Abstract
Renal cell carcinoma (RCC) carries a high risk of malignancy and metastasis. The inducible isoform of prostaglandin synthase, cyclooxygenase (COX)-2, and ICAM-1 may be involved in tumor metastasis. CCN3, also called nephroblastoma overexpressed gene (NOV), has been found to regulate the proliferation and differentiation of cancer cells. The effects of NOV on RCC cell migration and expression of COX-2 and ICAM-1 have not described yet in detail. But here, NOV was found to promote the migration and expression of COX-2 and ICAM-1 in human RCC cells. Akt inhibitor was found to interfere with this NOV-induced migration and up-regulation of COX-2 and ICAM-1 in RCC cells. NOV stimulation was here found to promote the phosphorylation of Akt. RCC tissue chips were subjected to IHC staining, which showed COX-2 expression in RCC tissues to be a significantly closely correlated with NOV expression, with significance determined using Pearson correlation testing (P < 0.05). The results of the current work indicate that NOV activates COX-2 and ICAM-1 through Akt, promoting the migration of RCC cells.
Keywords: NOV, renal cell carcinoma, cell motility, COX-2, ICAM-1, akt pathway
Introduction
Renal cell carcinoma (RCC) is a common type of kidney cancer. Metastasis often goes undetected until the time of diagnosis or until the patient develops distant metastases after removal of the primary tumor. Metastatic, locally unresectable RCC is extremely difficult to treat [1]. Better therapeutic options must be based on a thorough understanding of the basic biology underlying RCC.
Most studies of the molecular foundation of cancer focus on the causes of oncogenic transformation and tumor development [2]. However, this approach may overlook the complexities of tumor cell invasion. Invading cells render themselves more motile by inducing changes in the extracellular matrix (ECM), altering their cell-cell adhesion properties, reorganizing their own cytoskeletons, and suppressing anoikis [3]. The rate-limiting step in the conversion of arachidonic acid to prostaglandins (PGs) is controlled by cyclooxygenases (COXs). The catalyst COX-2 is an inducible enzyme activated by extracellular stimuli including proinflammatory cytokines and growth factors [4]. Overexpression of COX-2 is associated with poor prognosis and short survival times. COX-2 is overexpressed in many types of cancer, including breast, colon, pancreatic, lung, renal, head, and neck cancers [5-7]. This indicates that COX-2 may affect tumorigenesis, and disrupting its expression may prevent metastasis.
Intercellular adhesion molecule 1 (ICAM-1, also called CD54) is a cell surface glycoprotein in the immunoglobulin superfamily and mediates adhesion-dependent cell-to-cell interactions [8-10]. Previous studies have shown that ICAM-1 has been associated with negative prognostic features in various cancer types, such as breast and lung cancer [11,12]. Knockdown of the ICAM-1 expression decreased the invasive activity of breast cancer cells [11]. Moreover, ICAM-1 antibody or antisense ICAM-1 cDNA has also been reported to rescue the invasiveness of breast cancer cells [13]. Thus, ICAM-1 may play a critical role in tumorigenesis and tumor cell motility.
NOV is a cysteine-rich matrix cellular protein in the CCN (Cyr61, CTGF, Nov) family. Recent studies have shown that proteins from the CCN family also play important roles in various processes associated with tumorigenesis, including cancer cell adhesion, survival, proliferation, and invasion [14,15]. Most CCN are secreted and associated with the extracellular matrix. They may connect signaling pathways and facilitate crosstalk between the stroma and epithelium [16]. The NOV molecule is expressed in the blood vessels, and in the nervous and musculoskeletal systems, though it may perform different functions in each. When first described, NOV was believed to be antiproliferative and associated with differentiation and arrest of growth in Wilm’s tumor, rhabdomyosarcomas and chondrosarcomas [17-19]. However, it is now believed to be associated with increased proliferative index in 3T3 fibroblasts and in carcinomas of the prostate and kidney [20,21]. CCN1 (Cyr61) and CCN2 (CTGF) modulate cell migration and invasion in human cancer cells and promotes migration among RCC cells [22,23], and our published paper also indicated that CCN3 (NOV) regulates proliferation, adhesion, migration and invasion in RCC cells [24]. However, the underlying mechanisms of this regulation have not described yet in detail. NOV was here found to promote migration and to upregulate COX-2 and ICAM-1 expression in human RCC cells, with the Akt signaling pathway involved.RCC tissue chips were subjected immunohistochemistry (IHC) staining, which showed COX-2 expression to be significantly closely correlated with NOV expression in RCC tissues.
Materials and methods
Materials
Rabbit polyclonal antibodies specific to β-actin, COX-2, p-Akt, ICAM-1 and Akt were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.). Akt inhibitor was purchased from Calbiochem (San Diego, CA, U.S.). Recombinant human NOV was purchased from PeproTech (Rocky Hill, NJ, U.S.). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, U.S.).
Cell lines
The RCC cell lines (786-O cell and ACHN cell) were purchased from the cell bank of the Chinese Academy of Sciences. The cells were kept in RPMI-1640 medium supplemented with 20 mM HEPES and 10% heat-inactivated FCS, streptomycin (100 mg/ml) and 2 mM-glutamine, penicillin (100 U/ml) at 37°C with 5% CO2.
Real-time RT-PCR analysis
Quantitative real-time RT-PCR was carried out using SYBRGreen and a standard protocol (Roche Diagnostics GmbH, Mannheim, Germany). Total RNA was isolated and then cDNA was reverse-transcribed from 1 μg of RNA using RT-PCR standard methods. Real-time PCR was carried out in triplicate using gene expression assays on an ABI 7500 machine (Applied Biosystems, Foster City, CA, U.S.). Real-time PCR primers for COX-2 were 5’-GCACCCCGACATAGAGAGC-3’ (forward) and 5’-TTCCACGTCAAAGGACTGCAT-3’ (reverse). ICAM-1 was 5’-ATGCCCAGACATCTGTGTCC-3’ (forward) and 5’-GGGGTCTCTATGCCCAACAA-3’ (reverse). PCR products were detected by measuring the fluorescence emitted at the end of each reaction cycle. The threshold cycle (Ct) here refers with the number of cycles required to detect a fluorescence signal greater than baseline. The 786-O cell line served as a calibrator and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an endogenous control. A negative control was prepared without a cDNA template and run with each assay. Data analysis was performed with SDS version 1.3.0.
Migration assay
Migration assays were performed with Transwell in 24-well dishes (Costar, NY, U.S.; pore size, 8 mm). The RCC cell lines 786-O and ACHN were pretreated for 12 h with NOV at different concentrations and for 30 min with Akt inhibitor, the COX-2-specific inhibitor NS-398, or vehicle control (0.1% DMSO). Approximately 2×104 cells were seeded in 100 ml of serum-free RPMI-1640 medium in the upper chamber. Approximately 300 ml of the same medium containing 10% FBS was added to the lower chamber. Plates were incubated for 4 h at 37°C in 5% CO2. Cells that remained adherent to the upper sides of the filters were removed using cotton-tipped swabs. Inserts were fixed in methanol and stained H&E. The cells that came to attach to the other side of the insert were counted in 8 random fields under a light microscope at a magnification of 200. Each clone was plated three times per experiment, and each experiment was performed at least three times [25].
Western blot analysis
Cellular lysates were prepared as described in previous works [26]. Proteins were resolved on SDS-PAGE and then transferred to Immobilon polyvinyldifluoride (PVDF) membranes. Concentrations were determined using a BCA protein assay kit and Bradford protein method (Sigma, St Louis, MO, U.S.). Approximately 30 μg protein was loaded onto a pre-cast Bis-Tris polyacrylamide gel (8%), transferred to a PVDF membrane, and blotted with rabbit anti-human antibodies against CAM-1, Akt, p-Akt (1:1000), COX-2, and (HRP)-conjugated secondary antibodies (Abcam) (1:2000). The immunoblots were visualized using enhanced chemiluminescence. Quantitative data were recorded using ImageQuant software Image J and a computing densitometer.
IHC staining on RCC tissue chips
The study cohort included 40 patients who had undergone radical or partial nephrectomy for clear cell renal cell carcinoma (ccRCC) at the Shandong Provincial Hospital between 1993 and 2005. This protocol was approved by the Shandong Provincial Hospital ethical committee. A custom tissue array (TMA) was built using samples collected from these 40 patients. Two benign renal tissue samples served as controls. The immunohistochemical procedures were performed using an IHC kit (Santa Cruz, ImmunoCruz™ Rabbit ABC Staining System: sc-2018). Levels of NOV and COX-2 expression were measured using Image-Pro Plus software. The correlation between NOV expression and COX-2 was studied here.Furthermore, ICAM-1 expression were measured either, but our data did not show so significant difference between each tissues.
Statistical analysis
Results are here presented as means ± SD. Statistical analyses, including the student’s t-test, were performed using the Statistical Package for the Social Sciences (SPSS for Windows release 10.0; SPSS Inc., Chicago, IL, U.S.) as indicated in the results section and figure legends. P < 0.05 was set as the threshold of statistical significance. The associations among clinicopathological factors NOV, and COX-2 were evaluated using the χ2 test or Fisher’s exact test where appropriate. Correlations among variables were assessed using the Spearman correlation test.
Results
NOV-directed RCC cell migration
First ACHN and 786-O cells pretreated with NOV (Figure 1), NS-398, and Akt inhibitor were counted in transwell chambers (Figure 2). Cells pretreated with NOV showed a significant increase in cell motility over parental cells (P < 0.01) (Figure 2). The Akt inhibitor NS-398 reduced the magnitude of this NOV-induced increase in cell migration (P < 0.05) (Figure 2).
Figure 1.
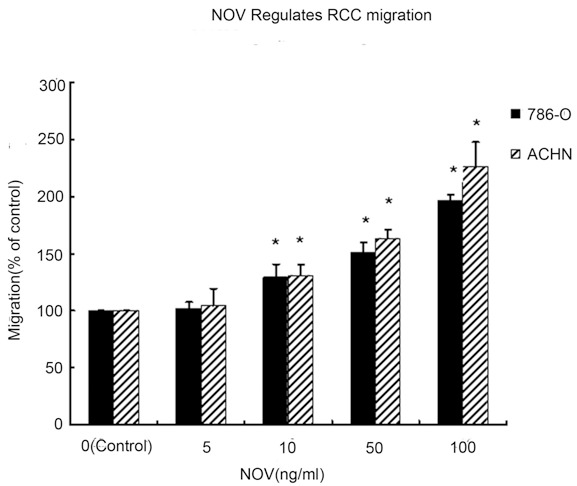
Wound healing migration assays were performed to determine the motility of 786-O and OS-RC-2 cells. RCC cells 786-O and ACHN were treated with different dose of NOV. Untreated cells were used as control. Quantification was performed by measuring the area migrated at same time point, 8 h after handled with NOV. Values represent the means ± SD of 3 independent experiments. *P < 0.05 vs. Control.
Figure 2.

Transwell migration assays were performed to determine the motility of 786-O and OS-RC-2 cells. A.The COX-2 specific inhibitors NS-398 and Akt pathway inhibitor were used in RCC cells 786-O and ACHN. The dose of NOV was keeped in 100 ng/ml. Untreated cells were used as control. Values represent the means ± SD of 3 independent experiments (*P < 0.05 vs. Control, #P < 0.05 vs. NOV treated cells.). B. Migration assays were performed using transwell chambers. The cells were pretreated with NOV, NOV+NS398 or NOV+Akt inhibitor (Untreated cells were used as control) for 8 h and then trypsinized and plated in the upper wells in serum-free medium at a concentration of 1×105/well. After 4 h, the numbers of cells migrating to the lower wells were counted following H&E staining. Left panels show representative images of the lower wells. Right panels show the means ± SD of the cell numbers in 8 random fields for 3 independent experiments. *P < 0.05 vs. Control.
NOV-directed RCC cell migration and upregulation of COX-2 and ICAM-1
Previous works have shown that COX-2 can mediate the motility of human cancer cells [27,28]. It was here hypothesized that COX-2 may be involved in NOV-mediated RCC migration. When RCC cells were treated with NOV the rate of expression of COX-2 mRNA and protein increased (Figure 3). Over-expression of COX-2 was found to increase the motility of RCC cells (Figure 3). The COX-2 specific inhibitors NS-398 were used to confirm that it was COX-2 that mediated NOV-induced cell migration. Administration of NS-398 reduced the magnitude of NOV-induced increases in cell migration (Figure 2). Similar trend has occurred to ICAM-1.We measured at different time and dose, though, here, exposure time haven’t yet to be the critical factor, when treated with NOV, the rate of ICAM-1 protein and mRNA expression increased with the dose of NOV (Figure 4). And the COX-2 specific inhibitors NS-398 have nothing with ICAM-1 expression. These results suggest that NOV-induced migration of cancer cells may involve upregulation of COX-2 and ICAM-1.
Figure 3.

COX-2 expression in 786-O and ACHN cells was assessed by Western blotting and Real-time PCR. 786-O and ACHN cells were incubated with same dose of NOV (100 ng/ml) for 24 h. Western blotting and Real-time PCR were used to detect the expression of COX-2 protein and mRNA. 0 h treated cells were used as control. Bars indicate the means ± SD of 3 independent experiments (*P < 0.05 versus control).
Figure 4.
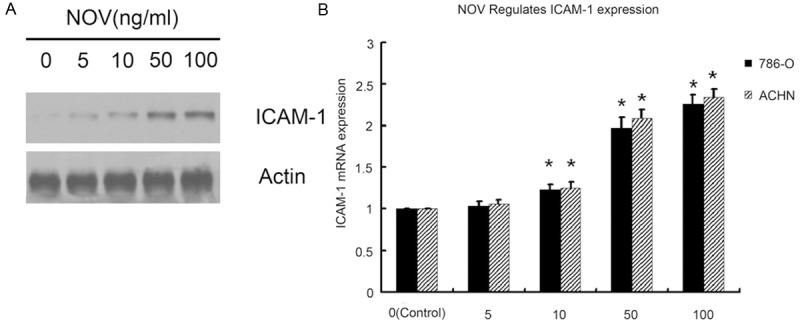
ICAM-1 expression in 786-O and ACHN cells was assessed by Western blotting and Real-time PCR. 786-O and ACHN cells were treated with different dose of NOV (0 ng/ml, 5 ng/ml, 10 ng/ml, 50 ng/ml, 100 ng/ml) for 8 h. Western blotting and Real-time PCR were used to detect the expression of ICAM-1 protein and mRNA. 0h treated cells were used as control. Untreated cells were used as control. Values represent the means ± SD of 3 independent experiments. *P < 0.05 vs. Control.
Akt signaling pathway, ICAM-1, NOV-mediated upregulation of COX-2 and migration of RCC
As indicated by the results of the migration assays, exposure to Akt inhibitor interfered with NOV-induced increases in cell migration. This suggested that the Akt signaling pathway may have been involved. To confirm this, p-Akt expression was measured in 786-O cells overexpressing NOV, 786-O and in ACHN RCC cell lines pretreated with NOV. Results showed higher levels of p-Akt expression than in parental cells (P < 0.01) (Figure 5). Articularly, the variation has similar trend with ICAM-1. That is to say, through Akt pathway, NOV also influences ICAM expression to increase cell migration (Figure 5). Data also showed that COX-2 expression was also upregulated in these cells. When the Akt pathway was partially blocked by the inhibitor, there was less NOV-mediated COX-2 upregulation (Figure 5), suggesting that the Akt signaling pathway affects NOV-mediated COX-2 upregulation and migration of RCC.
Figure 5.
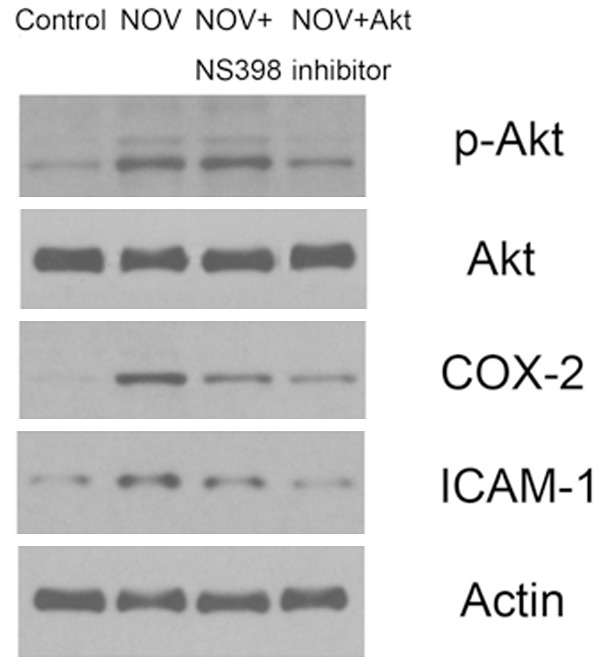
Western blotting were performed to determine the expression of p-Akt, Akt, COX-2 and ICAM-1 in 786-O cells. The COX-2 specific inhibitors NS-398 and Akt pathway inhibitor were used in RCC cells 786-O.The dose of NOV was keeped in 100 mg/nl. Untreated cells were used as control. The intensities of proteins were quantified by densitometry analysis using Image J software. Bars indicated the means ± SD from 3 independent experiments.NS-398 and Akt pathway inhibitor treated cells p-AKT, COX-2 and ICAM-1 expression were different with Control or cells treated with Akt pathway inhibitor (P < 0.01). Differences between Control and cells treated with Akt pathway inhibitor were not significantly (P > 0.05).
Immunohistochemical expression, clinicopathological variables, and associations among NOV and COX-2 expression (Table 1)
Table 1.
Immunohistochemical expression, clinicopathological variables, and associations among NOV and COX-2 expression
| Characteristic | No. of patients (N=40) | NOV | COX-2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High | Low | P-value | High | Low | P-value | ||
| Total number | 40 | 22 | 18 | 27 | 13 | ||
| Gender | |||||||
| male | 26 | 14 | 12 | 18 | 8 | ||
| female | 14 | 8 | 6 | 0.842 | 9 | 5 | 0.548 |
| Tumor size (pT) | |||||||
| T1a | 16 | 4 | 12 | 7 | 9 | ||
| T1b | 12 | 8 | 4 | 9 | 3 | ||
| T2 | 9 | 7 | 2 | 8 | 1 | ||
| T3 | 3 | 3 | 0 | P<0.05 | 3 | 0 | P<0.05 |
| Lymph node Metastasis (N) | |||||||
| negative | 20 | 6 | 14 | 8 | 12 | ||
| positive | 20 | 16 | 4 | 0.003 | 19 | 1 | 0.004 |
T1a: limited to kidney <4 cm); T1b: limited to kidney >4 cm, <7 cm; T2 (limited to kidney >7 cm; T3: tumor/tumor thrombus extension into major veins or perinephric tissues, but not into adrenal gland or beyond.
Examples of reactivity to NOV and COX-2 are given in (Figure 6).
Figure 6.

Expression of NOV and COX-2 in ccRCC tissues. Some of the proteins show null/low or high levels of expression (immunohistochemical staining; original magnification ×200). The immunohistochemical procedures were performed using an IHC kit (Santa Cruz, ImmunoCruz™ Rabbit ABC Staining System: sc-2018). Levels of NOV and COX-2 expression were measured using Image-Pro Plus software. The correlation between NOV expression and COX-2 was studied her.
Protein expression values and clinicopathological data for each patient are summarized in Table 1. As shown, levels of NOV and COX-2 expression showed no association with gender.
NOV expression was noted in 27 patients (74%), and high levels of NOV expression were observed in 22 cases (55%). There was significantly more expression in larger, later-stage tumors (pT) (P < 0.05). NOV was also significantly higher in patients with lymph node metastasis (P = 0.003).
COX-2 expression was noted in 32 of 40 cases (80%). COX-2 expression was found to be significantly closely correlated with both tumor stage and tumor size (P < 0.05). There was significantly more NOV expression in cases with lymph node metastasis (P = 0.004).
Another strong correlation was found between COX-2 and NOV expression in ccRCC tissues (R = 0.393, P < 0.001).
Discussion
Renal cell carcinoma is a malignant, high-grade renal neoplasm. Existing chemotherapies are not sufficiently effective, and 20% of all patients die of metastasis. Effective adjuvant therapies capable of preventing RCC metastasis must be developed. It is here hypothesized that NOV and NOV receptors may direct the migration of RCC cells. The results of the current work showed that NOV could promote the migration of human ccRCC cells. Previous results showed that overexpression of NOV could induce an increase in the migration of ccRCC cells by as much as 5-fold. The results of the current work were verified by pretreating ccRCC cells with NOV protein. Transcriptional upregulation of COX-2 and ICAM-1 and activation of Akt pathways was found to be one of the mechanisms underlying the effects of NOV on migration. Treatments involving COX-2 and ICAM-1 selective inhibitors may be suitable for the prevention of ccRCC metastasis.
The extracellular domain of ICAM-1 is crucial for leukocytes and has been shown to be correlated with the transendothelial migration of leukocytes from the capillary bed to the surrounding tissue [29]. ICAM-1 may also promote the migration of other cell types [30,31]. It has been reported that ICAM-1 plays an important role in lung cancer cell invasion [32]. In oral cancer, prostaglandin E2 (PGE2) induced cell motility through ICAM-1 up-regulation [33]. And our data shows that ICAM-1 expression had a considerable correlation with NOV expression, which is the key director of cell motility. All in all, these results suggest that ICAM-1 is an important target for treating cancer metastasis, especially for our renal cancer.
COX-2 is a pleiotropic enzyme. It mediates physiological functions such as augmentation of angiogenesis, inhibition of cell apoptosis, and increases in cell motility. Reports have indicated that COX-2 expression is associated with metastasis in human cancer cells [27,28]. Here, overexpression of COX-2 was found to induce cell motility in ccRCC. Human ccRCC cells treated with NOV showed more COX-2 expression. However, COX-2 inhibitors were found to antagonize NOV-induced reductions in cell motility. It is here reported that NOV can increase COX-2-related production of PGD2, PGI2, and PGE2 production in osteosarcoma cells. COX-2-induced PGs may be NOV-responsive mediators. They may promote cancer migration and metastasis. This conclusion will be confirmed in ccRCC in a subsequent study.
The results of the current work demonstrate that pretreatment of ccRCC with Akt inhibitor can antagonize increases in cell migration and COX-2 expression under NOV stimulation conditions. This was confirmed when exposure to Akt inhibitor was found to reduce the magnitude of the increase in COX-2 expression and cell migration under NOV stimulation conditions. Current results also showed that the cytoplasmic kinase Akt can be activated by NOV stimulation in patients with ccRCC. These effects indicate that Akt activation is part of in NOV-mediated induction of COX-2 expression and cell migration. These results indicate that the Akt pathway might be a key to the ability of NOV to induce COX-2 activation in human ccRCC cells.
The data collected here also demonstrate that increased expression of COX-2 and NOV was correlated with tumor stage and lymph node metastasis. In ccRCC tissues, COX-2 expression showed a considerable correlation with NOV expression, which is consistent with conclusions drawn in previous works. This indicated that COX-2 and NOV are an important part of ccRCC migration and invasion, that they promote the metastasis of ccRCC cells.
Because of this, the prognosis of patients with distant ccRCC metastasis is very poor. Preventing human ccRCC metastasis is highly important. The current study shows that NOV can increase the expression of COX-2 and ICAM-1 through the Akt pathway, so promoting the migration of human ccRCC cells. The discovery of the NOV-mediated pathway may facilitate understanding of the mechanism of human ccRCC metastasis and may foster development of effective therapies.
Acknowledgements
At the point of finishing this paper, I’d like to express my sincere thanks to all those who have lent me hands in the course of my writing this paper. Furthermore, thanks for the Shandong Provincial Natural Science Foundation (Y2007C067 and ZR2014HP015), Science and Technology Development Plan Project of Shandong Province, China (2014GGH218036), and Medical and health technology development projects of Shandong Province (2014WS0341), the source of our financial support.
Disclosure of conflict of interest
None.
References
- 1.Ek ET, Choong PF. The role of high-dose therapy and autologous stem cell transplantation for pediatric bone and soft tissue sarcomas. Expert Rev Anticancer Ther. 2006;6:225–37. doi: 10.1586/14737140.6.2.225. [DOI] [PubMed] [Google Scholar]
- 2.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–53. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–9. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 5.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–9. [PubMed] [Google Scholar]
- 6.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–4. [PubMed] [Google Scholar]
- 7.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–60. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman T, Blanco FJ. Inhibitors targeting the LFA-1/ICAM-1 cell-adhesion interaction: Design and mechanism of action. Curr Pharm Des. 2008;14:2128–2139. doi: 10.2174/138161208785740225. [DOI] [PubMed] [Google Scholar]
- 9.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 10.Van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 11.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 12.Grothey A, Heistermann P, Philippou S, Voigtmann R. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1, CD54) in patients with non-small-cell lung cancer: Correlation with histological expression of ICAM-1 and tumour stage. Br J Cancer. 1998;77:801–807. doi: 10.1038/bjc.1998.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 14.Kleer CG, Zhang Y, Pan Q, Merajver SD. (CCN6) is a secreted tumor suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia. 2004;6:179–85. doi: 10.1593/neo.03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleer CG, Zhang Y, Pan Q, van Golen KL, Wu ZF, Livant D. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene. 2002;21:3172–80. doi: 10.1038/sj.onc.1205462. [DOI] [PubMed] [Google Scholar]
- 16.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–73. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevalier G, Yeger H, Martinerie C, Laurent M, Alami J, Schofield PN. novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol. 1998;152:1563–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C, Le AT, Yeger H, Perbal B, Alman BA. NOV (CCN3) regulation in the growth plate and CCN family member expression in cartilage neoplasia. J Pathol. 2003;201:609–15. doi: 10.1002/path.1468. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Liu XJ, Crowe PD, Kelner GS, Fan J, Barry G. Nephroblastoma overexpressed gene (NOV) codes for a growth factor that induces protein tyrosine phosphorylation. Gene. 1999;238:471–8. doi: 10.1016/s0378-1119(99)00364-9. [DOI] [PubMed] [Google Scholar]
- 21.Maillard M, Cadot B, Ball RY, Sethia K, Edwards DR, Perbal B. Differential expression of the ccn3 (nov) proto-oncogene in human prostate cell lines and tissues. Mol Pathol. 2001;54:275–80. doi: 10.1136/mp.54.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan TW, Yang WH, Lin YT, Hsu SF, Li TM, Kao ST. Cyr61 increases migration and MMP-13 expression via alphavbeta3 integrin, FAK, ERK and AP-1-dependent pathway in human chondrosarcoma cells. Carcinogenesis. 2009;30:258–68. doi: 10.1093/carcin/bgn284. [DOI] [PubMed] [Google Scholar]
- 23.Tan TW, Lai CH, Huang CY, Yang WH, Chen HT, Hsu HC. CTGF enhances migration and MMP-13 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Biochem. 2009;107:345–56. doi: 10.1002/jcb.22132. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Liu Z, Bi DB, Yuan XD, Liu XW, Ding ST, Lu JJ, Niu ZH. CCN3 (NOV) regulates proliferation, adhesion, migration and invasion in clear cell renal cell carcinoma. Oncol Lett. 2012;35:1099–1104. doi: 10.3892/ol.2012.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang JY, Yang WH, Chen HT, Huang CY, Tan TW, Lin YT. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009;220:418–26. doi: 10.1002/jcp.21783. [DOI] [PubMed] [Google Scholar]
- 26.Chiu YC, Lin CY, Chen CP, Huang KC, Tong KM, Tzeng CY. Peptidoglycan enhances IL-6 production in human synovial fibroblasts via TLR2 receptor,focal adhesion kinase, Akt, and AP-1- dependent pathway. J Immunol. 2009;183:2785–92. doi: 10.4049/jimmunol.0802826. [DOI] [PubMed] [Google Scholar]
- 27.Yang SF, Chen MK, Hsieh YS, Chung TT, Hsieh YH, Lin CW. PGE2/EP1 signaling pathway enhances ICAM-1 expression and cell motility in oral cancer cells. J Biol Chem. 2010;285:29808–29816. doi: 10.1074/jbc.M110.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu JF, Fong YC, Chang CS, Huang CY, Chen HT, Yang WH. Cyclooxygenase-2 enhances alpha2beta1 integrin expression and cell migration via EP1dependent signaling pathway in human chondrosarcoma cells. Mol Cancer. 2010;9:43. doi: 10.1186/1476-4598-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duperray A, Languino LR, Plescia J, McDowall A, Hogg N, Craig AG, Berendt AR, Altieri DC. Molecular identification of a novel fibrinogen binding site on the first domain ofICAM-1 regulating leukocyte-endothelium bridging. J Biol Chem. 1997;272:435–441. doi: 10.1074/jbc.272.1.435. [DOI] [PubMed] [Google Scholar]
- 30.Rauch BH, Muschenborn B, Braun M, Weber AA, Schror K. ICAM-1 and p38 MAPK mediate fibrinogen-induced migration of human vascular smooth muscle cells. Eur J Pharmacol. 2007;577:54–57. doi: 10.1016/j.ejphar.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 31.Roche Y, Pasquier D, Rambeaud JJ, Seigneurin D, Duperray A. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb Haemost. 2003;89:1089–1097. [PubMed] [Google Scholar]
- 32.Huang WC, Chan ST, Yang TL, Tzeng CC, Chen CC. Inhibition of ICAM-1gene expression, monocyte adhesion and cancer cell invasion by targeting IKKcomplex: molecular and functional study of novel alpha-methylene-gammabutyrolactone derivatives. Carcinogenesis. 2004;25:1925–1934. doi: 10.1093/carcin/bgh211. [DOI] [PubMed] [Google Scholar]
- 33.Tang CH, Lu ME. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-kappaB pathways. Prostate. 2009;69:1781–1789. doi: 10.1002/pros.21029. [DOI] [PubMed] [Google Scholar]


