Abstract
It has been suggested that nuclear expression of maspin (mammary serine protease inhibitor; also known as SERPINB5) in colorectal cancer (CRC) is associated with proximal colonic tumor location, mucinous and poorly differentiated histology, microsatellite instability-high (MSI-H), and poor prognosis. Based on these findings, there may be a potential association between nuclear maspin expression and the CpG island methylator phenotype (CIMP) in CRC, but no study has elucidated this issue. Here, we evaluated maspin protein expression status by immunohistochemistry in 216 MSI-H CRCs. CIMP status was also determined by methylation-specific quantitative PCR method (MethyLight) using eight CIMP markers (MLH1, NEUROG1, CRABP1, CACNA1G, CDKN2A (p16), IGF2, SOCS1, and RUNX3) in 216 MSI-H CRCs. Associations between maspin expression status and various pathological, molecular, and survival data were statistically analyzed. Among the 216 MSI-H CRCs, 111 (51%) cases presented nuclear maspin-positive tumors. Nuclear maspin-positive MSI-H CRCs were significantly associated with proximal tumor location (P = 0.003), tumor budding (P < 0.001), lymphovascular invasion (P = 0.001), perineural invasion (P = 0.008), absence of peritumoral lymphoid reaction (P = 0.045), lymph node metastasis (P = 0.003), distant metastasis (P = 0.005), advanced AJCC/UICC stage (stage III/IV) (P = 0.001), and CIMP-high (CIMP-H) status (P < 0.001). Patients with nuclear maspin-positive tumors showed worse disease-free survival than patients with nuclear maspin-negative tumors (log-rank P = 0.025). In conclusion, nuclear maspin expression is molecularly associated with CIMP-H rather than MSI-H, and clinicopathologically correlates with tumor aggressiveness in CRC.
Keywords: Maspin, SERPINB5, CpG island methylator phenotype, microsatellite instability, colorectal cancer, prognosis
Introduction
Maspin (mammary serine protease inhibitor; also known as SERPINB5) is a member of the serine protease inhibitor superfamily. Based on experimental studies, it may be a tumor suppressor protein associated with the inhibition of cancer cell growth and metastasis [1]. However, clinicopathological implications of maspin expression in human malignancies are not consistent across different tissue types and different subcellular localizations, and therefore, controversial results have been frequently reported. For instance, maspin expression is associated with tumor-suppressive features in breast cancer, but is associated with tumor-progressive features in colorectal and pancreatobiliary cancers [2]. Furthermore, maspin positivity in tumor cell nuclei is associated with favorable survival in patients with breast cancer, non-small cell lung cancer or laryngeal cancer [3-5], whereas nuclear maspin expression correlates with poor prognosis in patients with colorectal cancer (CRC) or malignant melanoma [6-8].
In CRC, the prognostic significance of maspin expression depends on its subcellular localization in tumor cells. Nuclear maspin expression consistently indicates poor prognosis [6,7,9]. In addition, according to previous studies, nuclear maspin expression is significantly associated with predilections for right-sided tumor location, poor tumor differentiation, mucinous histology, and microsatellite instability-high (MSI-H) in CRC [10-12]. These features are reminiscent of clinicopathological characteristics of the CpG island methylator phenotype (CIMP) in CRC. CIMP-high (CIMP-H) is one of the major molecular subtypes in CRC and is molecularly and clinicopathologically characterized by extensive promoter CpG island hypermethylation of many tumor-related genes and is highly correlated with female gender, proximal tumor location, poor differentiation, BRAF mutation, and MSI-H status [13,14]. In this context, although previous studies indicated a relationship between nuclear maspin expression and MSI-H in CRC, we hypothesized that the significant molecular association of nuclear maspin expression in CRC might be linked to CIMP-H rather than MSI-H. Therefore, to investigate the association between maspin expression and epigenetic alterations, we decided to evaluate maspin protein expression and CIMP status through immunostaining and DNA methylation analysis in a large series of MSI-H CRCs. Additionally, to confirm that the clinicopathological features and prognostic significance of nuclear maspin expression are maintained in MSI-H CRCs, the correlations between maspin expression and various clinical, histopathological, molecular, and survival data were statistically analyzed.
Materials and methods
Tissue samples and MSI analysis
Initially, 218 formalin-fixed, paraffin-embedded (FFPE) MSI-H CRC tissue samples were collected from the depositories of the pathology departments of Seoul National University Hospital, Seoul, Korea and Seoul National University Bundang Hospital, Seongnam, Korea. Between 2004 and 2008, DNA testing for MSI determination was performed by the molecular pathology laboratory of our hospitals using genomic DNA samples extracted from tumor and normal tissues of a consecutive series of 2957 patients who underwent curative surgery for CRC at our hospitals. MSI analysis was performed by PCR and capillary electrophoresis-based methods using five microsatellite markers recommended by the National Cancer Institute (BAT-25, BAT-26, D5S346, D17S250, and D2S123) [15,16]. MSI-H tumor was diagnosed when two or more markers among the five markers showed instability in tumor DNA. Among the 2957 CRC samples subjected to MSI analysis, 237 specimens were determined as MSI-H. Of these, 218 specimens were suitable for use, and the FFPE tissues were used for tissue microarray (TMA) construction. After immunohistochemistry (IHC) using TMA sections, two cases were suboptimal for interpretation of maspin IHC. Thus, 216 cases were finally included in this study. This study was approved by institutional review board (IRB No. H-1203-072-402).
Clinical data collection and histopathological assessment
The clinical data for the 216 MSI-H CRC patients were collected by review of medical records. The clinical parameters included age, gender, tumor location, tumor multiplicity, gross tumor type, TNM cancer stage (AJCC/UICC 7th edition), and times of death, tumor recurrence and the last clinical follow-up for disease-free survival (DFS) data. Through microscopic examination of the hematoxylin and eosin-stained tissue slides of the 216 MSI-H CRCs, a histopathological assessment was performed independently by two gastrointestinal pathologists (J.H.K. and G.H.K.) blinded to the clinical and molecular data. The histopathological parameters included tumor border, lymphovascular invasion, perineural invasion, tumor budding, tumor differentiation, mucinous histology, signet ring cell histology, medullary histology, serrated histology, cribriform comedo histology, and peritumoral lymphoid reaction. Conflicting assessment results between the two pathologists were reviewed and discussed, and a consensus was reached.
Immunohistochemistry
TMA construction was performed as previously described [17]. Three different tumor areas in each of the 218 MSI-H CRC case specimens were extracted as three tissue cores (2 mm in diameter) for TMA construction. In this study, all IHC processes were automatically conducted using a BenchMark XT immunostainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s protocol. Immunostaining for MLH1, MSH2, MSH6, and PMS2 was performed and assessed as previously described [17]. Maspin IHC was performed on TMA sections using an anti-maspin antibody (Leica Biosystems, Newcastle Upon Tyne, UK; 1:30). Maspin IHC was evaluated independently by two pathologists (J.H.K. and K.J.K.) blinded to the clinicopathological and molecular data. Maspin expression status in all of the MSI-H CRC specimens was classified into negative or positive according to criteria defined in previous studies [6,10]. Initially, nuclear maspin expression in each specimen was graded as one of the four scores based on staining intensity: absent staining (0), weak staining (1+), moderate staining (2+), and strong staining (3+). Next, tumors showing moderate to strong staining (2+/3+) were categorized into the true positive group for nuclear maspin expression. As a minimum requirement for the determination of positivity, the nuclear staining pattern of maspin should be observed in more than 10% of tumor cells in each tissue core. The highest score among the results from the three tissue cores for each case was adopted as the final score of nuclear maspin expression for that particular case. As described above, two cases were excluded due to the suboptimal quality of the tissue cores on the TMA sections, and 216 cases were finally assessed for maspin expression. Conflicting assessment results between the two pathologists were reviewed and discussed, and a consensus was reached.
CIMP analysis
Genomic DNA isolation from microdissected tumor tissues and sodium bisulfite modification of the genomic DNA were conducted as previously described [14,16]. DNA methylation analysis for the determination of CIMP status was performed as previously described [14,18]. Promoter CpG island methylation of eight CIMP marker genes (MLH1, NEUROG1, CRABP1, CACNA1G, CDKN2A (p16), IGF2, SOCS1, and RUNX3) was measured using the methylation-specific real-time PCR method (MethyLight) in bisulfite-modified DNA samples of the 216 MSI-H CRCs. A methylated CpG island locus was determined when the percentage of methylated reference value was > 4. CIMP-H tumors were defined when five or more markers were methylated. CIMP-low (CIMP-L) tumors were determined when promoter methylation was detected in one to four markers, and CIMP-negative (CIMP-0) tumors were diagnosed when promoter methylation was not detected in all markers. The results of all DNA methylation analyses in this study were confirmed by at least three independent experiments.
KRAS/BRAF mutations analysis
Mutations in KRAS codons 12 and 13 and BRAF codon 600 were analyzed by PCR-restriction fragment length polymorphism and confirmative direct sequencing methods as previously described [14,16].
Statistical analysis
Comparisons of the categorical data were conducted using the chi-square test or Fisher’s exact test. Comparisons of DFS rates between patient subgroups according to maspin expression status were performed using the Kaplan-Meier analysis with the log-rank test. To identify independent prognostic factors, multivariate analysis was carried out using the Cox proportional hazards regression model. All P values were two-sided, and P values of less than 0.05 indicated statistical significance. All statistical analyses were performed using IBM SPSS Statistics version 20 software (Chicago, IL, USA).
Results
Clinicopathological features of nuclear maspin-positive CRCs
By immunohistochemical analysis, nuclear maspin positivity (2+/3+) was detected in 111 (51%) out of the 216 MSI-H CRC cases. Representative immunohistochemical images of nuclear maspin-positive and nuclear maspin-negative CRCs are shown in Figure 1. The clinicopathological features according to nuclear maspin expression status in the 216 MSI-H CRCs are summarized in Table 1. Nuclear maspin-positive tumors were significantly associated with proximal tumor location (cecum, ascending colon, or transverse colon; P = 0.003), advanced stage (AJCC/UICC TNM stage III or IV; P = 0.001), lymph node metastasis (pN1 or pN2 stage; P = 0.003), distant metastasis (M1 stage; P = 0.005), lymphovascular invasion (P = 0.001), perineural invasion (P = 0.008), tumor budding positivity (buds ≥ 5 at the invasive margin; P < 0.001), and absence of peritumoral lymphoid reaction (P = 0.045) in the MSI-H CRC cases.
Figure 1.
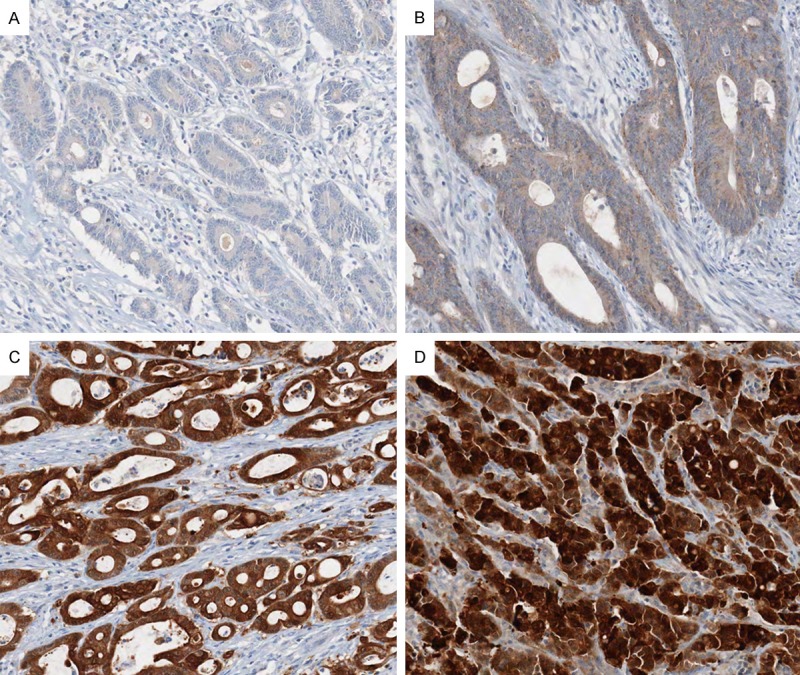
Representative photomicrographs of maspin IHC in MSI-H CRCs. A. A case showing the absence of maspin staining in tumor cells (0) (× 200). B. A case showing faintly granular maspin staining in tumor cell nuclei (1+) and weak to moderate maspin staining in tumor cell cytoplasm (× 200). C. A case showing moderate nuclear maspin staining (2+) (× 200). D. A case showing strong nuclear maspin staining (3+) (× 200). Tumors with moderate to strong nuclear staining (2+/3+) were regarded as nuclear maspin-positive cases.
Table 1.
Clinicopathological features according to nuclear maspin expression status in MSI-H CRCs (n = 216)
| Clinicopathological factors | Total cases | Nuclear maspin-positive | Nuclear maspin-negative | P value | |
|---|---|---|---|---|---|
| Agea | < 58 years | 102 | 52 (47%) | 50 (48%) | 0.91 |
| ≥ 58 years | 114 | 59 (53%) | 55 (52%) | ||
| Gender | Male | 115 | 55 (50%) | 60 (57%) | 0.264 |
| Female | 101 | 56 (50%) | 45 (43%) | ||
| Tumor location | Proximal | 139 | 82 (74%) | 57 (54%) | 0.003 |
| Distal | 77 | 29 (26%) | 48 (46%) | ||
| Tumor multiplicity | Solitary | 194 | 97 (87%) | 97 (92%) | 0.225 |
| Multiple | 22 | 14 (13%) | 8 (8%) | ||
| Gross tumor type | Polypoid | 31 | 17 (15%) | 14 (13%) | 0.678 |
| Ulcerative | 185 | 94 (85%) | 91 (87%) | ||
| Tumor border | Expanding | 35 | 17 (15%) | 18 (17%) | 0.716 |
| Infiltrative | 181 | 94 (85%) | 87 (83%) | ||
| AJCC/UICC TNM stage | Stage I/II | 139 | 60 (54%) | 79 (75%) | 0.001 |
| Stage III/IV | 77 | 51 (46%) | 26 (25%) | ||
| Lymph node metastasis | Absent | 143 | 63 (57%) | 80 (76%) | 0.003 |
| Present | 73 | 48 (43%) | 25 (24%) | ||
| Distant metastasis | Absent | 198 | 96 (86%) | 102 (97%) | 0.005 |
| Present | 18 | 15 (14%) | 3 (3%) | ||
| Lymphovascular invasion | Absent | 159 | 71 (64%) | 88 (84%) | 0.001 |
| Present | 57 | 40 (36%) | 17 (16%) | ||
| Perineural invasion | Absent | 199 | 97 (87%) | 102 (97%) | 0.008 |
| Present | 17 | 14 (13%) | 3 (3%) | ||
| Tumor budding | Negative | 171 | 77 (69%) | 94 (90%) | < 0.001 |
| Positive | 45 | 34 (31%) | 11 (10%) | ||
| Tumor differentiation | WD/MD | 171 | 87 (78%) | 84 (80%) | 0.769 |
| PD | 45 | 24 (22%) | 21 (20%) | ||
| Mucinous histology | Absent | 93 | 41 (37%) | 52 (50%) | 0.062 |
| Present | 123 | 70 (63%) | 53 (50%) | ||
| Signet ring cell histology | Absent | 196 | 97 (87%) | 99 (94%) | 0.08 |
| Present | 20 | 14 (13%) | 6 (6%) | ||
| Medullary histology | Absent | 209 | 106 (95%) | 103 (98%) | 0.447 |
| Present | 7 | 5 (5%) | 2 (2%) | ||
| Serrated histology | Absent | 193 | 98 (88%) | 95 (90%) | 0.602 |
| Present | 23 | 13 (12%) | 10 (10%) | ||
| Cribriform comedo histology | Absent | 202 | 104 (94%) | 98 (93%) | 0.914 |
| Present | 14 | 7 (6%) | 7 (7%) | ||
| Peritumoral lymphoid reaction | Absent | 16 | 12 (11%) | 4 (4%) | 0.045 |
| Present | 194 | 95 (89%) | 99 (96%) |
Abbreviations: AJCC/UICC, American Joint Committee on Cancer/International Union against Cancer; TNM, tumor-node-metastasis; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated;
Age subgroups were dichotomously classified using a cutoff value of the average age (58 years) of study patients.
Molecular correlations of nuclear maspin-positive CRCs
A summary of the underlying molecular features, including expression of DNA mismatch repair proteins, CIMP status, and KRAS/BRAF mutations, depending on nuclear maspin expression status in the 216 primary MSI-H CRCs is presented in Table 2. Maspin-positive tumors were significantly associated with CIMP-H (P < 0.001; Table 2) in the MSI-H CRCs. In addition, among the eight CIMP markers, promoter CpG island methylation of seven markers (MLH1, NEUROG1, CACNA1G, CRABP1, p16, IGF2, and RUNX3) was also significantly related to nuclear maspin-positive status (Table 2). The other molecular factors, including MLH1/MSH2/MSH6/PMS2 expression and KRAS/BRAF mutations, demonstrated no significant correlation with nuclear maspin expression (Table 2).
Table 2.
Molecular features according to nuclear maspin expression status in MSI-H CRCs (n = 216)
| Molecular factors | Total cases | Nuclear maspin-positive | Nuclear maspin-negative | P value | |
|---|---|---|---|---|---|
| MLH1 expression | Loss | 138 | 74 (67%) | 64 (61%) | 0.382 |
| Retained | 78 | 37 (33%) | 41 (39%) | ||
| MSH2 expression | Loss | 66 | 33 (30%) | 33 (31%) | 0.786 |
| Retained | 150 | 78 (70%) | 72 (69%) | ||
| MSH6 expression | Loss | 73 | 39 (35%) | 34 (32%) | 0.669 |
| Retained | 143 | 72 (65%) | 71 (68%) | ||
| PMS2 expression | Loss | 145 | 77 (69%) | 68 (65%) | 0.471 |
| Retained | 71 | 34 (31%) | 37 (35%) | ||
| CIMP status | CIMP-H | 56 | 40 (36%) | 16 (15%) | < 0.001 |
| CIMP-L/0 | 160 | 71 (64%) | 89 (85%) | ||
| MLH1 methylation | Methylated | 64 | 43 (39%) | 21 (20%) | 0.003 |
| Unmethylated | 152 | 68 (61%) | 84 (80%) | ||
| NEUROG1 methylation | Methylated | 62 | 42 (38%) | 20 (19%) | 0.002 |
| Unmethylated | 154 | 69 (62%) | 85 (81%) | ||
| CACNA1G methylation | Methylated | 59 | 39 (35%) | 20 (19%) | 0.008 |
| Unmethylated | 157 | 72 (65%) | 85 (81%) | ||
| CRABP1 methylation | Methylated | 112 | 65 (59%) | 47 (45%) | 0.043 |
| Unmethylated | 104 | 46 (41%) | 58 (55%) | ||
| p16 methylation | Methylated | 88 | 56 (50%) | 32 (30%) | 0.003 |
| Unmethylated | 128 | 55 (50%) | 73 (70%) | ||
| IGF2 methylation | Methylated | 58 | 40 (36%) | 18 (17%) | 0.002 |
| Unmethylated | 158 | 71 (64%) | 87 (83%) | ||
| RUNX3 methylation | Methylated | 62 | 43 (39%) | 19 (18%) | 0.001 |
| Unmethylated | 154 | 68 (61%) | 86 (82%) | ||
| SOCS1 methylation | Methylated | 84 | 44 (40%) | 40 (38%) | 0.816 |
| Unmethylated | 132 | 67 (60%) | 65 (62%) | ||
| KRAS mutation | Wild type | 168 | 89 (82%) | 79 (79%) | 0.63 |
| Mutant | 41 | 20 (18%) | 21 (21%) | ||
| BRAF mutation | Wild type | 189 | 96 (87%) | 93 (89%) | 0.77 |
| Mutant | 26 | 14 (13%) | 12 (11%) |
Abbreviations: CIMP, CpG island methylator phenotype; CIMP-H, CIMP-high; CIMP-L/0, CIMP-low or CIMP-negative.
Prognostic significance of nuclear maspin expression in MSI-H CRCs
Kaplan-Meier survival analysis with log-rank test of all the 216 patients with MSI-H CRC revealed that the patient subgroup with nuclear maspin-positive tumor was significantly associated with worse DFS in comparison with the patient subgroup presenting nuclear maspin-negative tumors (P = 0.025; Figure 2A). However, in a survival analysis of patients with stage II or III MSI-H CRC receiving post-operative fluoropyrimidine-based adjuvant chemotherapy (n = 124), the tendency towards worse DFS of the nuclear maspin-positive subgroup was maintained, but not significant (P = 0.274; Figure 2B). Finally, in a multivariate analysis based on the Cox proportional hazard regression model, nuclear maspin expression failed to be an independent prognostic factor in MSI-H CRCs (hazard ratio, 1.41; 95% confidence interval, 0.77 to 2.56; P = 0.265; Table 3).
Figure 2.
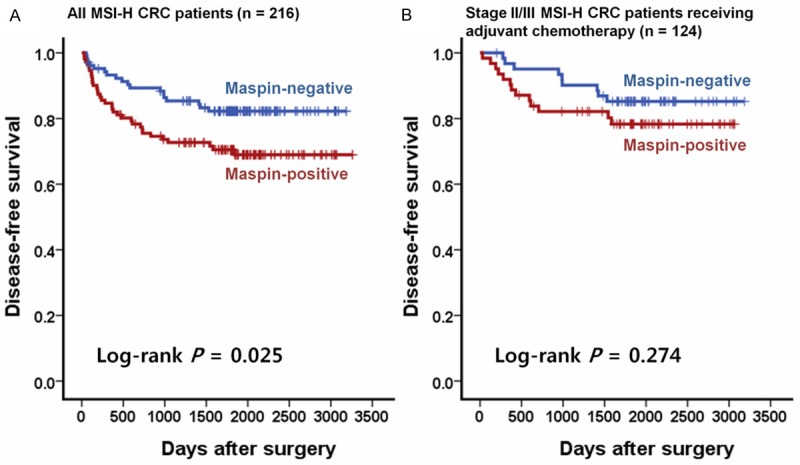
Kaplan-Meier survival analysis with log-rank test. A. A comparison of DFS rates between nuclear maspin-positive and -negative subgroups was performed in all MSI-H CRC patients (n = 216). B. A comparison of DFS rates between nuclear maspin-positive and -negative subgroups was performed in stage II/III MSI-H CRC patients treated with fluoropyrimidine-based adjuvant chemotherapy (n = 124).
Table 3.
Cox proportional hazard regression model-based multivariate analysis of patients with MSI-H colorectal cancer (n = 216)
| Variables | n | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|---|
|
|
|
||||
| H.R. (95% C.I.) | H.R. (95% C.I.) | ||||
| Nuclear maspin expression | |||||
| Maspin-negative (0/1+) | 105 | 1 (reference) | 1 (reference) | ||
| Maspin-positive (2+/3+) | 111 | 1.91 (1.08-3.4) | 0.027 | 1.41 (0.77-2.56) | 0.265 |
| AJCC/UICC TNM stage | |||||
| Stage I/II | 139 | 1 (reference) | 1 (reference) | ||
| Stage III/IV | 77 | 4.34 (2.44-7.72) | < 0.001 | 3.26 (1.77-6.02) | < 0.001 |
| Tumor differentiation | |||||
| WD/MD | 171 | 1 (reference) | 1 (reference) | ||
| PD | 45 | 3.01 (1.71-5.29) | < 0.001 | 1.95 (1.08-3.52) | 0.027 |
| CIMP | |||||
| CIMP-L/0 | 160 | 1 (reference) | 1 (reference) | ||
| CIMP-H | 56 | 2.36 (1.35-4.12) | 0.003 | 1.34 (0.68-2.63) | 0.401 |
| Age | |||||
| Younger (< 58 years) | 102 | 1 (reference) | 1 (reference) | ||
| Older (≥ 58 years) | 114 | 1.63 (0.92-2.87) | 0.094 | 1.33 (0.69-2.58) | 0.394 |
Abbreviations: H.R., Cox hazard ratio; 95% C.I., 95% confidence interval of H.R.; AJCC/UICC, American Joint Committee on Cancer/International Union against Cancer; TNM, tumor-node-metastasis; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; CIMP, CpG island methylator phenotype; CIMP-L/0, CIMP-low or CIMP-negative; CIMP-H, CIMP-high.
Discussion
Although previous studies reported the association of nuclear maspin expression with MSI-H status, poor survival, and beneficial response to adjuvant chemotherapy in CRC [6,10,19], a more detailed clinical and molecular analysis of maspin expression in CRC still remained to be elucidated. In our present study, we successfully revealed the significant correlation between nuclear maspin expression and CIMP-H status in CRC, and this finding provides important clues for the molecular basis of subcellular alteration of maspin expression in CRC. In our study, using a large series of MSI-H CRC samples, we excluded the statistical effect of MSI and focused on the pure relationship between CIMP and maspin expression in CRC. Therefore, previous observations regarding the significant association between nuclear maspin positivity and MSI-H status in CRC might represent confounding results due to the substantial overlap between CIMP-H and MSI-H in CRC.
As noted previously, the prognostic significance of maspin expression in CRC has been hypothesized to depend on its nuclear predominant expression pattern [6,7,10,19]. This feature is not surprising, as similar results have been observed in other malignancies such as malignant melanoma [8]. However, in breast cancer, nuclear maspin expression has been reported to play a tumor-suppressive role and is associated with improved patient survival. This favorable prognostic effect of nuclear maspin expression in breast cancer has also been supported by cell line experiments, which demonstrated the anti-proliferative effect of maspin protein on breast cancer cells when localized in the nucleus, but not on normal breast epithelial cells [3]. These paradoxical prognostic effects of nuclear maspin expression, depending on different tumor types, indicate that nuclear maspin expression may be one of the causal molecular alterations in carcinogenesis of several organs such as the breast, whereas it may be a consequential molecular event in some cancers such as CRC. Although maspin alteration may not be a critical causal factor in colorectal carcinogenesis, the significant value of nuclear maspin expression as a prognostic marker in CRC has been consistently confirmed by independent series of investigations, including our present study. Notably, on the basis of our data, nuclear maspin expression can distinguish a distinct prognostic subgroup associated with aggressive pathological factors and CIMP-H molecular status in CRC. According to several previous studies, CIMP-H is associated with poor prognosis in CRCs, including microsatellite-stable tumors as well as MSI-H tumors [20,21]. Thus, the significant interrelationship among nuclear maspin expression, CIMP-H status, and poor prognosis in CRC is plausible. Therefore, nuclear maspin expression can be a simple and useful screening marker, indicating both a clinically aggressive subgroup and a molecularly hypermethylated phenotype among CRCs.
The most notable result of our survival analysis is that there was no significant difference in terms of DFS based on maspin expression status in stage II/III MSI-H CRC patients receiving fluoropyrimidine-based adjuvant chemotherapy, although the tendency towards a worse survival of maspin-positive patients was maintained (Figure 2B). This finding can be interpreted as a blunting of the adverse prognostic effect of nuclear maspin expression after adjuvant chemotherapy or a relative chemo-resistant feature of the maspin-negative phenotype in CRC. Both of these interpretations are supported by previous data. Dietmaier et al. suggested that nuclear maspin expression could predict the response to 5-fluorouracil chemotherapy in CRC patients [6]. The clinical value of nuclear maspin expression as a chemotherapy predictive marker in CRC should be further evaluated.
In conclusion, nuclear maspin expression in CRC is significantly related to CIMP-H, but not to MSI-H. The association of poor prognostic and aggressive pathological features with nuclear maspin expression is also confirmed in MSI-H CRCs. Additional efforts to elucidate molecular interactions between maspin and various epigenetic factors, including CpG island methylation, in CRC are needed.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Education, Korea (2013R1A1A2059080), the Korean Health Technology R & D Project funded by the Ministry of Health and Welfare, Korea (HI13C1804), the Priority Research Centers Program through the National Research Foundation funded by the Ministry of Education, Science and Technology, Korea (2009-0093820), and the National Research Foundation grant funded by the Ministry of Science, ICT and Future planning, Korea (2011-0030049).
Disclosure of conflict of interest
None.
References
- 1.Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: molecular mechanisms and therapeutic implications. Cancer Metastasis Rev. 2012;31:529–551. doi: 10.1007/s10555-012-9361-0. [DOI] [PubMed] [Google Scholar]
- 2.Berardi R, Morgese F, Onofri A, Mazzanti P, Pistelli M, Ballatore Z, Savini A, De Lisa M, Caramanti M, Rinaldi S, Pagliaretta S, Santoni M, Pierantoni C, Cascinu S. Role of maspin in cancer. Clin Transl Med. 2013;2:8. doi: 10.1186/2001-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machowska M, Wachowicz K, Sopel M, Rzepecki R. Nuclear location of tumor suppressor protein maspin inhibits proliferation of breast cancer cells without affecting proliferation of normal epithelial cells. BMC Cancer. 2014;14:142. doi: 10.1186/1471-2407-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardi R, Santinelli A, Onofri A, Brunelli A, Pierantoni C, Pisa E, Pagliacci A, Stramazzotti D, Zuccatosta L, Mazzanti P, Sabbatini A, Gasparini S, Bearzi I, Cascinu S. Maspin expression is a favorable prognostic factor in non-small cell lung cancer. Anal Quant Cytol Histol. 2012;34:72–78. [PubMed] [Google Scholar]
- 5.Marioni G, Blandamura S, Giacomelli L, Calgaro N, Segato P, Leo G, Fischetto D, Staffieri A, de Filippis C. Nuclear expression of maspin is associated with a lower recurrence rate and a longer disease-free interval after surgery for squamous cell carcinoma of the larynx. Histopathology. 2005;46:576–582. doi: 10.1111/j.1365-2559.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 6.Dietmaier W, Bettstetter M, Wild PJ, Woenckhaus M, Rummele P, Hartmann A, Dechant S, Blaszyk H, Pauer A, Klinkhammer-Schalke M, Hofstadter F. Nuclear Maspin expression is associated with response to adjuvant 5-fluorouracil based chemotherapy in patients with stage III colon cancer. Int J Cancer. 2006;118:2247–2254. doi: 10.1002/ijc.21620. [DOI] [PubMed] [Google Scholar]
- 7.Markl B, Arnholdt HM, Jahnig H, Schenkirsch G, Herrmann RA, Haude K, Spatz H, Anthuber M, Schlimok G, Oruzio D. Shift from cytoplasmic to nuclear maspin expression correlates with shorter overall survival in node-negative colorectal cancer. Hum Pathol. 2010;41:1024–1033. doi: 10.1016/j.humpath.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Martinoli C, Gandini S, Luise C, Mazzarol G, Confalonieri S, Giuseppe Pelicci P, Testori A, Ferrucci PF. Maspin expression and melanoma progression: a matter of sub-cellular localization. Mod Pathol. 2014;27:412–419. doi: 10.1038/modpathol.2013.157. [DOI] [PubMed] [Google Scholar]
- 9.Gurzu S, Szentirmay Z, Popa D, Jung I. Practical value of the new system for Maspin assessment, in colorectal cancer. Neoplasma. 2013;60:373–383. doi: 10.4149/neo_2013_049. [DOI] [PubMed] [Google Scholar]
- 10.Bettstetter M, Woenckhaus M, Wild PJ, Rummele P, Blaszyk H, Hartmann A, Hofstadter F, Dietmaier W. Elevated nuclear maspin expression is associated with microsatellite instability and high tumour grade in colorectal cancer. J Pathol. 2005;205:606–614. doi: 10.1002/path.1732. [DOI] [PubMed] [Google Scholar]
- 11.Fung CL, Chan C, Jankova L, Dent OF, Robertson G, Molloy M, Bokey L, Chapuis PH, Lin BP, Clarke SJ. Clinicopathological correlates and prognostic significance of maspin expression in 450 patients after potentially curative resection of node-positive colonic cancer. Histopathology. 2010;56:319–330. doi: 10.1111/j.1365-2559.2010.03479.x. [DOI] [PubMed] [Google Scholar]
- 12.Snoeren N, Emmink BL, Koerkamp MJ, van Hooff SR, Goos JA, van Houdt WJ, de Wit M, Prins AM, Piersma SR, Pham TV, Belt EJ, Bril H, Stockmann HB, Meijer GA, van Hillegersberg R, Holstege FC, Jimenez CR, Fijneman RJ, Kranenburg OW, Rinkes IH. Maspin is a marker for early recurrence in primary stage III and IV colorectal cancer. Br J Cancer. 2013;109:1636–1647. doi: 10.1038/bjc.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae JM, Kim JH, Kang GH. Epigenetic alterations in colorectal cancer: the CpG island methylator phenotype. Histol Histopathol. 2013;28:585–595. doi: 10.14670/HH-28.585. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009;455:485–494. doi: 10.1007/s00428-009-0857-0. [DOI] [PubMed] [Google Scholar]
- 15.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 16.Lee DW, Kim KJ, Han SW, Lee HJ, Rhee YY, Bae JM, Cho NY, Lee KH, Kim TY, Oh DY, Im SA, Bang YJ, Jeong SY, Park KJ, Park JG, Kang GH, Kim TY. KRAS Mutation is Associated with Worse Prognosis in Stage III or High-risk Stage II Colon Cancer Patients Treated with Adjuvant FOLFOX. Ann Surg Oncol. 2015;22:187–94. doi: 10.1245/s10434-014-3826-z. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim KJ, Rhee YY, Oh S, Cho NY, Lee HS, Kang GH. Expression status of wild-type HSP110 correlates with HSP110 T17 deletion size and patient prognosis in microsatellite-unstable colorectal cancer. Mod Pathol. 2014;27:443–453. doi: 10.1038/modpathol.2013.160. [DOI] [PubMed] [Google Scholar]
- 18.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, Koh H, Simms L, Barker M, Leggett B, Levine J, Kim M, French AJ, Thibodeau SN, Jass J, Haile R, Laird PW. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 19.Gurzu S, Szentirmay Z, Toth E, Jung I. Possible predictive value of maspin expression in colorectal cancer. Recent Pat Anticancer Drug Discov. 2013;8:183–190. [PubMed] [Google Scholar]
- 20.De Sousa EMF, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, Rodermond H, van der Heijden M, van Noesel CJ, Tuynman JB, Dekker E, Markowetz F, Medema JP, Vermeulen L. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 21.Bae JM, Kim MJ, Kim JH, Koh JM, Cho NY, Kim TY, Kang GH. Differential clinicopathological features in microsatellite instability-positive colorectal cancers depending on CIMP status. Virchows Arch. 2011;459:55–63. doi: 10.1007/s00428-011-1080-3. [DOI] [PubMed] [Google Scholar]


