Abstract
Background: To investigate whether hyperbaric oxygen (HBO) intervention affects the expressions of inflammatory cytokines, HMGB1/TLR4/NF-κB, and arrests secondary spinal cord injury (SCI). Methods: One hundred and twenty healthy adult SD rats were randomly divided into four groups: sham, sham + HBO, SCI, and SCI + HBO. Each group was then randomly divided into five subgroups of 6 rats each according to the following time points: 1, 2, 3, 7, and 14 d post injury. Functional recovery of the hindlimb was assessed by Basso, Beattie, and Bresnahan (BBB) scores at different time points after SCI. The expression of HMGB1, TLR4, and NF-κB in the spinal cord tissue was determined by fluorescence quantitative PCR, western blot, immunohistochemistry, and ELISA. Results: The gene expressions of TLR4, HMGB1, and NF-κB (P < 0.01) and the TLR4 protein expression were significantly high after SCI. HBO intervention significantly decreased all the four parameters at 3, 7, and 14 d post injury (P < 0.05). A significant positive correlation (P < 0.01) was observed between the following: HMGB1 mRNA, TLR4 mRNA and TLR4 protein; HMGB1 mRNA and NF-κB mRNA; and TLR4 protein and NF-κB mRNA. BBB score was negatively correlated with HMGB1, TLR4 protein and NF-κB levels. HBO intervention significantly improved the BBB scores at 7 and 14 d post injury (P < 0.05). Conclusions: Hyperbaric oxygen reduced the expressions of HMGB1, TLR4, and NF-κB and reduced secondary SCI as measured using BBB scores.
Keywords: Hyperbaric oxygen, spinal cord injury, toll-like receptor 4
Introduction
Spinal cord injury (SCI) is a common traumatic injury that imposes several complications and poses a serious threat to human health. The incidence of SCI increases with economic growth over the past years. Although the primary injury is not reversible, the secondary injury is a dynamically regulated inflammatory process that affects the prognosis of patients. Excessive inflammation hinders the normal process of nerve repair and regeneration [1-3]. Therefore, reduction of secondary injury becomes the focus in the treatment of SCI [4]. The current treatments of SCI mainly include conventional drugs, surgery, and hyperbaric oxygen (HBO) intervention. HBO is effective and has become an indispensable method in the treatment of SCI [5,6].
Studies have shown that HBO can significantly improve spinal cord tissue oxygen tension and oxygen diffusing capacity, reduce tissue edema and hemorrhage, promote the recovery of nerve tract functions [7], and reverse various pathophysiological processes after SCI. More recent studies confirm that the application HBO therapy (HBO combined with drugs, surgery, and other treatments) after SCI plays a key role in reducing neurological deficits after SCI, thereby improving its function and the patient’s quality of life [8-10].
High mobility group protein B1 (HMGB1) is a late-acting mediator of sepsis and has long-lasting effects on the inflammatory damage [11,12]. Nuclear protein HMGB1 can interact with RAGE and TLR receptor pathways and activate nuclear factor κB (NF-κB), thereby activating TNF-α, IL-1, and other inflammatory cytokines [13].
Toll-like receptor 4 (TLR4) is the first TLR-associated protein to be discovered and is distributed in almost all cell types. TLR4 is a key factor in the inflammatory response pathway and can activate TLR4/NF-κB signaling via myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent signaling pathways [14]. Moreover, HMGB1 activation can significantly increase the release of TNF-α from macrophages. In turn the increased levels of TNF-α and IL-1β can promote the secretion of HMGB1 from macrophages. This positive feedback mechanism, HMGB1-macrophage-TNF-α-macrophage-HMGB1, plays an important role in the inflammatory process after injury [15].
This study was designed to clarify the impact of HBO intervention on the changes in HMGB1/TLR4/NF-κB inflammatory signaling pathways in rats after acute SCI and to explore the underlying mechanisms of HBO in the reduction of secondary SCI.
Materials and methods
Experimental animals and groups
One hundred and twenty healthy adult SD rats, weighing 250-300 g, were provided by Beijing Experimental Animal Center of Military Medical Sciences. The rats were randomly divided into four experimental groups: SCI, SCI + HBO, sham, and sham + HBO. The four groups were further divided into five subgroups of six according to the following time points: 1 (D1), 2 (D2), 3 (D3), 7 (D7), and 14 (D14) days post injury.
An animal model of spinal cord injury
The impact SCI model was generated using Allen’s weight-dropping (WD) method [4]. Briefly, SD rats were anesthetized with 0.3% chloral hydrate (intraperitoneal injection) and secured at a prone position. A mid-dorsal incision of approximately 5 cm was made around the T10 spinal region and its surrounding area was sterile-cleaned. The paraspinal muscles were stripped, the T10 spinous process and lamina were removed, and the underneath dura (1.0 cm × 0.5 cm) was exposed. SCI was performed using a NYU impactor with the following parameters: an impact rod weighing 10 g (2-mm bottom diameter); a 25 mm height of fall; and injury energy of 25 g·cm. A successful injury was indicated by spinal tissue edema, hemorrhage, and purple but intact dural membrane. The rat had wagging tail reflex, bilateral lower extremity retraction flutter, and flaccid paralysis. After the injury, rats were housed in single cages with the ambient temperature of 21-24°C and humidity of 40-50%. Sodium penicillin (400,000 U/rat) was given subcutaneously for 3 d. Urination was aided once in the morning and once in the evening daily for 7-10 d until recovery of micturition reflex.
BBB score
Functional recovery in rats with SCI was evaluated using Basso, Beattie, and Bresnahan (BBB) score system. The preoperative BBB scores of the rats in each group were 21 points. After the acute SCI, a successful model of SCI was characterized by the following parameters: abdomen touching the cage floor; body-dragging by using the forelimbs, loss of motor function of the hind limbs, dorsal hind feet touching the cage floor, loss of weight-bearing function, and urine retention, with a BBB score of 0. At 1, 2, 3, 7, and 14 d after injury, six rats from each group were randomly selected, and the BBB scores were evaluated by two laboratory personnel trained with the BBB score system and blinded to the treatments.
RNA isolation and real-time PCR
Total RNA was extracted from the spinal cord tissue using TriZol (Invitrogen Corporation, France). The first strand cDNA was synthesized using M-MLV reverse transcriptase (Bo, Hangzhou, China). Quantitative real-time PCR was performed using SYBR Green I BioEasy real-time PCR kit (Biomedical) with real-time PCR and gene sequence detector according to manufacturer’s instructions. PCR amplification consisted of 45 cycles and each cycle had the following temperature protocol: 95°C for 20 s, 60°C for 25 s, and 72°C for 30 s. Reaction specificity was confirmed by melting curve analysis. The SYBR Green PCR products were verified by gel electrophoresis. Primers used were as the follows: Rat GAPDH (forward): 5’-GGTGAAGGTCGGTGTGAACG-3’; Rat GAPDH (reverse): 5’-CTCGCTCCTGGAAGATGGTG-3’; HMGB1 (forward): 5’-CAAACCTGCCGGGAGGAGCA-3’; HMGB1 (reverse): 5’-TCTTTCATAACGAGCCTTGTCAGCC-3’; TLR4 (forward): 5’-TATCCAGAGCCGTTGGTGTA-3’; TLR4 (reverse): 5’-CCCACTCGAGGTAGGTGTTT-3’. qPCR results were standardized with GAPDH level. For quantitative analysis, relative mRNA levels were calculated according to the 2-ΔΔCt method.
Western blot
Protein lysate buffer was added to approximately 20 mg of frozen tissues (200 μl protein lysate/10 mg tissue). The protein concentration was determined using BCA Protein Assay Kit according to the manufacturer’s instructions. Each sample containing 20 μg total protein was loaded onto a 12% separating gel and transferred to membranes. The membranes were washed once in TBST for 5 min, and blocked in 5% skim milk in TBST for 4 h at room temperature. After wash, the membranes were incubated with the primary antibodies, anti-TLR4 (1:100), anti-HMGB1 (1:100) and anti-B-actin (1:2000), overnight at 4°C. After wash, the membranes were incubated with the HRP-labeled goat anti-mouse (1:10,000) and goat anti-rabbit (1:10,000) secondary antibodies (Jackson) for 4 h at room temperature. The color was developed using ECL chemiluminescence, and the images were analyzed with LabWorks gradation image analysis software. Each experiment was repeated thrice.
Enzyme-linked immunosorbent assay (ELISA)
The HMGB1, NF-κB, IL-1β, and TNF-α levels in the spinal cord tissue homogenates were detected using DuoSet® ELISA Development Systems (R & D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, 100 μl of Sample Diluent was added to each well followed by 100 μl of either standard or sample. All the samples and standards were run in duplicate and the optical density (OD) measured at 450 nm. The analyte concentration in the sample was interpolated from a standard curve. The levels of HMGB1, NF-κB, IL-1β, and TNF-α in the spinal cord tissue were expressed as pg/mg protein.
HBO intervention
The rats in the SCI + HBO group and sham + HBO group were placed into an animal hyperbaric chamber for HBO intervention 24 h after the injury. The rats were subjected to pure oxygen washing for 5 min, pressure increment for 10 min, stabilization of the pressure at 2.5 ATA, and oxygen uptake, for 45 min, and pressure decrement for 15 min. Continuous ventilation was maintained during HBO intervention with an oxygen flow rate of 8-10 L/min and chamber oxygen concentration above 95%. The intervention was conducted once daily.
Immunohistochemistry
Spinal cord tissues were prepared for immunohistochemistry analysis. The sections were deparaffinized and dehydrated in a gradient alcohol series. Sections were incubated with rabbit anti-TLR4 (1:250, Abcam, Cambridge, UK) at 4°C overnight. After wash, the sections were incubated with peroxidase (HRP) labeled secondary antibody (1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Positive cells were counted and analyzed in each section by a pathologist blinded to the experimental groups. Five fields on each slide, three slides for each rat, were randomly selected and observed under a light microscope (at × 100 and × 400 magnifications) (Eclipse E100, Nikon, Japan). Immunohistochemical assay was performed using ImageJ software.
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed using one-way analysis of variance (one-way ANOVA), independent samples t-test, and correlation analysis. All calculations were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
A spinal cord injury model and BBB scores
Rats in the sham and sham + HBO groups were able to stand and walk on hind limbs with a BBB score of 20-21 points, whereas rats in the SCI and SCI + HBO groups showed complete paralysis of both lower extremities, with a BBB score of 0-1, in accordance with the SCI hind limb behavioral standards for rats. The BBB scores post injury in the SCI and SCI + HBO groups were as follows: 0.28 ± 0.45 and 0.28 ± 0.44 on D1, 0.44 ± 0.31 and 0.69 ± 0.48 on D2, 0.71 ± 0.70 and 1.0 ± 0.76 on D3, 1.70 ± 0.90 and 4.81 ± 1.98 (P < 0.05) on D7, and 6.80 ± 2.93 and 10.82 ± 4.40 (P < 0.05) on D-14, respectively. These results indicated that a reliable animal model of spinal cord impact injury was successfully established using Allen’s weight drop method (Figure 1).
Figure 1.
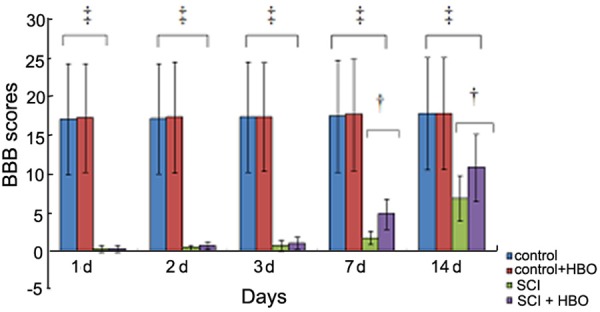
Basso, Beattie and Bresnahan (BBB) scores of rats in each group at different time points after injury. The BBB scores for hindlimb function were evaluated at 1, 2, 3, 7, and 14 days after sham surgery or spinal cord injury. n = 6/group for all the groups. Data were expressed as mean ± standard deviation (SD). ‡P < 0.01; †P < 0.05.
HBO intervention reduced the mRNA and protein expressions of TLR4 and HMGB1
The mRNA and protein levels of HMGB1 and TLR4 were significantly higher in the injury groups than in the sham and sham + HBO groups at different time points post injury (P < 0.01). Compared to the SCI group, in the SCI + HBO group, the TLR4 mRNA and protein expressions were slightly lower at 1 and 2 d post injury (P > 0.05); however, the reduction was significant at 3, 7, and 14 d post injury (P < 0.05). Immunohistochemistry showed the presence of a small number of TLR4-positive cells in the control groups, which increased upon SCI, but gradually reversed by HBO treatment. These results suggested that HBO intervention reduced the expressions of TLR4 and HMGB1 in the injured tissues (Figure 2 and Table 1).
Figure 2.
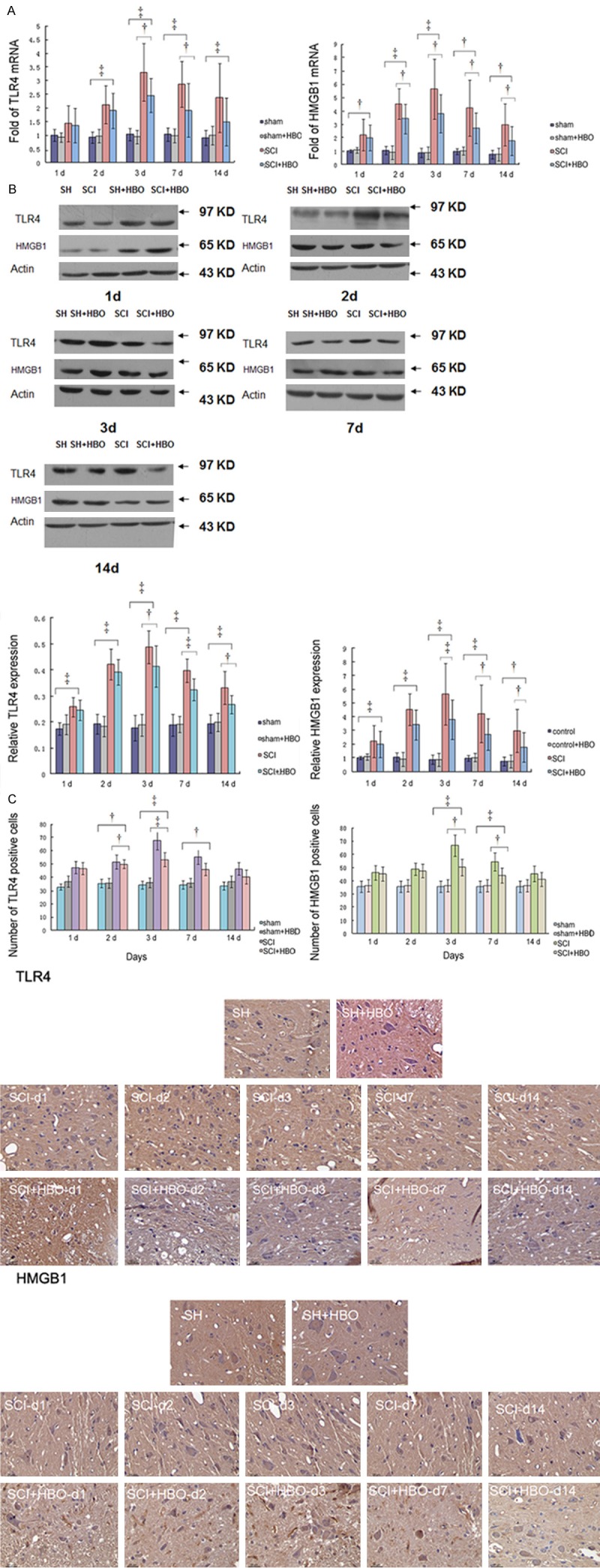
Expression of TLR4 and HMGB1 in each group. The mRNA expression (A) of TLR4 and HMGB1 at 1, 2, 3, 7, and 14 d post injury (n = 6). The protein levels (B) of TLR4 and HMGB1 at different times post injury (n = 6). The number of TLR4 and HMGB1 positive cells at different time points after injury (C). †P < 0.05.
Table 1.
Values of TLR4 mRNA and protein in groups of sham, sham + HBO, SCI and SCI + HBO by time course
| Time Points | Group | sham | sham + HBO | SCI | SCI + HBO | P value | F value |
|---|---|---|---|---|---|---|---|
| 1 d | |||||||
| mRNA | 1 | 0.92 ± 0.17 | 1.43 ± 0.66 | 1.36 ± 0.63 | 0.20 | 1.69 | |
| protein | 0.17 ± 0.02 | 0.19 ± 0.04 | 0.24 ± 0.04 | 0.25 ± 0.04 | 0.00 | 5.67 | |
| 2 d | |||||||
| mRNA | 0.92 ± 0.21 | 0.97 ± 0.24 | 2.11 ± 0. 70 | 1.90 ± 0.64 | 0.00 | 9.28 | |
| protein | 0.19 ± 0.04 | 0.18 ± 0.04 | 0.43 ± 0.06 | 0.39 ± 0.05 | 0.00 | 41.97 | |
| 3 d | |||||||
| mRNA | 1.0 ± 0.23 | 0.93 ± 0.24 | 3.31 ± 1.06 | 2.46 ± 0.62 | 0.00 | 19.63 | |
| protein | 0.18 ± 0.05 | 0.19 ± 0.04 | 0.49 ± 0.06 | 0.42 ± 0.08 | 0.00 | 41.64 | |
| 7 d | |||||||
| mRNA | 1.0 ± 0.24 | 0.99 ± 0.25 | 2.86 ± 0.84 | 1.91 ± 0.98 | 0.00 | 10.49 | |
| protein | 0.19 ± 0.04 | 0.19 ± 0.03 | 0.40 ± 0.05 | 0.33 ± 0.04 | 0.00 | 38.24 | |
| 14 d | |||||||
| mRNA | 0.91 ± 0.27 | 1.0 ± 0. 34 | 2.37 ± 1.26 | 1.49 ± 0.87 | 0.018 | 4.27 | |
| protein | 0.19 ± 0.04 | 0.20 ± 0.04 | 0.33 ± 0.06 | 0.27 ± 0.04 | 0.00 | 13.08 |
HBO interventions reduced HMGB1, NF-κB, IL-1β, and TNF-α expression
The HMGB1, NF-κB, IL-1β, and TNF-α levels in the injury groups were significantly higher than both the sham groups at different time points post injury (P < 0.01). The HMGB1 and NF-κB expressions decreased gradually after HBO intervention, and the reduction became significant at 3 and 7 d post injury (P < 0.01). These results suggested that HBO decreased the expressions level of HMGB1 and NF-κB (Figure 3 and Table 2).
Figure 3.
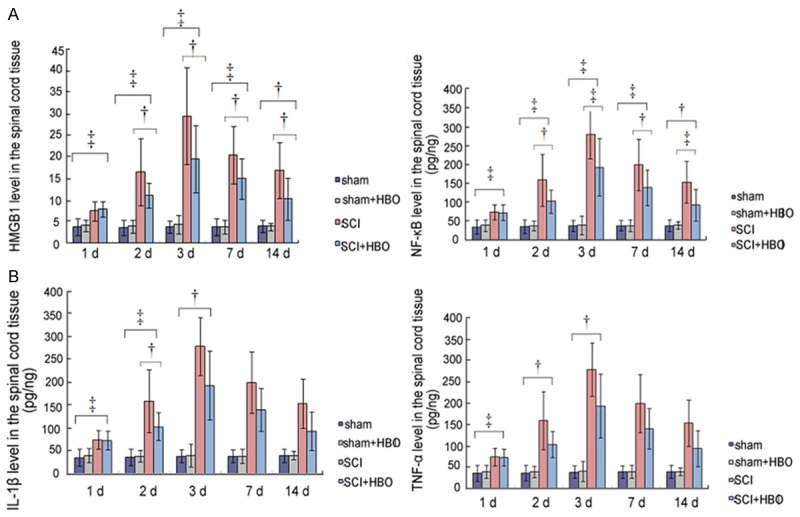
Changes in the expression of HMGB1, NF-κB, IL-1β, and TNF-α after hyperbaric oxygen intervention. A. Changes in the HMGB1 and NF-κB protein levels, B. Changes in IL-1β and TNF-α protein levels. Data expressed as mean ± SD.
Table 2.
HMGB1 level in the groups of sham, sham + HBO, SCI and SCI + HBO by time course
| Time Points | sham | Sham + HBO | SCI | SCI + HBO | P value | F value |
|---|---|---|---|---|---|---|
| 1 d | ||||||
| 3.82 ± 1.96 | 4.10 ± 1.37 | 7.47 ± 2.29 | 7.91 ± 1.78 | 0.00 | 7.95 | |
| 2 d | ||||||
| 3.58 ± 1.78 | 3.85 ± 1.49 | 16.49 ± 7.74 | 11.07 ± 2.87 | 0.00 | 12.97 | |
| 3 d | ||||||
| 3.80 ± 1.42 | 4.30 ± 2.15 | 29.5 ± 11.24 | 19.54 ± 7.75 | 0.00 | 11.15 | |
| 7 d | ||||||
| 3.80 ± 2.00 | 3.8 ± 1.52 | 20.47 ± 6.65 | 14.98 ± 4.70 | 0.00 | 22.91 | |
| 14 d | ||||||
| 0.19 ± 0.09 | 3.87 ± 0.82 | 16.85 ± 6.40 | 10.33 ± 4.88 | 0.00 | 13.59 |
Correlation of the expressions of HMGB1, TLR4, and NF-κB after spinal cord injury
After SCI, significant correlation (P < 0.01) was found between the following in the damaged tissues: HMGB1 and TLR4 protein expressions; HMGB1 and TLR4 mRNA expressions; HMGB1 and NF-κB levels; and TLR4 and NF-κB protein expressions. These data indicated that HBO regulated secondary SCI through the intervention of HMGB1, TLR4, and NF-κB pathways (Figures 4, 5, 6, 7 and 8; Tables 3, 4, 5, 6 and 7).
Figure 4.
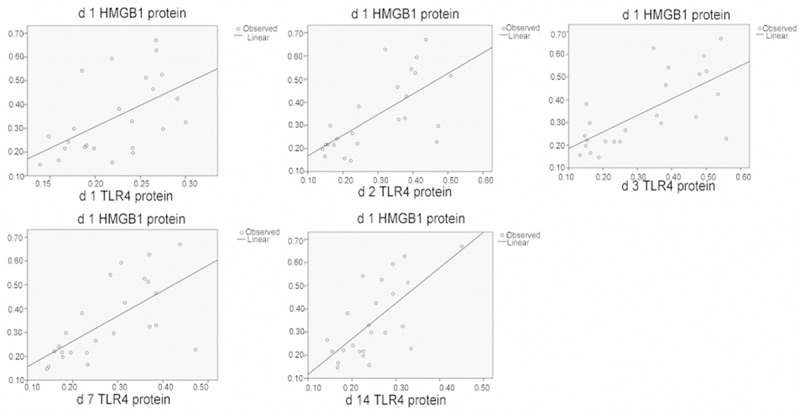
Correlation of HMGB1 and TLR4 protein levels in the spinal cord tissues.
Figure 5.
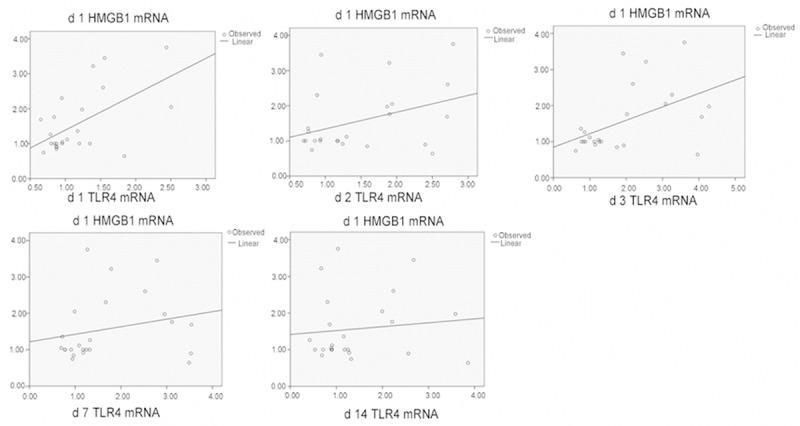
Correlation of HMGB1 mRNA and TLR4 mRNA levels in the spinal cord tissues.
Figure 6.
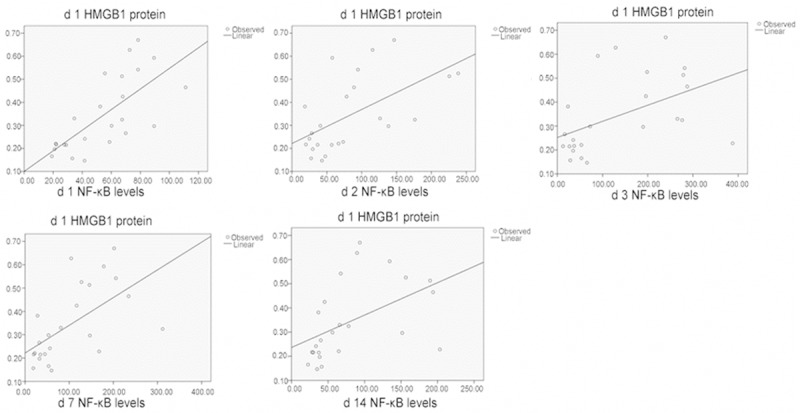
Correlation of HMGB1 protein and NF-κB levels in the spinal cord tissues.
Figure 7.
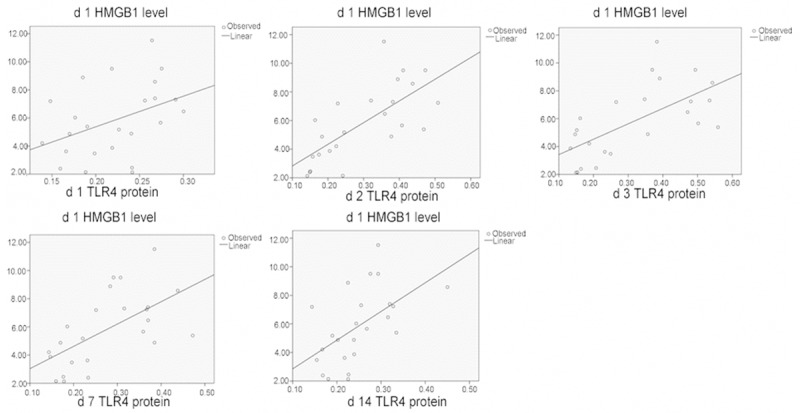
Correlation of HMGB1 l and TLR4 protein levels in the spinal cord tissues.
Figure 8.
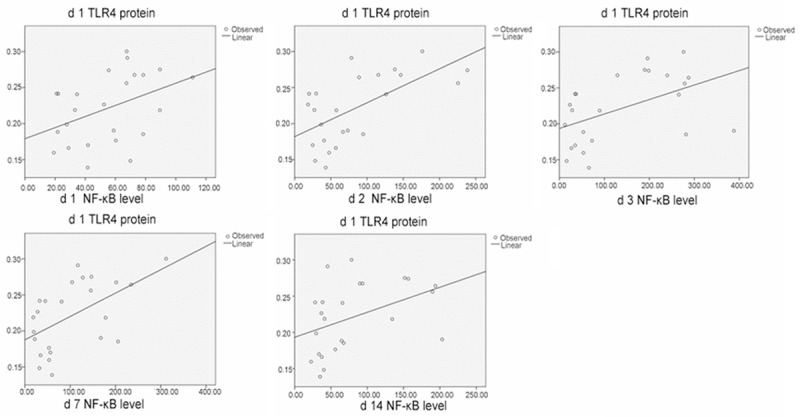
Correlation ofTLR4 protein and NF-κB levels in the spinal cord tissues.
Table 3.
Correlations of HMGB1 and TLR4 protein expression in the spinal cord tissue
| HMGB1 protein | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| TLR4 protein | ||||||||||
| 1 d | 0.29 | 0.00 | 0.44 | 0.00 | 0.53 | 0.00 | 0. 31 | 0.00 | 0.08 | 0.19 |
| 2 d | 0.45 | 0.00 | 0.57 | 0.00 | 0.69 | 0.00 | 0.55 | 0.00 | 0.49 | 0.00 |
| 3 d | 0.46 | 0.00 | 0.60 | 0.00 | 0.73 | 0.00 | 0.53 | 0.00 | 0.45 | 0.00 |
| 7 d | 0.42 | 0.00 | 0.51 | 0.00 | 0.75 | 0.00 | 0.46 | 0.00 | 0.43 | 0.00 |
| 14 d | 0.45 | 0.00 | 0.29 | 0.00 | 0.65 | 0.00 | 0.59 | 0.00 | 0.41 | 0.00 |
Table 4.
Correlations of HMGB1mRNA andTLR4 mRNA in the spinal cord tissue
| HMGB1 mRNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| TLR4 mRNA | ||||||||||
| 1 d | 0.33 | 0.00 | 0.38 | 0.00 | 0.04 | 0.34 | 0.099 | 0.13 | 0.14 | 0.068 |
| 2 d | 0.15 | 0.066 | 0.61 | 0.00 | 0.29 | 0.00 | 0.31 | 0.00 | 0.29 | 0.00 |
| 3 d | 0.24 | 0.015 | 0.65 | 0.00 | 0.53 | 0.00 | 0.53 | 0.00 | 0.26 | 0.01 |
| 7 d | 0.053 | 0.28 | 0.37 | 0.00 | 0.72 | 0.00 | 0.48 | 0.00 | 0.19 | 0.035 |
| 14 d | 0.013 | 0.60 | 0.48 | 0.00 | 0.65 | 0.00 | 0.50 | 0.00 | 0.075 | 0.196 |
Table 5.
Correlations of HMGB1 protein expression and NF-κB level in the spinal cord tissue
| HMGB1 Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| NF-κB | ||||||||||
| 1 d | 0.50 | 0.00 | 0.17 | 0.045 | 0.48 | 0.00 | 0. 49 | 0.00 | 0.37 | 0.00 |
| 2 d | 0.34 | 0.00 | 0.62 | 0.00 | 0.63 | 0.00 | 0.60 | 0.00 | 0.26 | 0.01 |
| 3 d | 0.23 | 0.02 | 0.45 | 0.00 | 0.58 | 0.00 | 0.40 | 0.00 | 0.45 | 0.00 |
| 7 d | 0. 35 | 0.00 | 0.28 | 0.01 | 0.65 | 0.00 | 0.56 | 0.00 | 0.53 | 0.00 |
| 14 d | 0.23 | 0.02 | 0.32 | 0.00 | 0.61 | 0.00 | 0.46 | 0.00 | 0.59 | 0.00 |
Table 6.
Correlations of HMGB1level andTLR4 protein expression in the spinal cord tissue
| HMGB1 level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| TLR4 protein | ||||||||||
| 1 d | 0.16 | 0.05 | 0.35 | 0.00 | 0.22 | 0.02 | 0. 32 | 0.00 | 0.18 | 0.04 |
| 2 d | 0.50 | 0.00 | 0.63 | 0.00 | 0.51 | 0.00 | 0.57 | 0.00 | 0.65 | 0.00 |
| 3 d | 0.40 | 0.00 | 0.50 | 0.00 | 0.55 | 0.00 | 0.64 | 0.00 | 0.50 | 0.00 |
| 7 d | 0.36 | 0.00 | 0.53 | 0.00 | 0.48 | 0.00 | 0.58 | 0.00 | 0.53 | 0.00 |
| 14 d | 0.30 | 0.00 | 0.44 | 0.00 | 0.32 | 0.00 | 0.53 | 0.00 | 0.38 | 0.00 |
Table 7.
Correlations of TLR4 protein expression and NF-κB level in the spinal cord tissue
| TLR4 protein | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| NF-κB level | ||||||||||
| 1 d | 0.17 | 0.046 | 0.49 | 0.00 | 0.42 | 0.00 | 0. 37 | 0.00 | 0.32 | 0.00 |
| 2 d | 0.39 | 0.00 | 0.57 | 0.00 | 0.49 | 0.00 | 0. 47 | 0.00 | 0.37 | 0.00 |
| 3 d | 0.26 | 0.016 | 0.72 | 0.00 | 0.67 | 0.00 | 0.75 | 0.00 | 0. 47 | 0.00 |
| 7 d | 0.30 | 0.006 | 0.55 | 0.00 | 0.61 | 0.00 | 0.56 | 0.00 | 0.50 | 0.00 |
| 14 d | 0.18 | 0.04 | 0.65 | 0.00 | 0.49 | 0.00 | 0.53 | 0.00 | 0.41 | 0.00 |
Correlation of HMGB1, TLR4, and NF-κB expression and BBB scores
Significant negative correlation (P < 0.01) was found between BBB scores and the following factors in the damaged tissues before and after HBO treatment: TLR4 protein expression and HMGB1 and NF-κB levels. These results indicated that after HBO intervention, the HMGB1, TLR4, and NF-κB expressions decreased, accompanied with the improvement of BBB scores (Tables 8, 9 and 10).
Table 8.
Correlations of HMGB1 level in the spinal cord tissue and BBB scores
| BBB scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| HMGB1 level | ||||||||||
| 1 d | -0.72 | 0.00 | -0.72 | 0.00 | -0.72 | 0.00 | -0.69 | 0.00 | -0.68 | 0.00 |
| 2 d | -0.79 | 0.00 | -0.80 | 0.00 | -0.79 | 0.00 | -0.83 | 0.00 | -0.78 | 0.00 |
| 3 d | -0.80 | 0.00 | -0.80 | 0.00 | -0.79 | 0.00 | -0.79 | 0.00 | -0.77 | 0.00 |
| 7 d | -0.85 | 0.00 | -0.85 | 0.00 | -0.79 | 0.00 | -0.87 | 0.00 | -0.88 | 0.00 |
| 14 d | -0.73 | 0.00 | -0.73 | 0.00 | -0.85 | 0.00 | -0.77 | 0.00 | -0.81 | 0.00 |
Table 9.
Correlations of TLR4 protein expression in the spinal cord tissue and BBB scores
| BBB scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| TLR4 protein | ||||||||||
| 1 d | -0.67 | 0.00 | -0.67 | 0.00 | -0.67 | 0.00 | -0.68 | 0.00 | -0.63 | 0.00 |
| 2 d | -0.92 | 0.00 | -0.92 | 0.00 | -0.92 | 0.00 | -0.92 | 0.00 | -0.90 | 0.00 |
| 3 d | -0.91 | 0.00 | -0.91 | 0.00 | -0.91 | 0.00 | -0.92 | 0.00 | -0.93 | 0.00 |
| 7 d | -0.89 | 0.00 | -0.89 | 0.00 | -0.89 | 0.00 | -0.91 | 0.00 | -0.93 | 0.00 |
| 14 d | -0.75 | 0.00 | -0.75 | 0.00 | -0.74 | 0.00 | -0.79 | 0.00 | -0.83 | 0.00 |
Table 10.
Correlations of NF-κB level in the spinal cord tissue and BBB scores
| BBB scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | 1 d | 2 d | 3 d | 7 d | 14 d | |||||
|
|
||||||||||
| R | P | R | P | R | P | R | P | R | P | |
| NF-κB level | ||||||||||
| 1 d | -0.71 | 0.00 | -0.71 | 0.00 | -0.71 | 0.00 | -0.69 | 0.00 | -0.69 | 0.00 |
| 2 d | -0.75 | 0.00 | -0.75 | 0.00 | -0.75 | 0.00 | -0.81 | 0.00 | -0.78 | 0.00 |
| 3 d | -0.88 | 0.00 | -0.88 | 0.00 | -0.88 | 0.00 | -0.91 | 0.00 | -0.91 | 0.00 |
| 7 d | -0.83 | 0.00 | -0.83 | 0.00 | -0.83 | 0.00 | -0.86 | 0.00 | -0.87 | 0.00 |
| 14 d | -0.73 | 0.00 | -0.73 | 0.00 | -0.74 | 0.00 | -0.77 | 0.00 | -0.81 | 0.00 |
Discussion
Our results show that SCI induces upregulation of HMGB1, TLR4, and NF-κB expressions, indicating the activation of HMGB1/TLR4/NF-κB inflammatory signaling pathway and HBO treatment can regulate this inflammatory signaling pathway through reductions in HMGB1, TLR4, and NF-κB expressions, thereby promoting recovery from SCI.
HBO treatment in experimental SCI started in 1965, when Maede et al [16] found a positive therapeutic effect. Holbach et al [17] first reported the clinical efficacy of HBO therapy in spinal cord injuries in 1977. In recent years, HBO therapy has gained increased attention in reducing the inflammatory response in secondary SCI [4,5,7,9,10].
Studies have shown that the serum inflammatory cytokines levels, including TNF-α, IL-6, IL-8, and IL-10, significantly decreased at 4 and 12 h and 1, 3, and 5 d after HBO therapy, suggesting that HBO can inhibit the activity of inflammatory cells in the peripheral blood and decrease the levels of inflammatory cytokines after SCI [18]. We know that HMGB1 level increases after an acute SCI, with the number of HMGB1-positive and RAGE-positive cells attaining peak levels at 48 and 24 h, respectively, indicating that the increase of HMGB1 is associated with neuronal apoptosis [19]. Another study showed that HBO therapy significantly reduced HMGB1 mRNA and protein expressions, at days 7 and 14 post injury, and NF-κB mRNA and protein expressions at days 3, 7, and 14 post injury, suggesting that HBO intervention can reduce HMGB1 and NF-κB expressions, ameliorate SCI, and improve BBB scores [20].
Our results demonstrate that a significant increase in HMGB1 and NF-κB in the damaged tissues negatively correlates with the BBB score at different time points after SCI. HMGB1 is mainly distributed in the nucleus. After an injury or inflammatory reaction, the activation of TNF-α and IL-1 induces HMGB1 translocation from the nucleus to the cytoplasm, followed by its subsequent release into the extracellular space. Extracellular HMGB1 binds to the membrane receptors, such as TLR4, resulting in the activation of NF-κB transcription via transmembrane signal pathways. Therefore, extracellular HMGB1 is an important mediator of inflammation and plays important roles in the activation of macrophages and mononuclear cells, and triggers the inflammatory immune response [12]. Extracellular HMGB1 becomes the target of studies and treatment plans [12]. Our study shows that HBO intervention can reduce extracellular HMGB1, thereby reducing NF-κB content and improving the BBB score.
Our study demonstrates that the TLR4 mRNA and protein expressions significantly increased at days 3, 7, and 14 after SCI; HBO intervention significantly reduced the TLR4 mRNA and protein expressions at days 3 and 7. TLR4 level in the damaged tissues was positively correlated with the HMGB1 mRNA and protein expressions. TLR4 plays an important role in the autoimmune and non-infectious immune response [12]. Lack of functional TLR4 reduces neuronal damage and microglial activation. TLR4 stimulates the microglial cell activity after oxygen/glucose deprivation. Lack of functional TLR4 significantly decreases the production of proinflammatory cytokines. TLR4-dependent activation of microglia cells regulates spinal cord ischemia-reperfusion injury [21].
Our results suggest that HBO intervention regulates HMGB1/TLR4/NFκB inflammatory signaling pathway through a reduction in the expressions of HMGB1, TLR4, NF-κB, TNF-α, and IL-1β. Studies show that the treatment of human peripheral blood mononuclear cells with purified HMGBl can significantly stimulate the production of TNF-α, interleukin-6 (IL-6) and other cytokines. The interaction between HMGBl and TNF-α can amplify the inflammation cascade and lead to increased tissue damage [22]. Accordingly, we speculate that HBO intervention in SCI can regulate inflammatory mediators in the inflammatory signaling pathways, thereby reducing secondary injury and promoting tissue repair.
In summary, HBO intervention regulates HMGB1/TLR4/NF-κB levels after SCI and ameliorates the inflammatory response while promoting functional recovery from SCI. The underlying mechanisms of HBO intervention in the SCI need to be further explored.
Acknowledgements
This research support by Chinese-Canada international Cooperation project.
Disclosure of conflict of interest
None.
References
- 1.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahel PF, Flierl MA. Targeted modulation of the neuroinflammatory response after spinal cord injury: the ongoing quest for the “holy grail”. Am J Pathol. 2010;177:2685–2687. doi: 10.2353/ajpath.2010.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- 5.Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strategies to promote neural repair and regeneration after spinal cord injury. Spine (Phila Pa 1976) 2005;30:S3–13. doi: 10.1097/01.brs.0000175186.17923.87. [DOI] [PubMed] [Google Scholar]
- 6.Li SH, Guo PD, Wang WJ. Current situation and progression in the treatment of spinal cord injury. Zhongguo Gu Shang. 2010;23:70–73. [PubMed] [Google Scholar]
- 7.Li N. Hyperbaric oxygen and spinal cord injury. Chongqing Medical. 2006;35:1729–1730. [Google Scholar]
- 8.Al-Waili NS, Butler GJ, Beale J, Abdullah MS, Hamilton RW, Lee BY, Lucus P, Allen MW, Petrillo RL, Carrey Z, Finkelstein M. Hyperbaric oxygen in the treatment of patients with cerebral stroke, brain trauma, and neurologic disease. Adv Ther. 2005;22:659–678. doi: 10.1007/BF02849960. [DOI] [PubMed] [Google Scholar]
- 9.Tai PA, Chang CK, Niu KC, Lin MT, Chiu WT, Lin CM. Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J Neurotrauma. 2010;27:1121–1127. doi: 10.1089/neu.2009.1162. [DOI] [PubMed] [Google Scholar]
- 10.Tian HC, Jin YC. The role of hyperbaric oxygen in adjuvant therapy for the patients with spinal cord injury. Modern Chinese Medicine. 2011;13:10–12. [Google Scholar]
- 11.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Li W, Goldstein R, Tracey KJ, Sama AE. HMGB1 as a potential therapeutic target. Novartis Found Symp. 2007;280:73–85. discussion 85-91, 160-164. [PubMed] [Google Scholar]
- 13.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YC, Lin S, Yang QW. Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflammation. 2011;8:134. doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maede N. Experimental studies on the effect of decompress prccedures and hyperbaric oxygenation for the treatment of spinal cord injury. J Nara Med Assoc. 1965;16:429–447. [Google Scholar]
- 17.Holbach KH, Wassmann H, Linke D. The use of hyperbaric oxygenation in the treatment of spinal cord lesions. Eur Neurol. 1977;16:213–221. doi: 10.1159/000114902. [DOI] [PubMed] [Google Scholar]
- 18.Xu ZQ, Sun YM, Wang HB. Effect of hyperbaric oxygen on inflammatory factors in spinal cord injury in rat. Modern Chinese Medicine. 2009;11:17–19. [Google Scholar]
- 19.Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine (Phila Pa 1976) 2010;35:1109–1115. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Liu X, Zhou Y, Wang G, Gao C, Su Q. Hyperbaric oxygen alleviates experimental (spinal cord) injury by downregulating HMGB1/NF-kappaB expression. Spine (Phila Pa 1976) 2013;38:E1641–1648. doi: 10.1097/BRS.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 21.Bell MT, Puskas F, Agoston VA, Cleveland JC Jr, Freeman KA, Gamboni F, Herson PS, Meng X, Smith PD, Weyant MJ, Fullerton DA, Reece TB. Toll-like receptor 4-dependent microglial activation mediates spinal cord ischemia-reperfusion injury. Circulation. 2013;128:S152–156. doi: 10.1161/CIRCULATIONAHA.112.000024. [DOI] [PubMed] [Google Scholar]
- 22.Li JM, Quan ZX, Liu B. Research progress of the NF-κB signaling pathway on acute secondary response to spinal cord injury. Journal of Traumatic Surgery. 2009;11:85–87. [Google Scholar]


