Abstract
Breast cancer bone metastases are attributed to multiple cellular and molecular interactions between the cancer cells and the bone microenvironment. Some breast cancers (about 10%) manifest predominant osteoblastic bone metastases. However, the effects of cancer cell-produced factors on osteoblastic differentiation are not fully understood. Semaphorin 3A (Sema 3A) is a newly identified regulatory factor of bone rebuilding. In the present study, we demonstrated that human breast cancer MCF-7 cells, which preferentially form osteoblastic bone metastases, exhibited increased Sema 3A expression levels. We also found that MCF-7 cell-derived Sema 3A stimulated osteoblastic differentiation and nuclear β-catenin accumulation, and these effects could be blocked by shRNA Sema 3A or a Sema 3A-neutralizing antibody. In conclusion, our data suggest that MCF-7 cell-derived Sema 3A plays a causative role in osteoblastic bone metastases progression by stimulating osteoblastic differentiation.
Keywords: Breast cancer, bone metastases, osteoblastic differentiation, semaphorin 3A
Introduction
Breast cancer (BCa) is the most common cancer diagnosed in the United States and the second leading cause of cancer deaths in women [1,2]. It metastasizes to bone in greater than 80% of patients with advanced disease [3]. Once bone metastases have occurred, they cannot be cured effectively, and the patient 5-year survival rate falls dramatically to 20% [4]. Breast cancer patients who develop bone metastases suffer increased morbidity and mortality, with enhanced bone pain, pathological fractures, spinal cord compression, and other nerve compression syndromes [5].
Cytokines and other factors produced by both tumor and bone cells interact through a process that has been described as a “vicious cycle” of bone metastases [6,7]. Mixed and osteoblastic bone metastases occur in a significant number of breast cancer patients [8], although osteolytic metastases are most common. It is widely recognized that cancer cells produce factors like bone morphogenic proteins (BMPs), endothelin-1 (ET1), and platelet-derived growth factor-BB (PDGF-BB) to contribute new bone formation [8-10]. However, the precise osteoblastic metastases mechanism is not fully understood. Additional factors likely function to induce osteoblastic bone injury at metastatic sites.
Sema 3A, a secreted axon guidance molecule, is correlated with cancer cell migration, invasion and angiogenesis [11-13]. Recently, Sema 3A was shown to stimulate osteoblastic differentiation through the canonical Wnt/β-catenin signaling pathway [14,15]. It is therefore conceivable that Sema 3A produced by breast cancer cells may significantly influence bone rebuilding in which cancer cells, osteoblasts, and osteoclasts interact and stimulate bone metastases development.
In this study, we examined the role of Sema 3A produced by human breast cancer MCF-7 cells in the regulating osteoblastic differentiation. We found that Sema 3A down-expression in MCF-7 cells by shRNA significantly decreased osteoblastic differentiation enhancement of MC3T3-E1 cells induced by MCF-7 conditioned medium (CM). Meanwhile, MCF-7 cells-derived Sema 3A promoted β-catenin activation via NRP1 during the osteoblastic differentiation process. Our results suggest that Sema 3A produced in MCF-7 cells is an important mediator in osteoblastic bone metastases development.
Materials and methods
Cell cultures
The MCF-7 and MDA-MB-231 human breast cancer cell line, mouse pre-osteoblast cell line MC3T3-E1 Subclone 14 were obtained from American Type Culture Collection. MCF-7, MDA-MB-231 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS). For breast cancer cells CM, MCF-7, and MDA-MB-231 cells (2 × 1066) were transferred into 75 cm2 cell culture flasks and incubated overnight in DMEM containing 10% FBS. The medium was then changed to 10 ml DMEM plus 0.5% FBS. CM was collected 24 h later, centrifuged at 2,500 rpm for 10 min to remove cell debris, and then stored at -80°C. Sema 3A in CM was depleted by incubating with 3 μg/ml Sema 3A neutralizing antibody (Santa Cruz, CA, USA) or 10 μg/ml IgG, followed by incubation with Protein G agarose beads. The incubated media was centrifuged to remove the beads, and the supernatant was used for experiments.
MC3T3-E1 cells were cultured in α-MEM medium supplemented with 10% FBS. For differentiation studies, MC3T3-E1 cells were cultured in osteogenic supplements (OS) containing 10 mM β-glycerol phosphate (Sigma, St. Louis, MO, USA) and 50 μg/ml L-ascorbic acid 2-phosphate (Sigma) with or without 10% (v/v) breast cancer cells CM. Cultures were supplemented with fresh medium every two days. Cells were analyzed for alkaline phosphatase (ALP) activity and staining after 7 days and Alizarin Red S staining after 14 days.
Transfection of Sema 3A shRNA
MCF-7 cells were transfected with shRNA lentiviral particles directed against Sema 3A (5’-TGCAGAAGATGGACAGTAT-3’), which substantially inhibited Sema 3A expression in our previous studies, using Polybrene (Sigma) according to manufacturer’s instruction (GenePharma, Shanghai, China). A non-targeted oligonucleotide was used as a negative control. Stably transfected cells of both types were selected by 2.5 μg/ml puromycin (Sigma), and Sema 3A expression was determined by western blotting using a specific Sema 3A antibody. All analyses were performed using cells that had been passaged less than four times.
Alkaline phosphatase (ALP) activity assay
ALP activity assays were performed using hydrolysis of p-nitrophenyl phosphate (pNPP). MC3T3-E1 cells were rinsed twice with PBS and lysed by 0.3% Triton-100. For each sample, 20 μl cell lysate was incubated with p-nitrophenyl phosphate (pNPP) as the substrate at 37°C. The absorbance was measured at 405 nm after 30 min incubation. The ALP activity was normalized to the total protein content and expressed in units of μmol/min/mg protein.
ALP and alizarin red S staining
ALP staining was performed using the BCIP/NBT alkaline phosphatase color development kit (Beyotime, Haimen, China) according to the manufacturer’s instructions. Alizarin Red S staining was determined as previously described [16]. Briefly, calcium deposits from MC3T3-E1 cells were detected by staining with 2% alizarin red S at pH 4.2 for 5 min and microphotographed using a microscope.
Immunofluorescence
MCF-7 cells and MDA-MB-231 cells were fixed in 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and blocked in 1% bovine serum albumin for 1 h. The slides were then incubated with Sema 3A antibody (1:50) overnight at 4°C, FITC second antibody for 1 h at room temperature, and DAPI for 3 min. Images were obtained using confocal microscopy (Leica, Wetzlar, Germany).
Western-blot analysis
Cell lysates were obtained using radioimmunoprecipitation assay buffer supplemented with 1 mM phenylmethylsulfonyl fluoride (Sigma, St. Louis, MO). Nuclear proteins for detecting β-catenin levels were prepared as previously described [17]. CM of MCF-7 and MDA-MB-231 cells were concentrated by ultrafiltration. Protein concentration was measured with Bicinchoninic Acid assay (Beyotime). For Western blot analysis, equal protein amounts were loaded and separated on 8% (w/v) sodium dodecyl sulfate polyacrylamide gels and subsequently blotted onto PVDF membrane. After blocking with TBST containing 5% (w/v) bovine serum albumin, the membranes were incubated with primary and horseradish peroxidase-labeled secondary antibodies, respectively. Then, the blots were detected with chemiluminescence (ECL-Kit, Bio-Rad, Cambridge, MA, USA) and quantified using Quantity One analysis software, Version 4.4 (Bio-Rad).
Quantitative real-time-PCR
MC3T3-E1 cells were maintained in osteogenic media for 7 days for detecting runt-related transcription factor 2 (RUNX2), ALP, and collagen Iα1 (Col Iα1) mRNA expression. The 14-day cultures were used for detecting Osterix and osteocalcin (OCN) mRNA expression. The total RNA was extracted using RNAiso Plus reagent (Takara, Dalian, China). cDNA was generated from 1 μg of total RNA using PrimeScript RT reagent kit (Takara) and amplified with the primer pairs for RUNX2, ALP, Col Iα1, Osterix, OCN, and β-actin. The primer sequences for the transcript analysis are listed in Table 1. The reaction data were collected in an ABI 7500 system (95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s), and relative mRNA levels were calculated using the 2-ΔΔCT method.
Table 1.
Primer sequences used for quantitative real-time-PCR
| Gene | Primer sequences (5’-3’) |
|---|---|
| ALP | F: ATGGTGAGCGACACGGACAAGA |
| R: GCCTGGTAGTTGTTGTGAGCGTAA | |
| RUNX2 | F: GCTGTTGTGATGCGTATTCCTGTA |
| R: GCTGTTGTTGCTGTTGCTGTTG | |
| Col1α1 | F: ACAGGCGAACAAGGTGACAGAG |
| R: AGAACCAGGAGAACCAGGAGGA | |
| OCN | F: CGCTCTGTCTCTCTGACCTCAC |
| R: CGGAGTCTGTTCACTACCTTATTC | |
| Osterix | F: AGCGACCACTTGAGCAAACATC |
| R: GCGGCTGATTGGCTTCTTCTTC | |
| β-actin | F: GATGTGGATCAGCAAGCAGGAGTA |
| R: GCTCAGTAACAGTCCGCCTAGAAG |
Statistical analysis
Differences between experimental groups were evaluated by two-sided unpaired Student’s t-test or one-way analysis of variance (ANOVA) using SPSS 17.0 software. Differences with a P-value < 0.05 were considered statistically significant. All figures shown in this article were obtained from at least three independent experiments with a similar pattern.
Results
MCF-7 cells promote osteoblastic MC3T3-E1 cell differentiation
To identify breast cancer cell effects on osteoblastic differentiation, osteoblastic progenitor MC3T3-E1 cells were treated with OS containing 10% CM of human breast cancer MCF-7 cells or MDA-MB-231 cells. MCF-7 cells cause osteoblastic bone metastases [18-20], whereas MDA-MB-231 cells form typical osteolytic bone metastases [6,21,22]. In keeping with previous studies, alkaline phosphatase activity, a specific marker of early osteoblastic differentiation, was promoted by MCF-7 CM after 7 days of culture, whereas it was inhibited by MDA-MB-231 CM (Figure 1A, 1B). Furthermore, MCF-7 CM significantly increased mineralization nodule formation in MC3T3-E1 cells (a specific late osteoblastic differentiation marker), as demonstrated by Alizarin Red S staining at 14 days (Figure 1C). Collectively, these findings indicate that MCF-7 cells promote osteoblastic MC3T3-E1 cell differentiation in vitro.
Figure 1.

Breast cancer MCF-7 cells augment osteoblastic differentiation. MC3T3-E1 cells were plated at a density of 2.5 × 104 cells/cm2 and incubated with OS with or without 10% (v/v) breast cancer cells CM for the indicated times. A. Representative ALP staining images (× 100). B. ALP activity quantitative data. C. Mineralization nodule formation in MC3T3-E1 cells was assessed by Alizarin Red-S staining at 14 days (× 100). *, P < 0.05, vs. the OS group. n = 3.
Sema 3A is highly expressed and produced in breast cancer MCF-7 cells
To explore Sema 3A’s role in breast cancer bone metastases, we examined Sema 3A expression in MCF-7 and MDA-MB-231 cells by Western blot. We found that MCF-7 exhibited higher Sema 3A expression levels than MDA-MB-231 cells (Figure 2A). Futhermore, the Sema 3A production of MCF-7 cells CM was significantly higher than that of MDA-MB-231 cells CM (Figure 2B). Consistent with the Western blot results, Sema 3A’s fluorescence intensity in MCF-7 cells was much greater than that in MDA-MB-231 cells (Figure 2C). Together, our results suggest that breast cancer cells with osteoblastic bone lesion potentials exhibit high Sema 3A expression levels.
Figure 2.
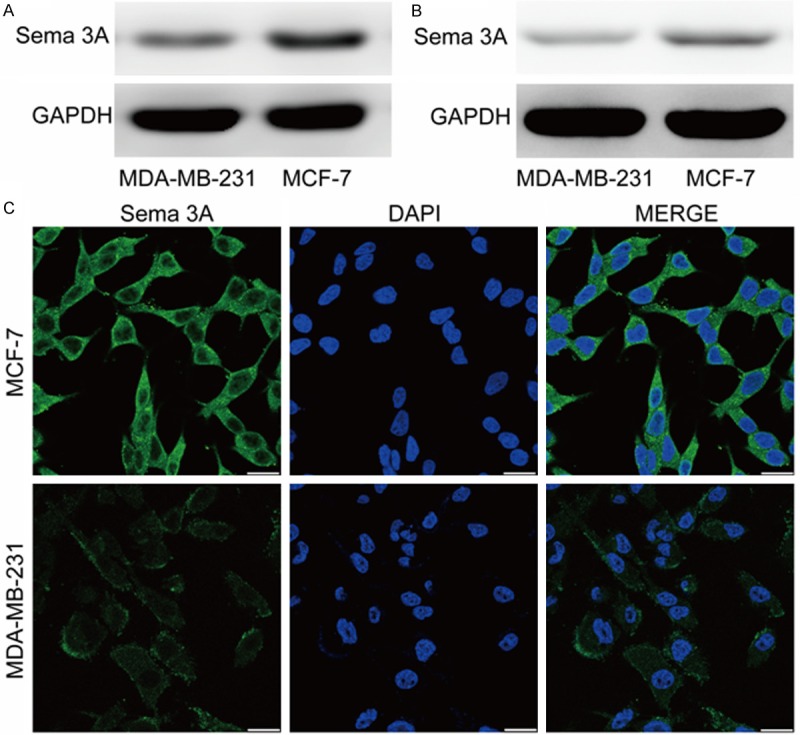
MCF-7 cells express and produce high amounts of Sema3A as assayed using western-blot and immunofluorescence analysis. A. Sema 3A expression in MCF-7 and MDA-MB-231 cells was compared via western-blot. B. Sema 3A production of MCF-7 and MDA-MB-231 cells CM was compared via western-blot. C. MCF-7 and MDA-MB-231 cells were plated on chamber slides and fixed, permeabilized, then incubated with Sema 3A antibody and FITC-conjugated antibody (green). Nuclei were stained with DAPI (blue). Scale bar is 25 μm. n = 3.
MCF-7 cell-derived Sema 3A stimulates osteoblastic differentiation in vitro
Recent studies demonstrated that Sema 3A exerts an osteoprotective influence by increasing osteoblastic differentiation. To further determine the role of MCF-7 cell-derived Sema 3A in the osteoblastic MC3T3-E1 cell differentiation, we examined whether down-regulating Sema 3A in MCF-7 cells may impact this process. MCF-7 cells were stably transfected with a Sema 3A shRNA (MCF-7Sema 3A-) or a non-targeted oligonucleotide (MCF-7NC). We observed that shRNA transfection did not affect the MCF-7 cell proliferation (data not shown). As expected, we found that the CM of MCF-7 cells expressing a Sema 3A shRNA, which exhibited obvious down-regulation of Sema 3A expression (Figure 3A), significantly decreased ALP activity in MC3T3-E1 cells compared to the blank control MCF-7 cells’ CM (MCF-7 CM, Figure 3B, 3C). As shown in Figure 3D, osteoblastic differentiation induction with MCF-7Sema 3A- CM, but not MCF-7NC CM, also resulted in obviously decreased mineralization nodule formation in MC3T3-E1 cells compared to MCF-7 CM. Furthermore, down-regulating Sema 3A expression diminished the promotion of Col1α1, ALP, RUNX2, OCN, and Osterix mRNA expression induced by MCF-7 CM (Figure 3E and 3F). More importantly, MCF-7 CM’s effects on ALP activity and mineralization nodule formation was also decreased by a Sema 3A-neutralizing antibody but not by a nonspecific polyclonal goat IgG (Figure 3G, 3H). Taken together, these results show that Sema 3A is indispensable for MCF-7 cell-induced osteoblastic formation of MC3T3-E1 cells.
Figure 3.
Down-regulating Sema 3A expression in MCF-7 cells reduces the potency of osteoblastic differentiation induced by MCF-7 cells CM. (A) The Sema 3A expression of MCF-7 cells transfected with lentiviral vector expressing Sema 3A shRNA or a non-targeted oligonucleotide was assessed by Western-blot analysis. (B, C) MC3T3-E1 cells were cultured with OS adding MCF-7 CM, or MCF-7NC CM, MCF-7 Sema 3A- CM for 7 days, then assayed for ALP activity (B), or stained for ALP (C, × 100). (D) MC3T3-E1 cells were incubated with OS and MCF-7 cells CM for 14 days, then stained with Alizarin Red S (× 100). (E, F) The relative mRNA expression levels of Col1α1, ALP, RUNX2 (E) and OCN, Osterix (F) were determined by quantitative Real-time-PCR. (G, H) MC3T3-E1 cells were cultured with MCF-7 cells CM in the presence of Sema 3A neutralizing antibody for the indicated times. Representative ALP staining images for 7 days (G, × 100), Alizarin Red S staining images for 14 days (H, × 100). *, P < 0.05, vs. the OS group; #, P < 0.05, vs. the MCF-7 CM group. n = 3.
MCF-7 cell-derived Sema 3A stimulates NRP1 expression and β-catenin nuclear accumulation in MC3T3-E1 cells
Neuropilin-1 (NRP1) is the exclusive Sema 3A receptor that supports Sema 3A signaling [23,24]. To examine NRP1’s importance for Sema 3A stimulating osteoblastic differentiation, we measured NRP1 expression levels in MC3T3-E1 cells with or without MCF-7 CM stimulation. It was found that NRP1 expression was low in the presence of OS alone. On the other hand, treating MC3T3-E1 cells with MCF-7 cells CM for 1 day greatly induced NRP1 expression, which was partially but significantly inhibited by Sema 3A shRNA down-regulation of MCF-7 cells Sema 3A expression (Figure 4A).
Figure 4.

MCF-7 cells-derived Sema 3A stimulates NRP1 expression and β-catenin nuclear accumulation in MC3T3-E1 cells. (A)MC3T3-E1 cells were incubated in OS with different CM of MCF-7 cells for 1 day. Total cellular proteins were extracted and the NRP1 expression of MC3T3-E1 cells was examined by Western blotting with a specific NRP1 antibody. (B, C) MC3T3-E1 cells were incubated in OS with different CM of MCF-7 cells for 2 days. Expression levels of cytosolic p-β-catenin (B) and β-catenin in nuclear (C) were determined by Western blotting. n = 3.
It has been reported that Wnt/β-catenin signaling is crucial to osteogenic differentiation [25,26], so we next assessed if Sema 3A is involved in β-catenin nuclear accumulation in MC3T3-E1 cells. MC3T3-E1 cells were cultured in OS with or without MCF-7 cells CM for 2 days, and the levels of cytosolic phosphorylated β-catenin (p-β-catenin, considered the inactive form of β-catenin) and nuclear β-catenin (considered the active form) were determined by Western blot. As shown in Figure 4B, p-β-catenin expression levels were dramatically increased by MCF-7Sema 3A- CM compared with MCF-7 CM and MCF-7NC CM. Meanwhile, we found that MCF-7 cells CM or MCF-7NC CM significantly increased nuclear β-catenin accumulation in MC3T3-E1 cells compared with OS alone, whereas MCF-7Sema 3A- CM had a weak effect on stimulating β-catenin nuclear accumulation (Figure 4C). Collectively, these findings indicate that Sema 3A produced by MCF-7 cells promotes β-catenin activation via NRP1 during the osteoblastic differentiation process.
Discussion
Bone is one of the most common sites of metastases in human breast cancer. Currently, there are few successful treatment options for patients with bone metastases that improve overall patient survival [27]. Understanding the molecules and their signaling mechanisms involved in bone metastases are critical for developing new and effective therapeutic approaches. In the present study, we have shown that the MCF-7 human breast cancer cells, which cause osteoblastic bone metastases in vivo [8,10,18-20], produce great amounts of Sema 3A, thereby promoting osteoblastic differentiation in vitro. Down-regulating Sema 3A expression in MCF-7 cells by shRNA significantly inhibited osteoblastic MC3T3-E1 cell differentiation induced by the MCF-7 CM. Consistent with these results, Sema 3A neutralizing antibody also reduced the stimulatory effect of the MCF-7 CM on osteoblastic differentiation. Simultaneously, we found that MCF-7 cell-derived Sema 3A promoted β-catenin activation and nuclear accumulation by binding with specific receptor NRP1 during the osteoblastic differentiation process. Taken together, these results strongly suggest that MCF-7 cell-derived Sema 3A has a causal role in breast cancer cell osteoblastic bone metastases development.
It has been recognized that some breast cancers (about 10%) manifest predominant osteoblastic bone metastases [8,10,19]. This suggests that osteoblastic bone metastases in breast cancer are not uncommon, whereas most cases of bone metastases in breast cancer are osteolytic [6,22]. Similar to osteolytic metastases pathophysiology, the vicious cycle hypothesis is also appropriate for osteoblastic bone metastases, which are attributable to tumor-produced factors that stimulate osteoblastic differentiation and bone formation. To assess Sema 3A’s potential role in pathological breast cancer bone metastases processes, the MC3T3-E1 cells were cultured in OM with or without MCF-7 CM. In the study, we showed that the MCF-7 cells, which have more osteoblastic potential, expressed higher Sema 3A levels compared to MDA-MB-231 cells. Down-regulating Sema 3A by shRNA or neutralizing antibody led to a trend of decreasing MCF-7 factor effectiveness in promoting osteoblast formation. Furthermore, Sema 3A derived from MCF-7 cells enhanced the nuclear β-catenin accumulation by binding NRP1. Sema 3A’s biological activity requires its interaction with NRP1 [28,29]. Sema 3A treatment induces NRP1 internalization and nuclear β-catenin accumulation through FRAP-2-mediated Rac1 activation [15], while the latter is crucial to osteoblastic differentiation [25,26]. These results indicate that Sema 3A derived by MCF-7 cells stimulates the osteoblastic MC3T3-E1 cell differentiation, which is at least partially mediated by the β-catenin signaling pathway. Finally, it should be noted that down-regulating Sema 3A expression could not completely decrease MCF-7 CM-induced osteoblastic differentiation indicators, suggesting that Sema 3A is necessary but not sufficient for MCF-7 CM’s osteogenic effects. Taken together, it is suggested that factors other than Sema 3A are responsible for the osteoblastic bone metastases caused by MCF-7 cells.
Sema 3A had been most extensively studied in the nervous system [23,30], but is now also recognized to be involved in multiple physiological and pathological processes. Recent reports demonstrated that Sema 3A may function as an inhibitor of angiogenesis by interfering with NRP1-mediated VEGF signaling, and it can also affect cancer cells directly by inhibiting cancer cell migration and invasion [11-13]. However, Sema 3A’s effect on tumor progression in vivo is still poorly understood. Meanwhile, we found that Sema 3A has no effect on breast cancer cell proliferation in vitro. Further studies are required to determine whether Sema 3A may affect cancer cell physiological function by an autocrine manner and osteoblastic differentiation by a paracrine manner in the bone metastases process.
In conclusion, our results reveal novel mechanisms underlying the direct breast cancer cell effects on osteoblastic differentiation in vitro. MCF-7 cells produce high Sema 3A levels, which promotes osteoblastic differentiation, and consequently stimulates osteoblastic bone lesions. As a result, Sema 3A is a potential therapeutic target for breast cancer osteoblastic bone metastases, but further studies including animal model and clinical specimens are necessary to determine whether our experimental findings described here can be extrapolated to osteoblastic bone metastases in breast cancer patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81271979).
Disclosure of conflict of interest
None.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F, Senkus-Konefka E, Fallowfield L, Costa A, Castiglione M, Group EG. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v15–19. doi: 10.1093/annonc/mdq160. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 6.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 7.Reddi AH, Roodman D, Freeman C, Mohla S. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res. 2003;18:190–194. doi: 10.1359/jbmr.2003.18.2.190. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad KS, Guise TA. Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res. 2003:S67–74. doi: 10.1097/01.blo.0000093047.96273.4e. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, Gallick GE, Maity SN, Lin SH. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011;71:5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi B, Williams PJ, Niewolna M, Wang Y, Yoneda T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002;62:917–923. [PubMed] [Google Scholar]
- 11.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 12.Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med. 2012;2:a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene. 2009;28:3537–3550. doi: 10.1038/onc.2009.204. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, Ma C, Xu C, Bando W, Fujita K, Shinomiya K, Hirai T, Asou Y, Enomoto M, Okano H, Okawa A, Itoh H. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 16.Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Li R, Xia W, Neuzil J, Lu Y, Zhang H, Zhao X, Zhang X, Sun C, Wu K. Bid integrates intrinsic and extrinsic signaling in apoptosis induced by alpha-tocopheryl succinate in human gastric carcinoma cells. Cancer Lett. 2010;288:42–49. doi: 10.1016/j.canlet.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Canon J, Bryant R, Roudier M, Branstetter DG, Dougall WC. RANKL inhibition combined with tamoxifen treatment increases anti-tumor efficacy and prevents tumor-induced bone destruction in an estrogen receptor-positive breast cancer bone metastasis model. Breast Cancer Res Treat. 2012;135:771–780. doi: 10.1007/s10549-012-2222-2. [DOI] [PubMed] [Google Scholar]
- 19.Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011;11:311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007;67:4157–4163. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 22.Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, Bonassar LJ, van der Meulen MC, Fischbach C. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res. 2013;28:2357–2367. doi: 10.1002/jbmr.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 24.Jiang SX, Whitehead S, Aylsworth A, Slinn J, Zurakowski B, Chan K, Li J, Hou ST. Neuropilin 1 directly interacts with Fer kinase to mediate semaphorin 3A-induced death of cortical neurons. J Biol Chem. 2010;285:9908–9918. doi: 10.1074/jbc.M109.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sottnik JL, Hall CL, Zhang J, Keller ET. Wnt and Wnt inhibitors in bone metastasis. Bonekey Rep. 2012;1:101. doi: 10.1038/bonekey.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Nannuru KC, Singh RK. Tumor-stromal interactions in bone metastasis. Curr Osteoporos Rep. 2010;8:105–113. doi: 10.1007/s11914-010-0011-6. [DOI] [PubMed] [Google Scholar]
- 28.Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol. 2013;24:163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci. 2012;6:28. doi: 10.3389/fncel.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development. 2009;136:1879–1888. doi: 10.1242/dev.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]



