Abstract
Objective: Acute lung injury (ALI) is a severe complication for patients undergoing cardiac surgery necessitating cardio-pulmonary bypass (CPB), however, the possible relationship between microRNAs change and ALI induced by CPB is still not completely understood. Objective: the aim of this study is to determine the microRNAs level changes in patients with ALI induced by CPB and its involved mechanism. Methods: We collected blood samples from 45 patients and performed microRNA microarray experiments to determine the microRNAs level changes in patients with ALI induced by CPB then the result was verified by quantitative real-time PCR (qRT-PCR). Plasma TNF-α level and respiration parameters including respiration index (RI) and oxygenation index (OI) were measured at five different time points before or after CPB. Meanwhile the correlationship between significantly changed microRNAs and TNF-α level and respiration parameters was analyzed. Further more, we transfected miR-320 mimic and inhibitor into A549 cells and observed the proliferation inhibition and apoptosis change caused by oxygen-glucose deprivation/reperfusion. Finally we using dual-luciferase reporter assay, qRT-PCR and western blot investigated the potential target of miR-320. Results: The level of miR-320 was higher in CPB caused ALI with the most significance. Correlation analysis found that the level of miR-320 was positively associated with TNF-α and RI (r = 0.649 and 0.564, P < 0.05), but negative correlated with OI (r = -0.638, P < 0.05). In A549 cells, up-regulated miR-320 induced proliferation inhibition and more apoptosis. SIRT1 may be a target of miR-320 and higher miR-320 resulted in lower expression of SIRT both in mRNA and protein level. Conclusion: miR-320 may mediate the ALI after CPB in which alveolar epithelial cells are injured via down-regulating SIRT1.
Keywords: Acute lung injury, cardio-pulmonary bypass, microRNA, SIRT1
Introduction
Cardiopulmonary bypass (CPB) assisted open heart surgery have become a frequent surgical procedures with decreasing postoperative complication and mortality [1]. However, the organ damage induced by CPB is still inevitable in spite of improving technology for organ protection or postoperative monitor [2-4], especially, acute lung injury (ALI) is a common and serious complication after cardiac surgery necessitating CPB. Only 2% of patients with ALI might worsen into acute respiratory distress syndrome (ARDS), But for that to happen, it could cause a mortality of 15% to 50% as reported [5]. The mechanism ALI caused by CBP is complicated, but can be summarized two reasons in generally, one is systemic inflammatory response, the other is ischemia reperfusion injury (IRI) [6-8]. Therefore, in a number of traditional study, inflammatory involved cytokines such as TNF-α and interleukin family were focused and utilize as a biological maker for lung injury due to their significant level change [9,10], nevertheless, in recent research, microRNAs changes are known to parallel the acute lung injury induced by hypoxia, oxidative stress and IRI. For instance, inhibition of miR-17 expression could result in FoxA1 overexpression that induced alveolar type II epithelial cells injury in vivo murine model [11]. miR-155 and miR-146 regulated the immune and inflammatory responses also served as novel therapeutic targets and biomarkers for ALI/ARDS [12]. In our previous study, we found that’s level was increased in ischemia reperfusion injury rat model and its higher expression could cause myocardial damage, however, whether miR-320 was associated with CPB-ALI is still not well understood. Therefore, in this pilot study, we using microarray detected the expression change of microRNAs in patients with ALI induced by CPB. Further more, the expression of Silent mating type information regulation 2 homolog-(SIRT1) was taken into account. SIRT1 is a NAD+-dependent deacetylase that exerts many of the pleiotropic effects involved with apoptosis, cell cycle, and DNA repair and so on. Moreover numerous studies discovered a higher expression of SIRT1 in the condition of energy deficiency or other stress [13,14]. As reported by Yamamoto and Pantazi, SIRT1 was related to cell injury caused by IRI [15,16]. In present study, we assumed that MicroRNAs mediated ALI after CPB and alveolar epithelial cells via regulating SIRT1’s expression.
Materials and methods
Study population
This prospective study selected 45 patients (27 males and 118 females, median age 50.2, range 8-65) undergone cardiac surgery necessitating CPB and diagnosed as ALI within 24 h after CPB between March 2013 and February 2014. The diagnosis of ALI met the American-European Consensus Criteria. Patients included must have abnormal liver or renal function; has no pulmonary inflammation or pulmonary edema before the CPB. Those have primary pulmonary disease, died because of cardiac dysfunction, relied on extracorporeal membrane oxygenation support after the operation, or refused to be studied should be excluded. All selected patients or their supervisors signed informed consents and research protocols were approved by the ethics committee of the People’s Hospital of Gansu province.
Surgery and CPB procedure
About 30 min before anesthesia, 0.01 mg/kg scopolamine and 0.1-0.2 mg/kg morphine were injected. Anesthesia was managed according to a standard protocol, including induction with ketamine (4 mg/kg) and midazolam (0.2 mg/kg) and maintenance with fentanyl (20 μg/kg). Regular monitoring was performed. CPB was also managed according to a standard protocol using JOSTRA system. Using lactated Ringer’s solution, 20% albumin, plasma, mannitol, packed red blood cells, heparin and 5% sodium bicarbonate primed CPB circuit. Core temperature of patients was controlled at 32-32°C and pump flow rates ranged from 2.0 to 2.5 L/min. Cardiac surgery was performed as normal and patients were transferred to the ICU immediately after operation. Arterial blood samples of patients were collected at 5 different time point including T0 (after thoracotomy but before CPB), T1 (1 h after reperfusion), T2 (4 h after reperfusion), T3 (8 h after reperfusion), T4 (16 h after reperfusion).
Microarray assay
The microRNA microarray assay was provided by Exiqon Life Sciences Company (Danmark).For analysis, total RNA from blood samples collected at T0 and T4 was extracted by Trizol. And then microRNAs were fractionated and purified by the Pure Link microRNA Isolation Kit (Life Technologies, USA). Tagged microRNAs were prepared and hybridization was performed for 12 h. Using Luxscan10K/A system (Capitalbio Biotechnology, China) and Array-Pro image analysis software dealt with hybridization images. Data was analyzed by Significance analysis of microarrays 2.0.
Real time quantitative PCR
According to the preliminary result shown by microarray assay, the level of has-miR-320 and has-miR-499 was significantly changed with lower P value. Hence, these two microRNAs expression changes were verified by qRT-PCR at 5 different time point. Reverse transcription was performed using TaqMan microRNA reverse transcription kit (Takara Biotechnology, Dalian, China) to product cDNA followed with the instruction of manufacturers. Using a SYBR® Premix Ex Taq™ II kit (Takara Biotechnology, Dalian, China) on a 7900HT fast real-time PCR system (Applied Biosystems, USA) to perform PCR procedure. The primers of has-miR-320 were ACA CTC CAG CTG GGA AAA GCT GGG TTG AGA (forward) and ACA CTC CAG CTG GGT CGC CCT C (reverse). The primers of has-miR-499: AGG CCG GTT AAG ACT TGC AGT (forward), CAT AAG CCA GCG CGA TCA G (reverse). U6 RNA was used as internal controls with the primers as follows: CTC GCT TCG GCA GCA CA (forward), AAC GCT TCA CGA ATT TGC GT (reverse). Using ΔΔ cycle threshold (2-ΔΔCt) method determined the fold change of has-miR-320 and has-miR-499.
Detection of TNF-α and other respiratory parameters
TNF-α level in blood samples collected from 5 different time point was detected using ELISA Kit with the recommended protocol by provider. Radial artery blood was also collected from patients at T0-T4 and blood gas analysis was carried out. Using the formula: respiration index (RI) = [(Pb-PH2O) × FiO2-PaO2-PaCO2]/PaO2 calculated RI and the formula Oxygenation index (OI) = PaO2/FiO2 calculated OI, In the formula, Pb means standard atmospheric pressure, PH2O means Saturated vapor pressure at room temperature.
Culture of A549 cells
As human alveolar type II epithelial cell, A549 cell line was purchased from Beijing Zhongyuan Ltd (Beijing, China). A549 cells were maintained in DMEM medium (Thermo Fisher Scientific, USA) supplemented with 10% fatal bovine serum (Tianhang Biological, Hangzhou, China) at 37°C in the 5% CO2 incubator (Thermo Fisher Scientific, USA).
miR-320 level change evaluation in injured A549 cells
In order to simulate the ischemia reperfusion injury in cells, an oxygen-glucose deprivation/reperfusion (OGD/R) cell model was built. In brief, A549 cells was cultured in serum-free and glucose-free DMEM in an anaerobic conditions condition (37°C, 10% H2, 85% N2) for 4 h and transferred in normal condition for 24 h. In control group, A549 cells were maintained conventionally. qRT-PCR determined the miR-320 level change in OGD/R group.
Up-regulating and down-regulating of miR-320
To up-regulate or down-regulate the miR-320 in A549 cells, miR-320 mimic or miR-320 inhibitor (GenePharma Co., Ltd, China) were transefected into A549 cells respectively. For transfection, LipofectamineTM 2000 transfection kit (Invitrogen, USA) was utilized followed the protocol in instruction. Preliminary experiment has confirmed that above protocol could effectively increase or decrease the miR-320’s level in A549 cells.
Proliferation and apoptosis assay
miR-320 up-regulated or down-regulated A549 cells were treated with the OGD/R protocol, as control, none-transefected cells were cultured in normal condition. Using MTT (Sigma-Aldrich, USA) assay calculated the cell viability combined with a morphological observation in inverted microscope. Furthermore, flow cytometer was used to determine the apoptosis rate of cells.
Bioinformatic prediction of miR-320 targets and Dual-luciferase assay
miR-320 target genes were predicted by bioinformatic software including Target Scan program, miRanda program and PicTar program. After integrating the obtained data, SIRT1 was considered as a potential target of miR-320. Based on this preliminary result, luciferase assay reporter plasmids containing a wild or mutant type 3’UTR of SIRT1 were designed and constructed by GenePharma Co., Ltd. Then the reporter plasmids were co-transfected into A549 cells with miR-320 mimic. After a 36 h culturing, dual-luciferase assay (Promega, USA) was performed and the relative luciferase activity was calculated (relative to cells transfected miR-320 alone). The Firely luciferase activity in each sample was normalized by Renilla reniformis luciferase activity.
Expression assay for SIRT1 in A549 cells
To determine the influence of miR-320 or OGD/R treatment on SIRT1 expression, qRT-PCR and western blot were performed to estimate the level changes of SIRT1 in miR-320 up-regulated or down-regulated A549 cells after OGD/R treatment. Primers of targeted genes and β-actin were as follows: SIRT1, 5’ GCC TCA TCT GCA TTT TGA TG ’3 (sense), 5’ TCT GGC ATG TCC CAC TAT CA ’3 (antisense). β-actin, 5’ CTC CAT CCT GGC CTC GCT GT ’3 (sense), 5’ GCT GTC ACC TTC ACC GTT CC ’3 (antisense). For western blot, Total protein was extracted by RIPA Lysis buffer with added 1% protease inhibitor cocktail (Beyotime Institute of Biotechnology, China). protein samples were separated using polyacrylamide gel electrophoresis and transferred to a PVDF Membrane (Applygen Technologies Inc, China), and then blocked with 5% non-fat milk powder for 2 h at 4°C. Membranes were incubated with the primary antibody of SIRT1 or β-actin (Abcam plc., UK) overnight at 4°C. After 45 min of incubation with secondary antibody, the proteins were visualized using an ECL detection system (Applygen Technologies Inc, China).
Statistical analysis
Using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA) performed statistical analysis.
Data were expressed as mean ± SEM and were compared between multiple groups or two groups using one-way analysis of variance and T test respectively. P < 0.05 was considered as statistically significant in this study.
Results
MicroRNA microarray result
Compared with T0 time point, in T4 11 changed microRNAs were found in patients’ blood samples according to the microRNA microarray data mining and differential analyses. More specifically, 5 microRNAs reduced and 6 microRNAs were up-regulated (Table 1; Figure 1). The most obvious expression change was occurred in miR-499 (0.25 fold) and miR-320 (3.56 folds).
Table 1.
MicroRNAs expressed differently in blood sample of patients with ALI
| signals of T0 | signals of T4 | Fold change | P value | |
|---|---|---|---|---|
| has-miR-451 | 1026 | 964 | 0.94 | 3.15E-9 |
| has-miR-499 | 3489 | 523 | 0.25 | 1.10E-12 |
| has-miR-133 | 10125 | 5670 | 0.56 | 4.54E-6 |
| has-miR-143 | 4785 | 3972 | 0.83 | 3.56E-4 |
| has-miR-214 | 4469 | 3307 | 0.74 | 6.54E-8 |
| has-miR-320 | 9655 | 34372 | 3.56 | 3.12E-9 |
| has-miR-494 | 1013 | 1246 | 1.23 | 2.44E-3 |
| has-miR-200c | 8896 | 13878 | 1.56 | 6.56E-3 |
| has-miR-205 | 4897 | 12634 | 2.58 | 1.16E-7 |
| has-miR-187 | 3397 | 4450 | 1.31 | 3.65E-8 |
| has-miR-192 | 689 | 751 | 1.09 | 4.21E-9 |
Figure 1.
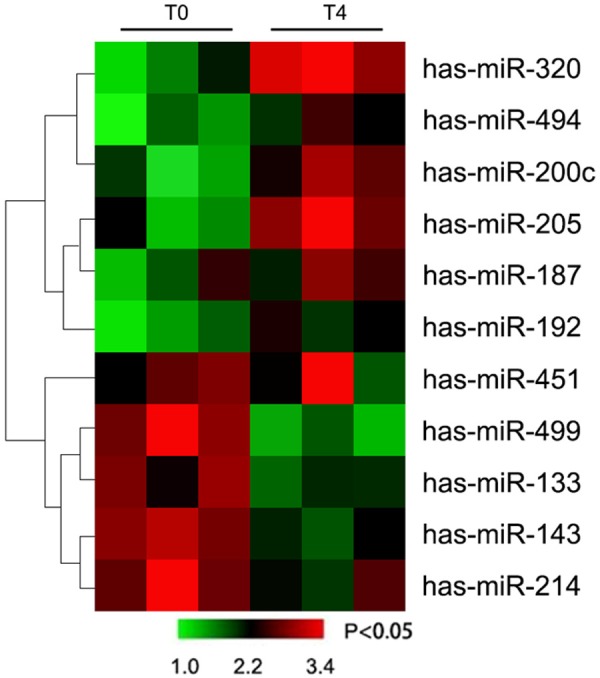
MicroRNAs signature in blood sample of patients with ALI.
Confirmation of the altered level of miR-499 and miR-320 by qRT-PCR
The accuracy of microarray results mentioned above was verified by qRT-PCR. miR-499 and miR-320 were selected because of its relatively lower P value in microarray result. As shown in Figure 2, miR-499 was gradually dropped from T0 to T5, however, the different was no significant (P > 0.05). miR-320 expression also was examined in 5 different time points, as shown in Figure 2, its level increased from T3 to T4 with statistical significance (P < 0.05). This result indicated that miR-320’s higher expression may be involved with ALI after CPB.
Figure 2.
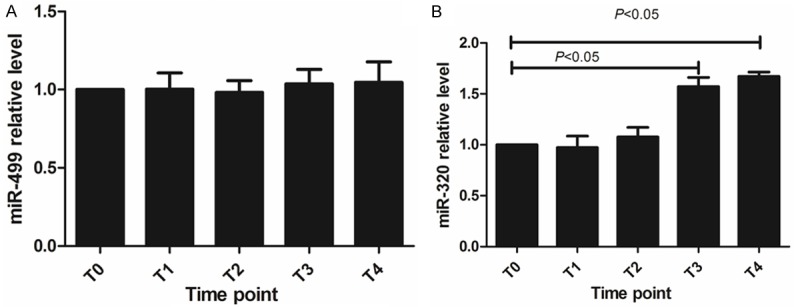
miR-499 and miR-320 level in blood sample from T0 to T1 (A) miR-499 relative level (B) miR-320 relative level.
TNF-α and respiratory parameters changes during CPB and their correlation with miR-320
TNF-α level in blood sample, the data of respiration index (RI) and Oxygenation index (OI) were analyzed in 5 different time points. As demonstrated in Figures 3 and 4, TNF-α level was raised during CPB, except for T2, in the other 4 points the difference was statistically significant compared with T0 (P < 0.05). The RI of patients that implies pulmonary ventilation function was increased in T4 (vs. T0, P < 0.05), meanwhile, the OI of patients that indicates oxygenation function was reduced in T4 (vs. T0, P < 0.05). The correlation analysis found that the level of miR-320 was positively associated with TNF-α and RI (r = 0.649 and 0.564, P < 0.05), but negative correlated with OI (r = -0.638, P < 0.05). This result suggested that higher miR-320 level might indicate poorer respiratory functions or more severe inflammatory reaction in these ALI patients.
Figure 3.
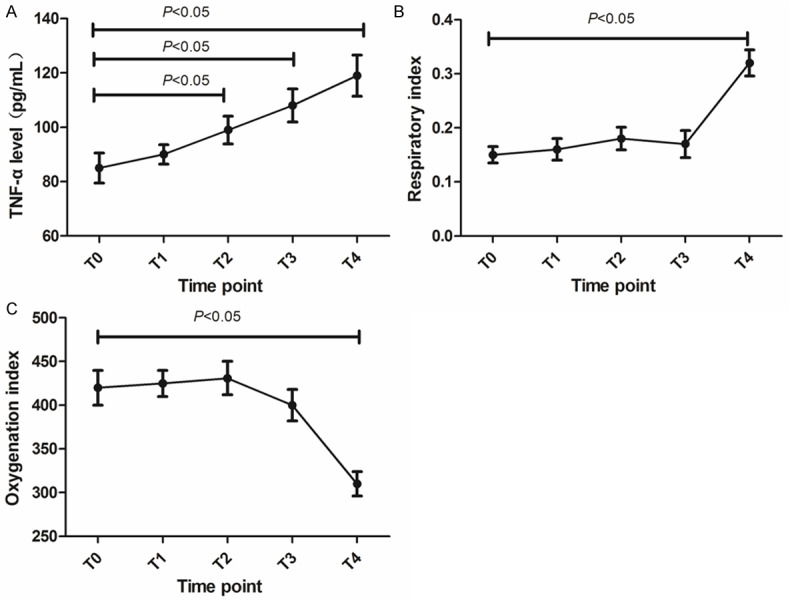
Lung injury indexes changes from T0 to T4 in ALI patients. (A) TNF-α level (B) respiration index (C) Oxygenation index.
Figure 4.
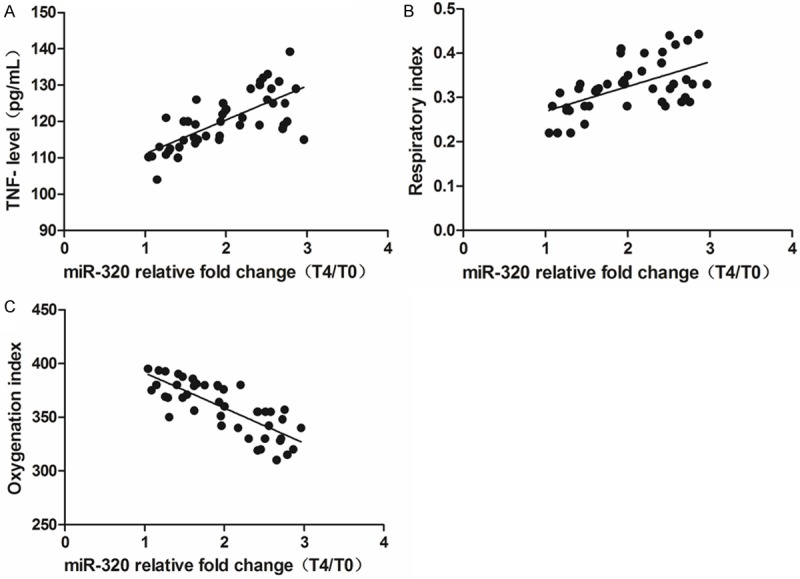
Correlation analysis for miR-320 level and lung injury indexes at T4. (A) Positive correlation between miR-320 level and TNF-α level (B) positive correlation between miR-320 level and respiration index (C) negative correlation between miR-320 level and oxygenation index.
Oxygen-glucose deprivation/reperfusion caused higher expression of miR-320 in A549 cells
As Figure 5 demonstrated, after oxygen-glucose deprivation and reperfusion treatment, miR-320 was significantly elevated in OGD/R model. Compared with control, the different was statistical (P < 0.05). It suggested that miR-320 may be related to alveolar epithelial cells injury.
Figure 5.
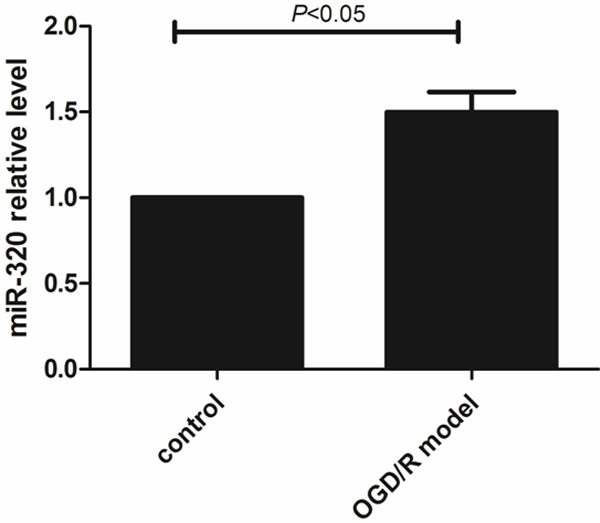
Higher miR-320 relative level in A549 cells of OGD/R model.
Up-regulating the expression miR-320 could attenuate proliferation and induce more apoptosis in injured A549 cells
After miR-320 mimic transfected, as MTT assay result shown (Figure 6), the proliferation of A549 cells in OGD/R model was inhibited more (vs. control and none-transfected cells, P < 0.05). miR-320 inhibitor transfected could attenuate this injury effect (vs. control and miR-320 mimic, P < 0.05). As well, morphological results shown that higher miR-320 level induced more cell death (Figure 7). Flow cytometer revealed that over-expression of miR-320 enhanced apoptosis dramatically caused by OGD/R (Figure 8).
Figure 6.
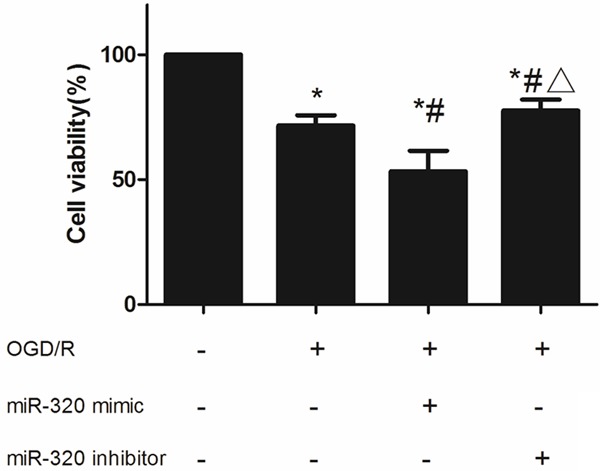
Cell viability of miR-320 up-regulated or down-regulated A549 cells after OGD/R treatment. *means compared with control P < 0.05, #means compared with none-transfected cells P < 0.05, Δmeans compared with miR-320 mimic P < 0.05.
Figure 7.
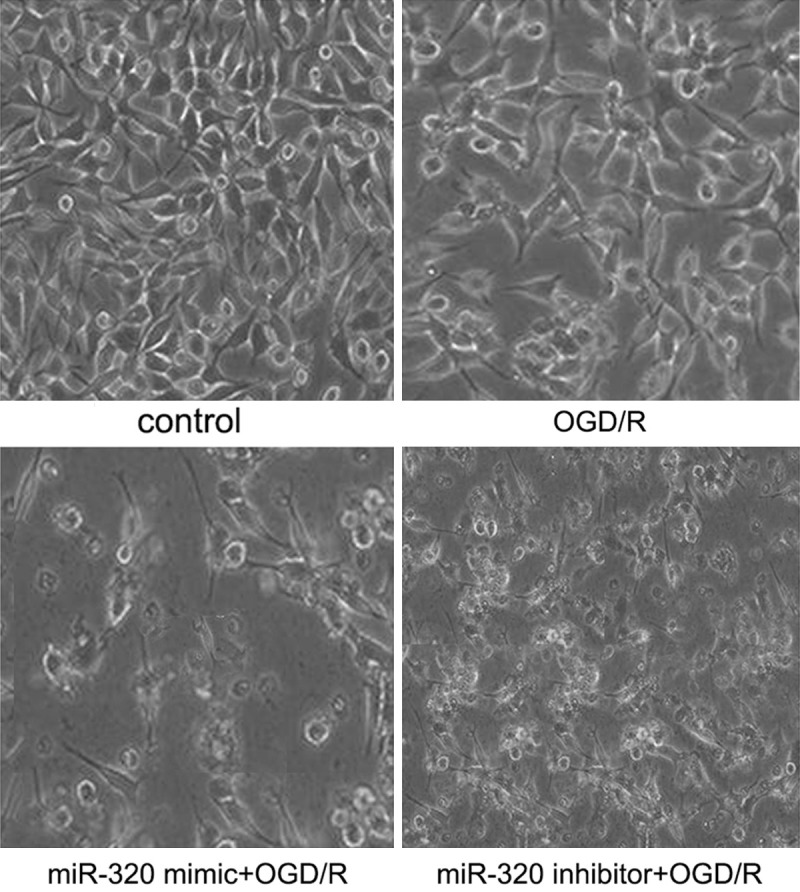
Inverted microscope image A549 cells after OGD/R treatment.
Figure 8.
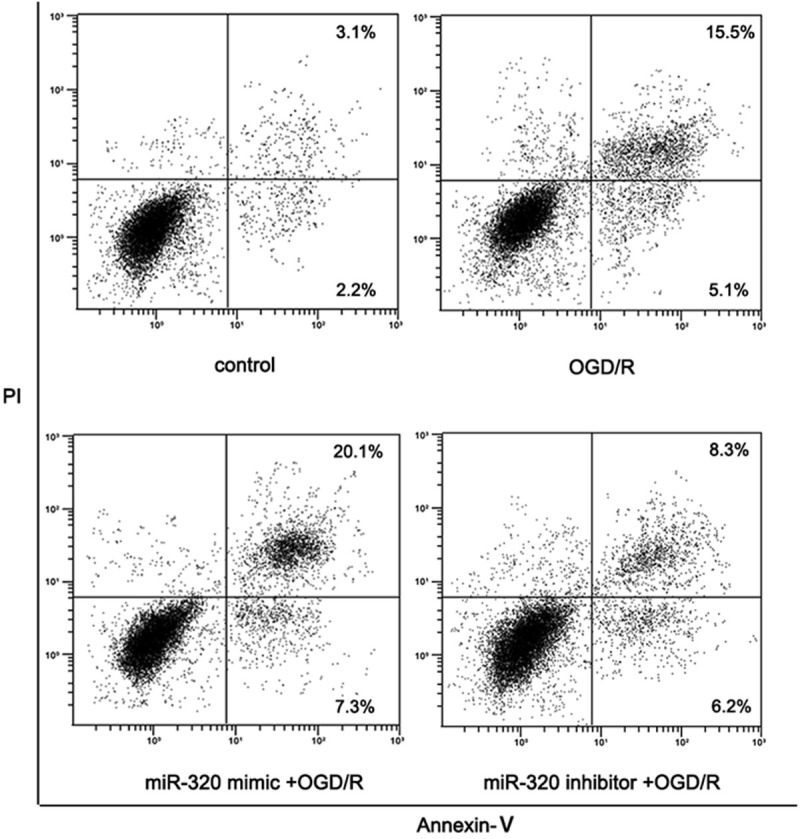
Cell apoptosis of miR-320 up-regulated or down-regulated A549 cells after OGD/R treatment.
SIRT1 is a target gene of miR-320 in A549 cells
According to the result of dual-luciferase assay (Figure 9), co-transfection a wild type 3’-UTR plasmid with miR-320 mimic significantly decreased the relative luciferase activity, in contrast, co-transfection a mutant type 3’-UTR plasmid with miR-320 mimic could induced higher luciferase activity, these different were all significant (P < 0.05). In A549 cells, OGD/R treatment could decreased the SIRT1 expression both in mRNA and protein level, more interestingly, miR-320 up-regulating caused a more lower SIRT1 expression (P < 0.05) while miR-320 down-regulating slightly retarding the increasing of SIRT1 induced by OGD/R treatment (P > 0.05) (Figures 10, 11). These results indicated that SIRT1 is a target gene of miR-320 and also involved with alveolar epithelial cells injury.
Figure 9.
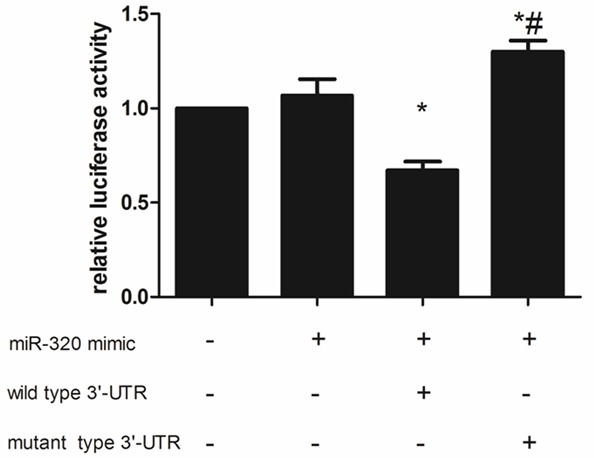
Dual-luciferase assay result.
Figure 10.
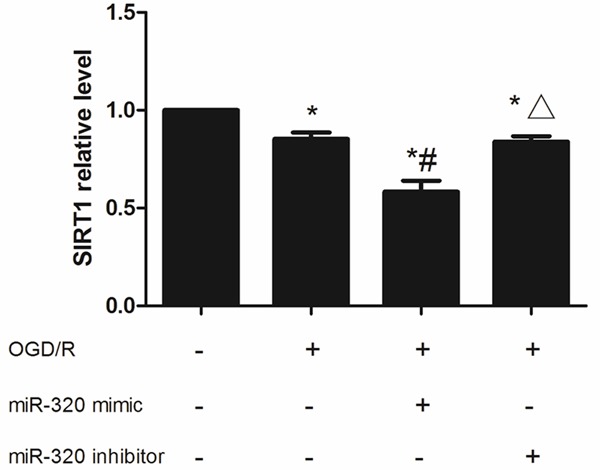
SIRT1 mRNA expression in miR-320 up-regulated or down-regulated A549 cells after OGD/R treatment. *means compared with control P < 0.05, #means compared with none-transfected cells P < 0.05, Δmeans compared with miR-320 mimic P < 0.05.
Figure 11.
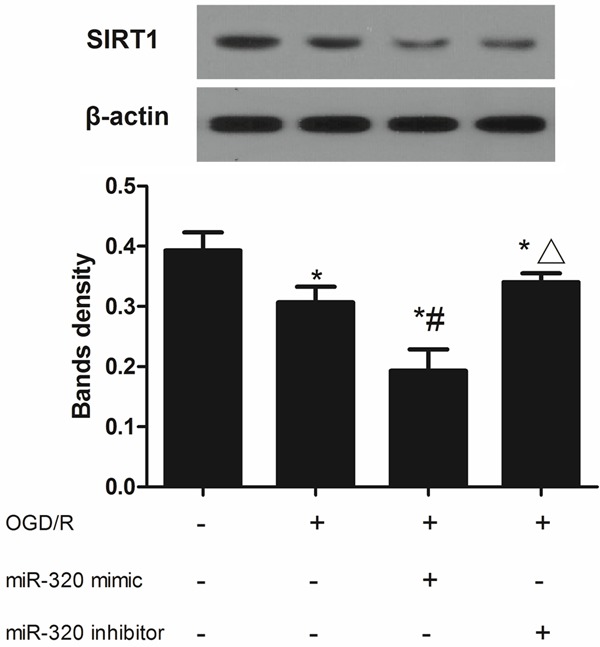
SIRT1 protein expression in miR-320 up-regulated or down-regulated A549 cells after OGD/R treatment. *means compared with control P < 0.05, #means compared with none-transfected cells, Δmeans compared with miR-320 mimic P < 0.05.
Discussion
It was traditionally considered that ALI after CPB was caused by IRI and systemic inflammation reaction syndrome. Recently several studies have suggested a functional role for microRNAs in ischemia and reperfusion or systemic inflammation [12]. Chassin et al. found that IRAK1 can be down-regulated by MicroRNA-146a, this effect protects small intestine of mouse and human from IRI [17]. As Aguado-Fraile’s report, KIF3B is one target of miR-127 which is involved with proximal tubule cells’ IRI [18]. miR-499 can protect cardiomyocyte against apoptosis through suppressing calcineurin-mediated dephosphorylation of dynamin-related protein-1, further, α- or β-isoforms of the calcineurin catalytic subunit are potential targets [19]. In inflammatory lung disease microRNAs’ possible critical role was proven by growing body of evidence. Roff suggested that miR-570-3p enhances various of cytokines and chemokines such as TNFα and IL-6 in airway epithelial cells [20]. More other researches shown that microRNA’s change mediates ALI/ARDS by numerous integrated pathways involved with genomic, proteomic, genetic and environmental information. In a mice ARDS model, induction of miR-146a, an anti-inflammatory microRNA targeting TLR4 signaling, could alternate macrophage activation and suppressed LPS-induced inducible NO synthase that resulted in amelioration of acid-induced lung injury [21]. a similar conclusion was reported by Zeng’s study, in briefly treatment with miR-146a mimic dramatically inhibited LPS-mediated TNF-α, IL-6, and IL-1β overexpression in alveolar macrophages, a key defender of the alveolar space, by suppressing IRAK-1 and TRAF-6 [21]. In murine with lung injury induced by oxidative stress, Sturrock demonstrated that miR133a and miR133b suppress GM-CSF expression and related to alveolar epithelial cells [22]. As shown in Adyshev’s study, in human lung endothelium, nonmuscle myosin light chain kinase isoform which plays a essential role in agonist-induced pulmonary endothelium cells barrier regulation could be increased by excessive mechanical stress, LPS, TNF-α, but its higher level could be attenuated by transfection with mimics of hsa-miR-374a, hsa-miR-374b, hsa-miR-520c-3p, or hsa-miR-1290 [23]. It implied that these microRNAs regulated lung injury and also represented a novel therapeutic strategy.
In present study, we provide clear evidence that several microRNAs’ expression change during ALI in CPB patients. The elevated levels of miR-320 were correlated with the severity of ALI. In fact the relationship between miR-320 and organ injury has been revealed by previous studies, these studies were united in agreement that miR-320 can promote apoptosis. Ren et al. claimed that miR-320 is involved in the regulation of I/R-induced cardiac injury and dysfunction via antithetical regulation of Hsp20 [24]. In rat heart injury model induced by ischemia-reperfusion miR-320 was significantly affected by both pre- conditioning (3 × 5 min of coronary occlusion) and post-conditioning (6×10 s of global ischemia-reperfusion at the onset of reperfusion) [22]. Although we using microarray combining with qRT-PCR detected the miR-320’s change in ALI patient after CPB, it is still unmet clinical needs, its underlying down-stream target should be also uncovered. In miR-320 over-expressed and OGD/R treated A549 cells, we observed more proliferation inhibition and increased apoptosis, even more important, we demonstrated that higher miR-320 significantly suppressed SIRT1’s expression. Combining with bioinformatics assay and Dual luciferase reporter assay, it’s indicated that SIRT1 may one target of miR-320 during ALI. SIRT1 is a member of the silent information regulator family and belongs to class III histone/protein deacetylase. It is known to play a pivotal roles in preventing cells from injury induced by various stress during IRI or inflammation [25]. For instance, a possible interaction between AMPK pathway and SIRT1 under ischemic conditions has been demonstrated to protect cerebral against ischemia [26]. The activity of SIRT1 influences the metabolism, survival of the cell through transcription regulation and post-translational modulation. SIRT1 triggered cellular protection depends on deacetylation, for example, SIRT1 promotes cell survival or inhibits cell death by deacetylating the p53, Ku70 and forkhead transcription factor [27-29]. Cytoprotection factor involved in this pathway were often adjusted by transcriptional silencing through histone modification, certainly, it might deacetylate non-histone proteins such as NF-κB in a context-specific manner [30]. In hypoxic liver cancer cells (Hep3B cell line) SIRT1 deacetylated lysine residues in the HIF2α protein, activating its transcription, another target of plays an essential role in caused by IRI is hypoxia inducible factors (HIFs) [31,32]. SIRT1 can also cope with oxidative stress through by means of transcriptional up-regulation of the antioxidant enzymes such as MnSOD [33]. Considerable amounts of groups also found that stress caused cell injury and dysfunction was attributed to with SIRT1 depletion [34]. Based on above mechanism, it’s not incomprehensible that lower SIRT1 induced by miR-320 results in ALI in a condition of stress after CPB.
In conclusion, our study demonstrates that miR-320 was increased in ALI induced by CPB. SIRT1 is a potential target of miR-320, Moreover, miR-320 indicated the severity of ALI and may mediate alveolar epithelial cell via down-regulation of SIRT1. miR-320 could be a potential biomarker for evaluating ALI in clinic.
Disclosure of conflict of interest
None.
References
- 1.Neves FH, Carmona MJ, Auler JO Jr, Rodrigues RR, Rouby JJ, Malbouisson LM. Cardiac compression of lung lower lobes after coronary artery bypass graft with cardiopulmonary bypass. PLoS One. 2013;8:e78643. doi: 10.1371/journal.pone.0078643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngatchou W, Surdeanu I, Ramadan AS, Essola B, Youatou P, Guimfacq V, Wauty P, Mols P. Penetrating cardiac injuries in Belgium: 20 years of experience in university hospitals in Brussels. Acta Chir Belg. 2013;113:275–280. doi: 10.1080/00015458.2013.11680927. [DOI] [PubMed] [Google Scholar]
- 3.Zheng J, Xiao Y, Chong M, Chen Y, Yao Y, Jin M, Liu Y, Han L. The effect of cardiopulmonary bypass duration on renal injury after congenital heart surgery in infants and young children. Adv Clin Exp Med. 2013;22:693–698. [PubMed] [Google Scholar]
- 4.Algra SO, Jansen NJ, Van der Tweel I, Schouten AN, Groenendaal F, Toet M, van Oeveren W, van Haastert IC, Schoof PH, de Vries LS, Haas F. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–233. doi: 10.1161/CIRCULATIONAHA.113.003312. [DOI] [PubMed] [Google Scholar]
- 5.Dong LY, Zheng JH, Qiu XX, Yu M, Ye YZ, Shi S, Yang DC, Xie YW. Ischemic preconditioning reduces deep hypothermic circulatory arrest cardiopulmonary bypass induced lung injury. Eur Rev Med Pharmacol Sci. 2013;17:1789–1799. [PubMed] [Google Scholar]
- 6.Krdzalic A, Klokocovnik T, Krdzalic G, Gaco S. The influence of cardiopulmonary by-pass on respiratory function in patients who underwent coronary disease surgery. Med Arch. 2013;67:97–100. doi: 10.5455/medarh.2013.67.97-100. [DOI] [PubMed] [Google Scholar]
- 7.Qu X, Li Q, Wang X, Yang X, Wang D. N-acetylcysteine attenuates cardiopulmonary bypass-induced lung injury in dogs. J Cardiothorac Surg. 2013;8:107. doi: 10.1186/1749-8090-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong J, Ye S, Wu ZK, Chen GX, Liang MY, Liu H, Zhang JX, Huang WM. Controlled oxygen reperfusion protects the lung against early ischemia-reperfusion injury in cardiopulmonary bypasses by downregulating high mobility group box 1. Exp Lung Res. 2012;38:183–191. doi: 10.3109/01902148.2012.662667. [DOI] [PubMed] [Google Scholar]
- 9.Liu K, Shen L, Wang J, Dong G, Wu H, Shao H, Jing H. The preventative role of curcumin on the lung inflammatory response induced by cardiopulmonary bypass in rats. J Surg Res. 2012;174:73–82. doi: 10.1016/j.jss.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang JF, Bian JJ, Wan XJ, Zhu KM, Sun ZZ, Lu AD. Association between inflammatory genetic polymorphism and acute lung injury after cardiac surgery with cardiopulmonary bypass. Med Sci Monit. 2010;16:CR260–265. [PubMed] [Google Scholar]
- 11.Xu Z, Zhang C, Cheng L, Hu M, Tao H, Song L. The microRNA miR-17 regulates lung FoxA1 expression during lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2014;445:48–53. doi: 10.1016/j.bbrc.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T, Garcia JG, Zhang W. Integrating microRNAs into a system biology approach to acute lung injury. Transl Res. 2011;157:180–190. doi: 10.1016/j.trsl.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada T, Furuta H, Doi A, Ariyasu H, Kawashima H, Wakasaki H, Nishi M, Sasaki H, Akamizu T. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway. Metabolism. 2014;63:469–474. doi: 10.1016/j.metabol.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Yao H, Sundar IK, Ahmad T, Lerner C, Gerloff J, Friedman AE, Phipps RP, Sime PJ, McBurney MW, Guarente L, Rahman I. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2014;306:L816–828. doi: 10.1152/ajplung.00323.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantazi E, Zaouali MA, Bejaoui M, Serafin A, Folch-Puy E, Petegnief V, De Vera N, Ben Abdennebi H, Rimola A, Roselló-Catafau J. Silent information regulator 1 protects the liver against ischemia-reperfusion injury: implications in steatotic liver ischemic preconditioning. Transpl Int. 2014;27:493–503. doi: 10.1111/tri.12276. [DOI] [PubMed] [Google Scholar]
- 17.Chassin C, Hempel C, Stockinger S, Dupont A, Kübler JF, Wedemeyer J, Vandewalle A, Hornef MW. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguado-Fraile E, Ramos E, Saenz-Morales D, Conde E, Blanco-Sánchez I, Stamatakis K, del Peso L, Cuppen E, Brüne B, Bermejo ML. miR-127 protects proximal tubule cells against ischemia/reperfusion: identification of kinesin family member 3B as miR-127 target. PLoS One. 2012;7:e44305. doi: 10.1371/journal.pone.0044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 20.Roff AN, Craig TJ, August A, Stellato C, Ishmael FT. MicroRNA-570-3p regulates HuR and cytokine expression in airway epithelial cells. Am J Clin Exp Immunol. 2014;3:68–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B, Stathopoulos EN, Margioris AN, Georgopoulos D, Tsatsanis C. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192:394–406. doi: 10.4049/jimmunol.1300959. [DOI] [PubMed] [Google Scholar]
- 22.Varga ZV, Zvara A, Farago N, Kocsis GF, Pipicz M, Gáspár R, Bencsik P, Görbe A, Csonka C, Puskás LG, Thum T, Csont T, Ferdinandy P. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol. 2014;307:H216–227. doi: 10.1152/ajpheart.00812.2013. [DOI] [PubMed] [Google Scholar]
- 23.Adyshev DM, Moldobaeva N, Mapes B, Elangovan V, Garcia JG. MicroRNA regulation of nonmuscle myosin light chain kinase expression in human lung endothelium. Am J Respir Cell Mol Biol. 2013;49:58–66. doi: 10.1165/rcmb.2012-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantazi E, Zaouali MA, Bejaoui M. Role of sirtuins in ischemia-reperfusion injury. World J Gastroenterol. 2013;19:7594–7602. doi: 10.3748/wjg.v19.i43.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petegnief V, Planas AM. SIRT1 regulation modulates stroke outcome. Transl Stroke Res. 2013;4:663–671. doi: 10.1007/s12975-013-0277-y. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 28.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 29.Motta MC, Divecha N, Lemieux M. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon H, Shin SH, Shin DH, Chun YS, Park JW. Differential roles of Sirt1 in HIF-1alpha and HIF-2alpha mediated hypoxic responses. Biochem Biophys Res Commun. 2014;444:36–43. doi: 10.1016/j.bbrc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Srisuttee R, Koh SS, Kim SJ, Malilas W, Boonying W, Cho IR, Jhun BH, Ito M, Horio Y, Seto E, Oh S, Chung YH. Hepatitis B virus X (HBX) protein upregulates beta-catenin in a human hepatic cell line by sequestering SIRT1 deacetylase. Oncol Rep. 2012;28:276–282. doi: 10.3892/or.2012.1798. [DOI] [PubMed] [Google Scholar]
- 33.Arunachalam G, Samuel SM, Marei I, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]


