Abstract
Mutidrug resistance (MDR) severly blocks the successful management of breast cancer. Overexpression of MDR1/p-gp accounts for the major factor in the development of MDR. β-arrestin 2 has been reported to widely involve in multiple aspects of tumor development. In order to verify whether β-arrestin 2 regulates mutidrug resistance in breast cancer, we analyzed the protein expression levels of β-arrestin 2 and MDR1/p-gp by immunohistochemistry in 106 paraffin-embedded human breast tissue samples. There was a positive correlation between β-arrestin 2 and MDR1/p-gp protein expression (P = 0.016). Changes in MDR1/p-gp mRNA and protein levels were examined by quantitative real-time reverse polymerase chain reaction (qRT-PCR) and western blotting. Silencing of β-arrestin 2 evidently down-regulated the expression of MDR1/p-gp in transfected ADM cells. In contrast, overexpression of β-arrestin 2 had the opposite changes in MDA-MB-231 and MCF-7 cells. MTS assay revealed that silencing of β-arrestin 2 increased the sensitivity to anti-cancer drugs to some extent. On the other hand, overexpression of β-arrestin 2 had the opposite effects. Our above data demonstrate that β-arrestin 2 plays a vital role in the regulation of MDR1/p-gp expression in Breast cancer.
Keywords: β-arrestin 2, mutidrug resistance (MDR), chemotherapy, MDR1/p-gp, breast cancer
Introduction
Chemotherapy is one of the most effective tools in breast cancer treatment [1]. However, the emergence of multidrug resistance (MDR) to a series of clinical chemotherapeutics with different structures or different target sites [2], severely blocks the successful management of breast cancer. The well recognized mechanism of classical MDR is the significant overexpression of human MDR1 gene encoding MDR1/p-gp [3-5]. The MDR/p-gp acts as an efflux pump in cell surface. Intracellular anti-cancer drugs increasingly flow from cells through the efflux pump [6,7], thus drug concentrations becomes lower and cancer cells becomes resistant to chemotherapeutic drugs, such as doxorubicin and paclitaxel [8]. Nowadays, modulators or inhibitors of p-gp, and gene therapy have been used to reverse MDR [9-11]. But, currently available P-gp inhibitors or modulators are nearly impossible to completely restore MDR because of their side effects [12-15]. Therefore, a reasonable method to reverse MDR in the cheomotherapy of breast cancer is to target genes widely involved in signaling pathways that play roles in the development of resistance to chemotherapeutic drugs.
Nonvisual β-arrestins, which include β-arrestin1 and β-arrestin 2, ubiquitously localize in the cytoplasm and plasma membrane [16]. They were firstly discovered as scaffold proteins involved in G protein-coupled receptors (GPCR) desensitization, sequestration, and internalization [16-18]. Recently, growing researches indicated that β-arrestin 2 plays roles in much pathological progress, especially get widely involved in many cancer developmental signaling pathways in the progression of malignant tumor. More recently, increasing evidences suggested a potential role of β-arrestin 2 in tumor viability and metastasis [17]. Mistre Alemayehu et al. discovered that β-arrestin 2 directly regulated LPA1- induced migration and invasion in breast cancer MDA-MB-231 cell lines [19]. Buchanan et al. also reported that β-arrestin 2 mediated metastasis in mouse colorectal tumor entity [17]. Besides, M. ZHAO et al. demonstrated that β-arrestin 2 has anti-apoptosis function in human breast cancer cells through casepase-8 pathways [18,20]. However, β-arrestin 2 has not been reported to mediate chemotherapy in breast cancer.
In our experimental work, we unintentionally found that the expression of β-arrestin 2 was closely related to MDR1/P-gp in our clinical samples of breast carcinoma. We hypothesized that the silence of β-arrestin 2 by plasmid-mediated expression of small interfering RNA (siRNA) may suppress the expression of MDR1/p-gp; Therefore, the multidrug resistance could be partially reversed by down-regulation of β-arrestin 2, which may improve the poor prognosis and increase survival rate of drug resistance patients with breast carcinoma. In this research, in order to prove our inference, we performed immunohistochemistry on 106 clinical breast cancer tissues to investigate protein expression of β-arrestin 2 and MDR1/p-gp. We also examined the functional relationships between β-arrestin 2 and MDR1/p-gp in three breast cancer cell lines by both downregulating and upregulating β-arrestin 2 .
Materials and methods
Tumor samples
We selected 106 paraffin-embedded samples diagnosed as invasive ductal breast carcinoma at the department of pathology, Qilu hospital from 2010 to 2011. Patient content and approval was obtained from the Institution Research Ethic Committee of Shandong Medical University.
Immunohistochemistry
Tissue arrays were deparaffinized in xylene and rehydrated in an ethanol gradient. Antigens were retrieved by heating slides in a pressure cooker in buffer (pH 6.0 sodium citrate buffer for MDR1/p-gp and EDTA buffer for β-arrestin 2). After antigen retrieval, slides were washed in PBS buffer and incubated with 3% H2O2 for 15 min at room temperature. Slides were washed in PBS buffer and blocked with protein-blocking buffer (normal goat serum, Zhongshan) for 15 min at room temperature. Tissues were incubated with primary antibodies overnight: anti-β-arrestin 2 mouse monoclonal antibody (ab54790; USA, diluted 1:200) and anti-P-gp mouse monoclonal antibody (mAb) (C494; Merck KgaA, Darmstadt, Germany, diluted 1:100). Following overnight incubation, slides were washed in washing buffer and incubated with PV900 (Zhongshan). Then Slides were incubated with substrate-chromagen solution, 3,3’-diaminobenzidine (DAB) (Zhongshan) for about 1 min, washed in distilled water, and counterstained with hematoxylin for 15 seconds. Slides were washed in washing buffer followed by distilled water and dehydrated in ethanol gradient and xylene baths. Cover slips were mounted and evaluated for positive staining. For negative controls, the antibodies were replaced with PBS.
Evaluation of immunohistochemical staining
β-arrestin 2 and MDR1/p-gp immunostaining signals were evaluated independently by two pathologists in a blinded manner. Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). Tumors were regarded as immune-positive if > 10% of tumor cells showed immunoreactivity. We considered any nuclear staining positive for β-arrestin 2, and any membranous staining positive for MDR1/p-gp.
Cells and cultures
Human breast cancer cells MCF-7 and MDA-MB-231 were purchased from the American Type Culture Collection (Manassas, VA, USA).The multidrug resistance cell line ADM were purchased from Tianjin Blood Institute. ADM cell line was derived from MCF-7 cell line and required higher concentration of paclitaxel to achieve cell death as compared to MCF-7 cell. MCF-7 was cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA) (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). MDA-MB-231 was maintained in Leibovitz’s L-15 (Gibco, Grand Island, NY, USA) medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). ADM was cultured in RPMI-1640 culture medium (Hyclone, Logan, UT, USA) containing 10% FBS. All cells were kept in a humidified atmosphere of 5% CO2 and were free from bacteria infection.
Constructions of plasmids
Full length expression vector pCDNA3.1-β-arrestin 2-GFP was favored by Professor Yin Deling, The United States east of the university of Tennessee.
siRNA targeted at β-arrestin 2 were purchased from Shanghai GenePharma Co., Ltd., gene sequences as follows: forward: 5’-GATCCCCGGGCTTGTCCTTCCGCAAAGACTTCAAGAGAGTCTTTGCGGAAGGACAAGCCCTTTTTGGAAA-3’ and reverse: 5’-AGCTTTTCCAAAAAGGGCTTGTCCTTCCGCAAAGACTCTCTTGAAGTCTTTGCGGAAGGACAAGCCCGGG-3’.
Small hairpin siRNA sequences were synthesized, annealed, and then cloned into the pSUPER. neo + GFP expression vector using T4-DNAligase to generate the pSUPER- siβ-arrestin 2 plasmid [21].
Non-targeting (Notarget) siRNAs were designed as negative controls using the sequences: Forward: 5’-GATCCCCACTCCTGGAGGAAGTTCTATTCAAGAGATAGAACTTCCTCCAGGAGTTTTTTGGAAA-3’; Reverse: 5’-AGCTTTTCCAAAAAACTCCTGGAGGAAGTTCTATCTCTTGAATAGAACTTCCTCCAGGAGTGGG-3’.
Transfection of breast cancer cells
Cells were seeded on 6-well plates at 50-60% confluence 24 h before transfection using 4 ml of respective media supplemented with 10% FBS per well. For each well, either 4 μg of pSUPER- siβ-arrestin 2 or pcDNA3.1-β-arrestin 2 plasmid DNA were diluted in 400 μl of serum-free respective media. Then, 8 μl of TurboFect in vitro Transfection Reagent (Fermentas, Burlington, Canada) was added to the diluted DNA and mixed by pipetting before incubating for 20 min at room temperature. 400 μl of the TurboFect/DNA mixture was added drop-wise to each well without removal of the previous culture medium. After 20 minutes, the plates were gently rocked to distribute the complexes evenly and were then incubated for 24 h. Non-target siRNA plasmids (pSUPER-siNC and pcDNA3.1-siNC) were used as controls. Three groups of breast cancer cells were transduced: ADMpSUPER-siβ-arrestin 2, ADMpSUPER-siNC, MCF-7pcDNA3.1-β-arrestin 2, MCF-7pcDNA3.1-NC, MDA-MB-231pcDNA3.1-β-arrestin 2, MDA-MB-231pcDNA3.1-NC, All cells were then grown for 24 h.
Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was extracted with An RNA Iso-Plus kit (Takara, Otsu, Japan) and the first strand DNA was synthesized with Omniscript Reverse Transcriptase (TOYOBO, Osaka, Japan) following the manufacturers’ instructions. We performed quantitative real-time polymerase chain reaction (qRT-PCR) reactions using eUltraSYBR Mixture (with ROX) (Beijing CoWin Bioscience Co., Ltd., Beijing, China), primers (Sangon), and cDNA in a 10 μl total reaction volume according to the manufacturer’s instructions. All samples were run in triplicate in three independent experiments, β-actin was used as the internal control. The qRT-PCR primers are: β-actin forward primer 5’- CTCCATCCTGGCCTCGC TGT-3’, and β-actin reverse primer 5’-GCTGTCACCTTCACCGTTCC-5’. MDR1 forward primer 5’-CCCATCATTGCAATAGCAGG-3’, and MDR1 reverse primer 5’-GTTCAAACTTCTGCTCCTGA-3’. β-arrestin 2 forward primer 5’-GTCGAGCCCTAACTGCAAG-3’, and β-arrestin 2 reverse primer 5’-ACAAACACTTTGCGGTCC TTC-3’.
Western blot analysis
Total cell protein extracts were lysed with RIPA lysis buffer containing a protease inhibitor on ice for half an hour. Then the complex was centrifuged at 12,000×g for 10 min at 4°C. Protein concentration could be quantified by a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). 40 μg of proteins were separated by electrophoresis on 10% SDS-PAGE, and then blotted onto PVDF membranes (Milipore, USA). Transferred blots were blocked with 5% fat-free milk powder in TBS at room temperature for 2 h. Blots were then incubated overnight at 4°C with the relevant primary antibodies, washed, and probed again with species-specific secondary antibodies coupled with horseradish peroxidase. Immunoreactivity was detected using an enhanced chemiluminescence (ECL) advance western blotting detection kit (Milipore, USA) [21]. The antibodies were specific for mouse mAb β-actin (TA-09; Zhongshan Goldenbridge Biotechnology, Beijing, China, diluted 1:1000); anti-P-gp mouse monoclonal antibody (mAb) (C494; Merck KgaA, Darmstadt, Germany, diluted 1:500), anti-β-arrestin 2 mouse monoclonal antibody (ab54790, USA, diluted 1:500).
Cytotoxicity assay for cell survival
The MTS assay was used to assess the effect of overexpression or silencing of β-arrestin 2 on the chemosensitivity of human breast cancer cells to anticancer drugs. Cells were plated in a 96-well plate at a density of 5×103 cells per well for 24 h, and then incubated with different concentrations of doxorubicin(Dalian Meilunbio Co., Ltd., China) for 24 h. Then 20 μl of MTS was added to every well and incubated at 37°C for 3 hour. Optical densities (ODs) were detected using a spectrometric absorbance of 570 nm against a background of 630 nm on a Bio-Rad microplate reader (Hercules, CA, USA). The value of (A570 anticancer drug +/A570 anticancer drug - ×100% indicated cell viability. Dose-response curves were plotted from three independent experiments.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). All experiments were performed in triplicate, and all data were presented as mean ± S.E.M. The Student’s t-test was used to determine statistical significance. Comparisons between two groups were performed using the paired t-test, and the Pearson Chi-Square tests or Fisher’s Exact Test was used to evaluate the correlation between β-arrestin 2 expression and the MDR1/P-gp, if appropriate. P < 0.05 was considered statistically significant.
Results
β-arrestin 2 expression correlated with MDR1/p-gp in human breast cancer
The immunohistochemical staining of β-arrestin 2 and MDR1/p-gp in 106-samples microarray (TMA) human patients were shown in Figure 1. β-arrestin 2 expression was observed in 73 cases, and 33 samples did not express β-arrestin 2. MDR1/p-gp expression was observed in 66 cases, and 40 samples did not express MDR1/p-gp. The immunohistochemical expression of β-arrestin 2 was positively correlated with MDR1/p-gp. (P = 0.016), which was indicated by Spearman correlation analysis (r = 0.233, P = 0.016) (Table 1).
Figure 1.
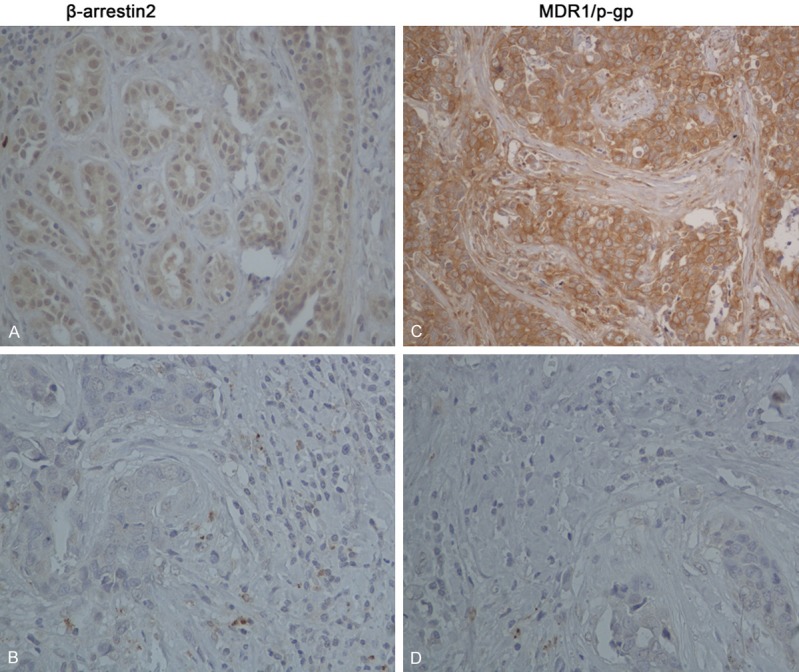
Expression of β-arrestin 2 and MDR1/p-gp in human breast cancer. Representative fields of view from the TMA cores show examples of positive β-arrestin 2 (A), positive MDR1/p-gp (C), negative β-arrestin 2 (B), and negative MDR1/p-gp (D) at ×400 magnification. β-arrestin 2 exhibited nuclear and cytoplasm immunoreactivity, MDR/p-gp membrane immunoreactivity. Correlations between β-arrestin 2 and MDR1/p-gp from these human cases were analyzed algorithmically and are depicted in Table 1.
Table 1.
Correlation between β-arrestin 2 and MDR1/p-gp in human breast cancer
| β-arrestin 2 | n | MDR1/p-gp expression | P value | Spearman | Value (r) | P value | |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| positive | negative | correlationa | |||||
| positive | 73 | 51 | 22 | 0.016 | 0.233 | 0.016 | |
| negative | 33 | 15 | 18 | ||||
The Spearman correlation was used to compare the degrees of correlation.
Positive numbers reflected direct correlation, and negative numbers reflected inverse correlation.
Relative expression of β-arrestin 2 and MDR1/p-gp in MDA-MB-231, MCF-7 and ADM cell lines
We previously detected the expression of β-arrestin 2 in the breast cancer cell lines MCF-7 and MDA-MB-231, as well as in the multidrug resistant subline MCF-7/ADM. β-arrestin 2 was found to be higher in mRNA and protein levels in ADM cells, compared to the sensitive cell lines MCF-7 and MDA-MB-231 (Figure 2). Western blotting showed that MDR1/p-gp was notably expressed in multidrug resistant ADM cells, but not in MDA-MB-231, MCF-7 cells.
Figure 2.

Relative expression of β-arrestin 2 and MDR1/p-gp in MDA-MB-231, MCF-7 and ADM cell lines. A. qRT-PCR results showed a stronger expression of β-arrestin 2 and MDR1 mRNA in ADM compared to MDA-MB-231, MCF-7 cell lines. B. Western blot results showed a higher level of β-arrestin 2 and MDR1/P-gp proteins expressed in ADM cells. C. Changes in Trps1 and MDR1/P-gp proteins were all significant, error bars indicate standard error. (**P < 0.01, ***P < 0.001, respectively).
Effects of decreased β-arrestin 2 expression on MDR1/P-gp mRNA and protein levels in ADM cells
To further explore the mechanism and effect of β-arrestin 2 on the expression of MDR1/P-gp, We transiently transfected β-arrestin 2 siRNA in ADM cells, which strikingly expresses high levels of β-arrestin 2 and MDR1/p-gp in mRNA and protein levels. We observed a dramatic decrease by 89.3% in MDR1/p-gp mRNA level by qRT-PCR when β-arrestin 2 was knocked down by 76.7%. Western blotting indicated that the MDR1/p-gp protein expression levels have the same trend as mRNA (Figure 3).
Figure 3.

Changes in β-arrestin 2 and MDR1/P-gp mRNA and protein expression in response to β-arrestin 2 knockdown. A. qRT-PCR for the expression of β-arrestin 2 and MDR1/P-gp mRNA in ADM cells (**P < 0.01, ***P < 0.001, respectively). B. Western blots showing a decrease in MDR1/P-gp and β-arrestin 2 expression in β-arrestin 2-silenced ADM cells. C. Changes in β-arrestin 2 and MDR1/P-gp proteins were all significant (**P < 0.01, ***P < 0.001, respectively).
Effects of increased β-arrestin 2 expression on MDR1/P-gp mRNA and protein levels in MCF-7 and MDA-MB-231 cells
In addition to detect the effects of β-arrestin 2 silence, We treated human breast cancer cell lines MCF-7 and MDA-MB-231 with pcDNA3.1-β-arrestin 2 vector to verify the effects on MDR1/P-gp mRNA expression. qRT-PCR showed MDR1/P-gp mRNA expression was up-regulated when β-arrestin 2 was overexpressed (Figure 4).
Figure 4.

Changes in β-arrestin 2 and MDR1/P-gp mRNA and protein expression in response to β-arrestin 2 overexpression. A. qRT-PCR for the expression of β-arrestin 2 and MDR1/P-gp mRNA in MDA-MB-231 and MCF-7 cells (**P < 0.01, ***P < 0.001, respectively). B. Western blots showing an increase in MDR1/P-gp and β-arrestin 2 expression in β-arrestin 2-overexpressed MDA-MB-231 and MCF-7 cells. C. Changes in β-arrestin 2 and MDR1/P-gp proteins were all significant (**P < 0.01, ***P < 0.001, respectively).
Western blot results for proteins showed the same trend as the mRNA levels. These results further indicated the effects of β-arrestin 2 on MDR in human breast carcinoma.
β-arrestin 2 regulates multidrug sensitivity of breast cancer cells
The evaluation of sensitivity to anti-cancer drugs was accomplished by means of MTS assay. The dose-response curves for drug concentrations and cell viability indicated an increase in sensitivity to doxorubicin in β-arrestin 2-silenced ADM cells (Figure 5). In addition, when we transfected full-length β-arrestin 2 plasmid into MDA-MB-231 and MCF-7 cell lines, sensitivity to doxorubicin was significantly decreased, indicating that β-arrestin 2 is responsible for MDR of breast cancer.
Figure 5.

Dose-response curves for doxorubicin concentrations and cell viability. Breast cancer cells were treated with four concentrations of doxorubicin for 48 h. Cell viability was calculated relative to untreated controls. A. TRPS1 knockdown resulted in an increase in doxorubicin cytotoxicity in siβ-arrestin 2 ADM cells. B and C. β-arrestin 2 transfection and upregulation of MDR1/P-gp decreased doxorubicin cytotoxicity in MDA-MB-231 and MCF-7 transfected cells. Data points represent averages from triplicates in a representative experiment, and their standard errors are depicted.
Discussion
As we all know, chemotherapy is a routine therapy method in the management of patients with breast carcinoma. However, cross-resistance to structurally and mechanistically unrelated anti-cancer drugs is a major impediment to the treatment of breast cancer [4,21,22], which leading to poor diagnosis. The mechanism of MDR has been investigated by researchers for many years and it is very complicated [23,24]. Overexpression of members of the adenosine triphosphate (ATP)-binding cassette (ABC) membrane transporter family accounts for the main factor [2,25]. MDR1/p-gp, known as a member of the (ABC) transporter family, is significantly overexpressed in multidrug resistance phenotype [2].
Several drugs targeted to MDR1/p-gp have been preliminarily used in clinical treatment, but with limited effect because of their cytotoxic effects and adverse pharmacokinetics [15,21], so it is very difficult to come to a satisfying result. Luckily, molecular therapy that targeted signaling pathways widely involved in the regulation of MDR1/p-gp has drawn more and more researcher’s attention in recent years [22].
By accident, we found a positive relationship between β-arrestin 2 and MDR1/p-gp. β-arrestin 2 was initially regarded as mediators of GPCR signaling [16,19,20,28]. Besides, growing studies demonstrated that β-arrestin 2 takes part in multiple aspects of tumor development, such as proliferation, apoptosis, migration and invasion [27,28]. However, whether β-arrestin 2 could mediate multidrug resistance has not been discovered in previous studies, so our unintentional experimental findings attract our attention.
In order to verify our findings, we selected 3 human breast cancer cell lines MDA-MB-231,MCF-7 and the multidrug resistant subline MCF-7/ADM to detect the relative expression of β-arrestin 2 and MDR1/p-gp by qRT-PCR and western blotting. We found that the expression of β-arrestin 2 in multidrug resistant subline ADM is evidently higher than chemotherapy sensitive cells MDA-MB-231 and MCF-7, which is consistent with the expression of MDR1/p-gp. We assumed that β-arrestin 2 could regulate multidrug resistance in human breast cancer, thus silence of β-arrestin 2 may restore multidrug resistance to some extent. To elucidate our assuming in depth, we transiently transfected small interfering RNA (siRNA) pSUPER- siβ-arrestin 2 in ADM and transfected full length plasmid pCDNA3.1-β-arrestin 2 in MDA-MB-231 and MCF-7 cells. Then we examined the mRNA and protein level of MDR1/p-gp after silence or overexpression of β-arrestin 2 in respective cell lines by q-PCR and western blotting. We discovered that the expression of MDR1/p-gp was consistent with the changes of β-arrestin 2. MTS cell viability assays showed that these cells exhibited statistically significant resistance to anti-cancer drugs. The cell toxicity of anti-cancer drugs in ADM with silencing of β-arrestin 2 was significantly lower than the control. By contrast, MDA-MB-231 and MCF-7 cells transfected with pCDNA3.1-β-arrestin 2 became more resistant to these anti-cancer drugs. So we concluded that the changes of β-arrestin 2 could affect the toxicity of anti-cancer drugs in cells. Immunohistochemistry indicate there is a positive correlation between β-arrestin 2 and MDR1/P-gp. All these results evidently verified our hypothesis.
Because of time limit, we only indicated that β-arrestin 2 could mediate multidrug resistance by regulating the expression of MDR1/p-gp. But, the mechanism about the regulating of MDR1/p-gp by β-arrestin 2 still remains unclear.
The Wnt/β-catenin signaling pathway involves in breast cancer development and plays a key role in chemoresistance, by regulating the transcription of multidrug resistance gene MDR1 [29-31]. Human MDR1 gene is a direct target of Wnt/β-catenin signaling pathway [21]. β-arrestin 2 has also shown to be an essential component of the Wnt/β-catenin signaling pathway [16,28]. In the classical Wnt/β-catenin signaling pathway, β-arrestin 2 interacts with intracellular protein Dishevelled (Dsh) and Axin, thus stabilizing the β-catenin and promoting its translocation to nucleus. Then β-catenin combines with TCF/LEF transcriptional complex and activate the target gene transcription [31,32]. So we infer that β-arrestin 2 may mediate multidrug resistance through Wnt/β-catenin signaling pathway by forming a complex with other proteins. In our future experiment work, we will investigate the mechanism in depth. We will carry out a series of trials to investigate whether β-arrestin 2 regulates multidrug resistance in breast cancer cells by interacting with β-catenin through Wnt/β-catenin signaling pathway.
In conclusion, we concluded that β-arrestin 2 was significantly overexpressed in multidrug resistance cell ADM, and the silencing of β-arrestin 2 by siRNA down-regulated the expression of MDR1/p-gp, consequently, sensitivity to anti-cancer drugs could be partially restored. We hope our findings will be regarded as a new feasible approach to reverse MDR. In particular, we suggest β-arrestin 2 as a new molecular target for reversing drug resistance in breast carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Li W, Song M. Expression of multidrug resistance proteins in invasive ductal carcinoma of the breast. Oncol Lett. 2014;8:2103–2109. doi: 10.3892/ol.2014.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao P, Zhou GY, Guo LL, Zhang QH, Zhen JH, Fang AJ, Lin XY. Reversal of drug resistance in breast carcinoma cells by anti-mdr1 ribozyme regulated by the tumor-specific MUC-1 promoter. Cancer Lett. 2007;256:81–89. doi: 10.1016/j.canlet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 6.Pokharel D, Padula MP, Lu JF, Tacchi JL, Luk F, Djordjevic SP, Bebawy M. Proteome analysis of multidrug-resistant, breast cancer-derived microparticles. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapse-Mistry S, Govender T, Srivastava R, Yergeri M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front Pharmacol. 2014;5:159. doi: 10.3389/fphar.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber M, Bordow SB, Haber PS, Marshall GM, Stewart BW, Norris MD. The prognostic value of MDR1 gene expression in primary untreated neuroblastoma. Eur J Cancer. 1997;33:2031–2036. doi: 10.1016/s0959-8049(97)00229-3. [DOI] [PubMed] [Google Scholar]
- 9.Anreddy N, Gupta P, Kathawala RJ, Patel A, Wurpel JN, Chen ZS. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules. 2014;19:13848–13877. doi: 10.3390/molecules190913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–1616. doi: 10.3892/etm.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Cabrero A, Wrasidlo W, Reisfeld RA. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS One. 2013;8:e73607. doi: 10.1371/journal.pone.0073607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusi F, Saponara S, Valoti M, Dragoni S, D'Elia P, Sgaragli T, Alderighi D, Kawase M, Shah A, Motohashi N, Sgaragli G. Cancer cell permeability-glycoprotein as a target of MDR reverters: possible role of novel dihydropyridine derivatives. Curr Drug Targets. 2006;7:949–959. doi: 10.2174/138945006778019336. [DOI] [PubMed] [Google Scholar]
- 13.Nobili S, Landini I, Giglioni B, Mini E. Ph-armacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 14.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, Caplen NJ, Fales HM, Ambudkar SV, Weinstein JN, Gottesman MM. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnans C, Flaceliere M, Grillet F, Dantec C, Desvignes JP, Pannequin J, Severac D, Dubois E, Bibeau F, Escriou V, Crespy P, Journot L, Hollande F, Joubert D. Essential requirement for beta-arrestin2 in mouse intestinal tumors with elevated Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:3047–3052. doi: 10.1073/pnas.1109457109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michal AM, Peck AR, Tran TH, Liu C, Rimm DL, Rui H, Benovic JL. Differential expression of arrestins is a predictor of breast cancer progression and survival. Breast Cancer Res Treat. 2011;130:791–807. doi: 10.1007/s10549-011-1374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, Wang D, Wu J, Jin J, Wei W, Sun W. Involvement of beta-arrestins in cancer progression. Mol Biol Rep. 2013;40:1065–1071. doi: 10.1007/s11033-012-2148-0. [DOI] [PubMed] [Google Scholar]
- 19.Alemayehu M, Dragan M, Pape C, Siddiqui I, Sacks DB, Di Guglielmo GM, Babwah AV, Bhattacharya M. beta-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One. 2013;8:e56174. doi: 10.1371/journal.pone.0056174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Zhou G, Zhang Y, Chen T, Sun X, Stuart C, Hanley G, Li J, Zhang J, Yin D. β-arrestin 2 inhibits opioid-induced breast cancer cell death through Akt and caspase-8 pathways. Neoplasma. 2009;56:108–113. doi: 10.4149/neo_2009_02_108. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang X, Wu X, Li W, Su P, Cheng H, Xiang L, Gao P, Zhou G. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/beta-catenin pathway. Cancer Lett. 2012;323:106–113. doi: 10.1016/j.canlet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Xie XH, Zhao H, Hu YY, Gu XD. Germacrone reverses Adriamycin resistance through cell apoptosis in multidrug-resistant breast cancer cells. Exp Ther Med. 2014;8:1611–1615. doi: 10.3892/etm.2014.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Z, Sun P, Chu H, Zhu H, Sun D, Chen J. Overexpression of sorcin in multidrug-resistant human breast cancer. Oncol Lett. 2014;8:2393–2398. doi: 10.3892/ol.2014.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007;98:1303–1310. doi: 10.1111/j.1349-7006.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia M, Hu J, Li W, Su P, Zhang H, Zhang X, Zhou G. Trps1 is associated with the multidrug resistance of osteosarcoma by regulating MDR1 gene expression. FEBS Lett. 2014;588:801–810. doi: 10.1016/j.febslet.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin Development: Emerging Roles for β-arrestins in Developmental Signaling Pathways. Developmental Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta P. Nicotine induces cell proliferation by -arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa A, Kruger WD. Suppression of tumor formation by a cyclooxygenase-2 inhibitor and a peroxisome proliferator-activated receptor gamma agonist in an in vivo mouse model of spontaneous breast cancer. Clin Cancer Res. 2008;14:4935–4942. doi: 10.1158/1078-0432.CCR-08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]


