W, Chen J. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol. 2014;9:159. doi: 10.1186/s13000-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525–1540. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 12.Qayyum S, Parikh JG, Zafar N. Primary renal angiosarcoma with extensive necrosis: a difficult diagnosis. Case Rep Pathol. 2014;2014:416170. doi: 10.1155/2014/416170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HM, Yan Y, Luo M, Xu YF, Peng B, Zheng JH. Primary angiosarcoma of the kidney: case analysis and literature review. Int J Clin Exp Pathol. 2014;7:3555–3562. [PMC free article] [PubMed] [Google Scholar]
- 14.Leggio L, Addolorato G, Abenavoli L, Ferrulli A, D’Angelo C, Mirijello A, Vonghia L, Schinzari G, Arena V, Perrone L, Citterio F, Bonomo L, Rapaccini GL, Capelli A, Barone C, Gasbarrini G. Primary renal angiosarcoma: a rare malignancy. A case report and review of the literature. Urol Oncol. 2006;24:307–312. doi: 10.1016/j.urolonc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga M. Angiosarcoma of the kidney with minute clear cell carcinomas: a case report. Pathol Res Pract. 2009;205:347–351. doi: 10.1016/j.prp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Fellegara G, Rosai J. Multifocal capillary hemangioma-like vascular proliferation of the kidney associated with clear cell renal cell carcinoma: a case report and review of the literature. Int J Surg Pathol. 2013;21:424–426. doi: 10.1177/1066896912474340. [DOI] [PubMed] [Google Scholar]
- 17.Jiang JG, Rao Q, Xia QY, Tu P, Lu ZF, Shen Q, Zhang RS, Yu B, Zhou XJ, Shi SS, Shi QL. Sporadic hemangioblastoma of the kidney with PAX2 and focal CD10 expression: report of a case. Int J Clin Exp Pathol. 2013;6:1953–1956. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Williamson SR, Yu J, Xia W, Li C, Zheng J, Zhu Y, Sun K, Wang Z, Cheng L. PAX8 expression in sporadic hemangioblastoma of the kidney supports a primary renal cell lineage: implications for differential diagnosis. Hum Pathol. 2013;44:2247–2255. doi: 10.1016/j.humpath.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Lamba G, Rafiyath SM, Kaur H, Khan S, Singh P, Hamilton AM, Ang DC. Malignant glomus tumor of kidney: the first reported case and review of literature. Hum Pathol. 2011;42:1200–1203. doi: 10.1016/j.humpath.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Kryvenko ON, Roquero L, Gupta NS, Lee MW, Epstein JI. Low-grade clear cell renal cell carcinoma mimicking hemangioma of the kidney: a series of 4 cases. Arch Pathol Lab Med. 2013;137:251–254. doi: 10.5858/arpa.2011-0615-OA. [DOI] [PubMed] [Google Scholar]


