Abstract
Among malignant tumors, the mortality rate of esophageal squamous cell carcinoma (ESCC) ranks sixth in the world. Late-stage diagnosis of ESCC increases the mortality. Therefore, more effective biomarkers for early diagnosis of ESCC are necessary. Unfortunately, appropriate biomarkers for clinical diagnosis and prognosis have not been identified yet. However, recent progresses in quantitative proteomics have offered opportunities to identify plasma proteins as biomarkers for ESCC. In the present study, plasma samples were analyzed by differential in-gel electrophoresis (DIGE) and differentially expressed proteins were identified by matrix assisted laser desorption ionization-time of flight/time of flight mass spectrometry (MALDI-TOF/TOF MS). A total of 31 proteins representing 12 unique gene products were identified, in which 16 proteins were up-regulated and 15 down-regulated in tumors. The up-regulated proteins were alpha-2-HS-glycoprotein (AHSG), leucine-rich alpha-2-glycoprotein (LRG), zinc-alpha-2-glycoprotein, alpha-1-antichymotrypsin, complement factor I and complement C4-B, whereas the down-regulated proteins were serum albumin, Ig alpha-2 chain C region, alpha-1-antitrypsin, fibrinogen gamma chain, haptoglobin and hemoglobin subunit alpha. Among all the differentially expressed proteins, AHSG and LRG were validated by ELISA. The results were consistent with the data from the proteomics results, further suggesting that AHSG and LRG may be employed as potential biomarkers for the early diagnosis of ESCC. In summary, this study was the first time to use DIGE combined MALDI-TOF/TOF platform to identify the potential plasma biomarkers for ESCC. The plasma AHSG and LRG showed great potential for ESCC screening.
Keywords: Esophageal squamous cell carcinoma, plasma, biomarkers, proteomics, differential in-gel electrophoresis, MALDI-TOF/TOF
Introduction
Esophageal cancer (EC) is the eighth most common incident cancer in the world because of its extremely aggressive nature and poor survival rate [1]. EC affects more than 450000 people worldwide and the incidence is rapidly increasing [2]. Moreover, about half of world’s EC cases newly diagnosed each year occurred in China [3], and esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype and accounts for nearly 90% of all EC [4]. Unfortunately, the majority of ESCC patients have advanced metastatic disease at initial diagnosis and are inappropriate for curative resection [5], the overall 5-year survival rate remains poor despite significant improvements in surgical techniques and adjuvant chemoradiation [6]. Taken together, late-stage diagnosis increases the mortality of ESCC. Therefore, it is imperative to search more effective biomarkers for early diagnosis of ESCC. However, the appropriate biomarkers for clinical diagnosis and prognosis have not been identified yet.
Recent progresses in quantitative proteomics have offered opportunities to discover plasma biomarkers for ESCC. To date, most studies on cancer proteomics have used tumor and adjacent non-tumor tissue samples as the primary source to search for biomarkers, followed by immortalized cell lines and malignantly transformed counterparts [7]. However, the greatest potential breakthrough will be through the identification of validated biomarkers, ideally in plasma. There has been growing interest in the use of proteomics methods on peripheral blood plasma to rapidly profile protein markers [8,9]. Liquid chromatography methods such as high performance liquid chromatography and two-dimensional liquid chromatography have been increasingly employed for protein separation, and mass spectroscopy techniques such as matrix assisted laser desorption ionization-time of flight/time of flight mass spectrometry (MALDI-TOF/TOF MS) have been used to analyze the proteins [9]. The surface enhanced laser desorption/ionization-time of flight (SELDI-TOF) MS technique, in which chromatographic separation is achieved on a solid surface and the proteins analyzed intact, has been investigated to identify serum and tissue proteomic profiles that could be used in clinical practice [9]. Differential in-gel electrophoresis (DIGE) is able to co-detect numerous samples in the same two-dimensional gel (2DE) to minimize gel-to-gel variation and compare the protein features across different gels by means of an internal standard [10], it has been used widely to search the biomarkers for numerous cancers.
In this study, we developed a proteomics-based approach that involves immune-depletion of high-abundance proteins, 2D-DIGE analysis, and subsequent MALDI-TOF/TOF MS identification to obtain a panel of differentially expressed plasma proteins from ESCC patients.
Materials and methods
Plasma sample preparation
For DIGE, 6 donors in a single hospital (The First Affiliated Hospital of Zhengzhou University, Henan, China) were enrolled in this study from Jan 2013 to Dec 2013, which were divided into ESCC patients group (n = 3) and healthy donors group (n = 3). The criteria to assess the presence of ESCC were based on the pathological diagnosis and guidelines proposed by the World Health Organization. None of the patients had received radiotherapy or chemotherapy before plasma collection. Healthy individuals were selected with no ESCC diagnosed clinically. An additional group of 45 ESCC patients and 45 healthy donors were selected for ELISA. All plasma samples were stored at -80°C before use. This study was approved by the Institutional Research Board and carried out according to the Helsinki Declaration Principles. Written informed consent was collected from all participating subjects. The albumin and immunoglobulin G in the collected plasma samples were depleted using an albumin and IgG removal kit (Sigma-Aldrich) in accordance with the manufacturer’s instructions. The depleted plasma samples were then precipitated with 2D Clean-up Kit (GE Healthcare) according to the manufacturer’s protocol and resuspended in Lysis Buffer (7 M urea, 2 M thiourea, 4% CHAPS, 30 mM Tris-HCl, at pH 8.5). Protein concentrations were determined by 2D Quant Kit (GE Healthcare) according to the manufacturer’s instructions. The purified samples were preserved at -80°C before DIGE.
DIGE analysis
The purified samples were labeled using the fluorescent cyanine dyes (CyDye) developed for DIGE technology following the manufacturer’s recommended protocols (GE Healthcare) [10]. In brief, the internal standard was prepared by combining equal portions of each of the pooled samples. Sample proteins (50 μg) were labeled with Cy3 or Cy5, and the internal standard mixture with the same protein amount was labeled with Cy2. Mixed solutions were incubated on ice for 30 min in the dark. The reactions were then quenched with the addition of 1 μl of 10 mM lysine for 10 min on ice in the dark. The quenched Cy3- and Cy5-labeled samples and the Cy2-labeled internal standard were pooled prior to 2-DE analysis. Meanwhile, a preparative gel, containing 800 μg of unlabeled internal standard mixture proteins, was prepared. The plasma samples arrangement for a triplicate DIGE experiment is shown in Table 1. The rehydration process was performed with immobilized non-linear pH gradient (IPG) strips (pH 3-10 NL, 24 cm) which were later rehydrated by CyDye-labeled samples in the dark at room temperature overnight. Isoelectric focusing was then performed using an IPGphor III apparatus (GE Healthcare) for a total of 60 kVh at 20°C. The gels were then run in an Ettan DALT Six gel tank (GE Healthcare) at 3 Watt per gel at 10°C until the dye front had completely run off the bottom of the gels. Afterward, the fluorescence 2DE gels were scanned directly between the low fluorescent glass plates using an Ettan DIGE Imager (GE Healthcare). Gel analysis was performed using DeCyder 2D Differential Analysis Software v7.0 (GE Healthcare) to co-detect, normalize and quantify the protein features in the images. Spots displaying 1.5 average-fold increase or decrease in abundance with P < 0.05 were selected for protein identification.
Table 1.
Arrangement of plasma samples for a triplicate DIGE experiment
| Gel NO. | Cy2 (50 μg) | Cy3 (50 μg) | Cy5 (50 μg) |
|---|---|---|---|
| Gel 1 | Pool | Healthy donor 1 | ESCC patient 1 |
| Gel 2 | Pool | ESCC patient 2 | Healthy donor 2 |
| Gel 3 | Pool | Healthy donor 3 | ESCC patient 3 |
Protein identification
Selected protein spots were excised from the gel using Ettan spot picker (GE Healthcare), subjected to in-gel digestion and protein IDs were determined by MS/MS analysis using a MALDI-TOF/TOF mass spectrometer, ABI-4800 (Applied Biosystems). For MS analyses, typically 800 shots were accumulated. MS/MS analyses were performed using air, at collision energy of 2 KV. MASCOT search engine (version 2.1, Matrix Science) was used to search all of the tandem mass spectra. GPS ExplorerTM software (version 3.6.2, Applied Biosystems) was used to create and search files with the MASCOT search for peptide and protein identification. Protein identities were obtained by using Mascot searching engine against Swiss-Prot non-redundant sequence databases selected for human taxonomy.
Bioinformatics analysis
The theoretical isoelectric point (pI) and molecular weight (MW) of protein spots were obtained through MALDI-TOF/TOF Mascot database searching. The function of the identified proteins was elucidated by UniProt knowledgebase (UniProtKB/Swiss-Prot).
ELISA
Enzyme immunoassay (EIA) polystyrene micro titration wells were coated with 50 µg of protein samples and incubated at 37°C for 2 h. The plate was washed for three times with phosphate buffered saline-Tween 20 (PBST) and three times with phosphate buffered saline (PBS). After the uncoated space was blocked with 100 µl of 5% skimmed milk in PBS at 37°C for 2 h, the plate was washed three times with PBST. AHSG antibody (Abcam) and LRG antibody (Abcam) solutions were added and incubated at 37°C for 2 h. After washing with PBST and PBS for 10 times in total, 100 µl of peroxidase-conjugated secondary antibodies in PBS was added for incubation at 37°C for 2 h. Following 10 washings, 100 µl of 3,3’,5,5’-tetramethyl benzidine (Pierce) was added. After incubation at room temperature for 30 min, 100 µl of 1 M H2SO4 was added to stop the reaction followed by measured absorbance at 450 nm using SpectraMax M5 (Molecular Devices).
Statistical analysis
Statistic features in DeCyder were used for evaluation of DIGE gels. Protein spots, which were found differentially expressed between groups (at least 50% change of ratios between groups and t-test P < 0.05) were extracted. For ELISA analysis, independent-samples t-test was used to determine mean differences between two groups and P < 0.05 was used to assess significance of differences with SPSS 13.0 software.
Results
DIGE analysis of differentially expressed plasma proteins between ESCC patients and healthy donors
According to the DeCyder software analysis, about 1500 protein spots were constantly detected in each gel and quantified, normalized and inter-gel-matched. Due to the CyDye DIGE Fluor detection limit and the CyDye linear response in protein concentrations over five orders of magnitude, 31 protein spots with significant differences were detected between ESCC patients and healthy honors. As shown in Figure 1, the differentially expressed protein figures were shown more than 1.5-fold changes in expression level with the Student t-test (P < 0.05).
Figure 1.
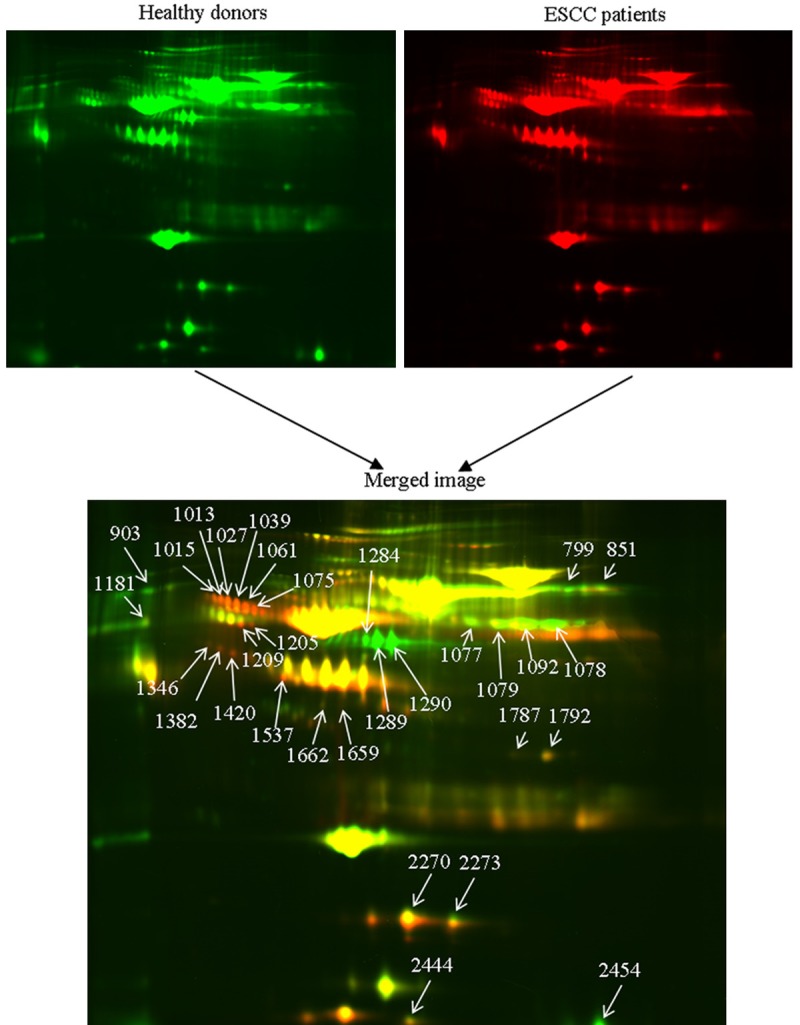
DIGE images of the plasma samples from ESCC patients and healthy donors were shown (upper images) as well as the merged pseudo-colored image processed with ImageQuant software (bottom image). The differentially expressed proteins were annotated with spot numbers.
Identification of differentially expressed proteins by MALDI-TOF/TOF MS
31 differentially expressed protein spots were excised and subsequently in-gel digested by trypsin, then successfully analyzed by MALDI-TOF/TOF MS. Among those 31 spots, 16 up-regulated and 15 down-regulated proteins were identified. The up-regulated proteins were leucine-rich alpha-2-glycoprotein, alpha-1-antichymotrypsin, alpha-2-HS-glycoprotein, zinc-alpha-2-glycoprotein, complement factor I and complement C4-B, whereas the down-regulated proteins were serum albumin, Ig alpha-2 chain C region, alpha-1-antitrypsin, fibrinogen gamma chain, haptoglobin and hemoglobin subunit alpha. Details of these proteins were listed in Table 2.
Table 2.
Identification of differentially expressed proteins by MALDL-TOF/TOF MS
| Spot No.a | Protein name | ACb | MWc | pId | Average ratiose |
|---|---|---|---|---|---|
| 799, 851, 903 | Serum albumin | P02768 | 71.3 | 5.92 | -2.27, -2.03, -3.64 |
| 1013, 1015, 1027, 1039, 1061, 1075 | Alpha-1-antichymotrypsin | P01011 | 47.8 | 5.33 | 1.96, 1.55, 1.99, 2.25, 2.20, 1.95 |
| 1077, 1078 | Ig alpha-2 chain C region | P01877 | 37.3 | 5.71 | -1.52, -9.08, |
| 1079, 1092 | -4.81, -4.93 | ||||
| 1181 | Alpha-1-antitrypsin | P01009 | 46.9 | 5.37 | -5.77 |
| 1205, 1209 | Alpha-2-HS-glycoprotein | P02765 | 40.1 | 5.43 | 3.20, 4.58 |
| 1284, 1289, 1290 | Fibrinogen gamma chain | P02679 | 52.1 | 5.37 | -11.39, -39.55, -39.58 |
| 1346, 1382, 1420 | Leucine-rich alpha-2-glycoprotein | P02750 | 38.4 | 6.45 | 1.62, 2.25, 3.06 |
| 1537 | Zinc-alpha-2-glycoprotein | P25311 | 34.1 | 5.57 | 1.89 |
| 1659, 1662 | Complement factor I | P05156 | 68.1 | 7.72 | 1.71, 1.62 |
| 1787, 1792 | Complement C4-B | P0C0L5 | 194.2 | 6.73 | 1.52, 1.73 |
| 2270, 2273, 2444 | Haptoglobin | P00738 | 45.9 | 6.13 | -36.64, -41.36, -24.13 |
| 2454 | Hemoglobin subunit alpha | P69905 | 15.3 | 8.72 | -10.73 |
Spot number corresponding to the annotation shown in Figure 1.
Accession number (SwissProt), theoretical molecular weight (kD) and isoelectric point were obtained by Mascot MS-MS ion searching following MALDI -TOF/TOF MS.
Accession number (SwissProt), theoretical molecular weight (kD) and isoelectric point were obtained by Mascot MS-MS ion searching following MALDI -TOF/TOF MS.
Accession number (SwissProt), theoretical molecular weight (kD) and isoelectric point were obtained by Mascot MS-MS ion searching following MALDI -TOF/TOF MS.
Average ratios between ESCC patients and healthy controls.
Bioinformatics analysis
These identified proteins are functionally involved in inflammatory response (38.7%), immune response (29%), transportation (19.4%) and coagulation (12.9%) (Figure 2).
Figure 2.
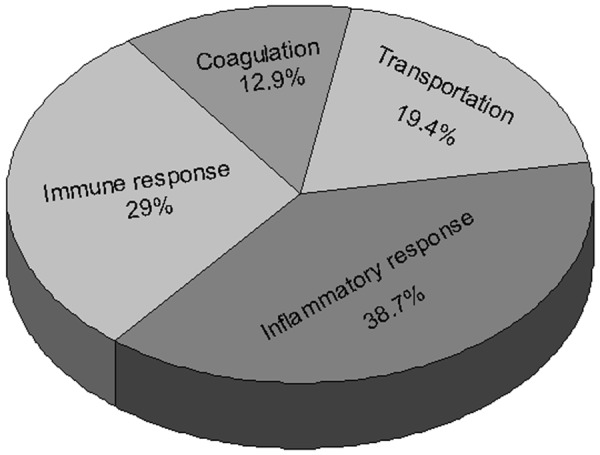
Classification of the differentially expressed proteins according to biological functions.
Validation by ELISA
As shown in Figure 3, AHSG and LRG were both significantly increased in the plasma of ESCC patients. To verify the abundances of proteins deduced from the proteomics results, the abundance levels of AHSG and LRG were investigated by ELISA respectively. Interestingly, these results were consistent with the data from the proteomics experiments (Figure 4).
Figure 3.
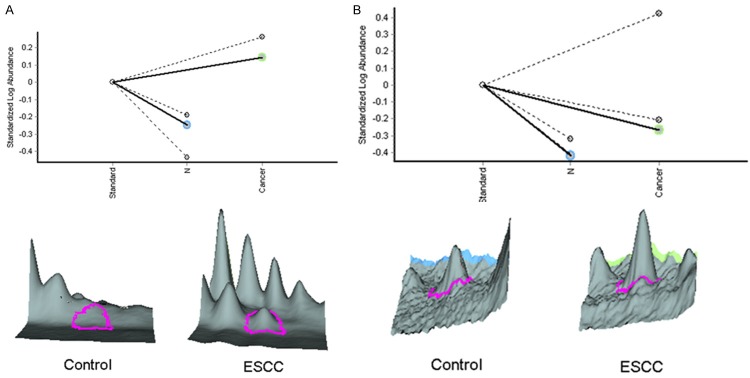
Relative abundance and 3D-view of AHSG (A) and LRG (B) analyzed by Decyder 2D software.
Figure 4.
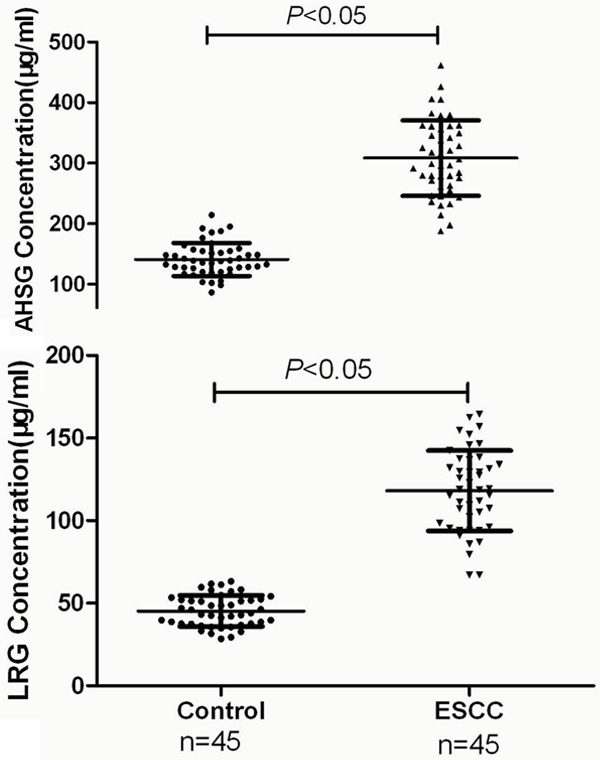
Verification of AHSG and LRG expression levels between ESCC patients and healthy donors by ELISA analysis. The scatter plots represented protein expression variations. Independent samples t-test was performed with SPSS 13.0 software (P < 0.05). The plasma concentration of AHSG in control group was 140.9 ± 27.3 μg/ml, but 308.4 ± 62.3 μg/ml in ESCC group. Similarly, LRG was 45.3 ± 9.4 μg/ml in control donors, but 118.1 ± 24.3 μg/ml in ESCC patients. These ELISA results were consistent with the data from 2D-DIGE, and further suggested that AHSG and LRG may be employed as potential biomarkers for the early diagnosis of ESCC.
Discussion
Differential proteomics have been widely used to identify all types of biomarkers in cancer research [11]. The application of serum biomarkers for the screening and diagnosis of ESCC was studied because of their advantages of less pain and wider accessibility [12]. Tumor biomarkers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and squamous cell carcinoma antigen (SCCA) have been widely investigated in the treatment of EC patients [13]. However, the application of these markers to the clinical diagnosis of EC is still limited by their low sensitivity and specificity. Therefore, it is necessary to search for new biomarkers with high sensitivity and specificity.
To our knowledge, we are the first to utilize DIGE and MALDI-TOF/TOF platform to look for potential plasma biomarkers for ESCC. Our proteomics analysis revealed 31 altered plasma proteins corresponding to 12 unique plasma proteins. These proteins were functionally involved in inflammatory response, immune response, transportation and coagulation. Interestingly, they have not been reported as biomarkers for ESCC in previous studies except haptoglobin. Accordingly, the combination of these identified proteins might be further evaluated as ESCC specific biomarkers.
Alpha-2-HS-glycoprotein (AHSG), also known as human fetuin-A, is a major serum glycoprotein synthesized and secreted by the liver. A number of studies suggest that AHSG is a multifunctional protein involved in calcium homeostasis, bone development and insulin sensitivity [14,15]. Additionally, AHSG has the characteristics of a negative acute-phase protein, in that the serum concentrations decrease significantly after major surgical procedures, trauma, burns, and severe inflammation [16]. Interestingly, AHSG can inhibit intestinal tumor progression by blocking TGF-β signal transduction [17]. In our study, AHSG is up-regulated in ESCC plasma, and validated by ELISA. The results suggested that AHSG may play a major role in ESCC tumorigenesis.
Leucine-rich alpha-2-glycoprotein (LRG) is a secreted glycoprotein. In previous literatures, LRG has been shown to be involved in cell adhesion and has been recognized as a biomarker for certain diseases including microbial infections [18], autoimmune diseases [19], lung cancer [20] and hepatocellular carcinoma [16], etc. Our experiment results revealed that LRG was up-regulated in ESCC plasma and validated by ELISA analysis. Our results are the first time to support LRG as a potential plasma marker for ESCC.
Zinc-alpha-2-glycoprotein (ZAG) is a glycoprotein secreted by a variety of normal epithelia and was recently shown to stimulate lipolysis in adipocytes, leading to the development of cachexia in animals with ZAG-producing tumors [21]. In our study, ZAG is up-regulated in ESCC plasma. We deemed that ZAG may play a major role in ESCC cachexia.
Alpha-1 antichymotrypsin (ACT), is a protease inhibitor thought to limit tissue damage produced by excessive inflammation-associated proteolysis [22]. ACT has been recognized as a biomarker for certain cancers including colorectal adenocarcinomas [23], breast cancer [24] and cervical carcinoma [25]. In our experiment, ACT is over-expressed in ESCC plasma. We speculate that ACT may be as a protease inhibitor to limit tissue damage produced by excessive inflammation-associated proteolysis.
Complement C4-B (C4B) is an isotype of complement C4. C4B functions more in the propagation of the activation pathways that lead to the formation of the membrane attack complex in attacking foreign antigens [26]. Complement factor I (CFI) is a plasma serine protein which plays a prominent role in the regulation of the complement system. The activated C4B is also cleaved by CFI, in the presence of C4 binding protein. Several studies reported that serum complement proteins may include some efficient biomarkers related to cancers, such as ovarian cancer, breast cancer and bladder cancer, etc [27]. In our study, CFI and C4B were both up-regulated, which may play important roles in elimination of damaged tumor cells.
Serum albumin (ALB), the main protein of plasma, is synthesized and secreted by the liver. Low serum albumin has also been shown to be an independent indicator for prognosis in cancer patients with unknown primaries [28]. Our study revealed that ALB was down-regulated in ESCC plasma, indicates that malnutrition and inflammation of ESCC may suppress ALB synthesis.
Ig alpha-2 chain C region (IGHA2) belongs to the major immunoglobulin class in body secretions. High blood Ig and circulating immune complex values in patients with cancer have been repeatedly reported and suggested as tumor markers with varying regulation patterns in numerous cancers [29]. In our study, however, the decreased plasma level of IGHA2 was contrary to the previous reports about other cancers, revealing a potential biomarker of ESCC.
Alpha-1-antitrypsin (A1AT), a member of the serpin superfamily, is highly expressed by hepatocytes. It inhibits neutrophil proteases to protect host tissues from nonspecific injury associated with episodes of inflammation. A number of studies suggest that A1AT may be a potential biomarker in many kinds of cancers [30,31]. Decreased level of A1AT in our study indicates that the A1AT level of plasma may be a useful protein biomarker of ESCC.
Fibrinogen gamma chain (FGG) is one of fibrinogen degradation products generated during fibrinolysis. FGG has a COOH-terminal globular domain to which several integrin cell adhesion receptors bind. In addition, fibrinogen and its gamma chain containing many specific binding sites and can regulate cellular adhesion and invasion. Recently, the correlation of fibrinogen and its degradation products with carcinogenesis was reported in oral squamous cell carcinoma [32]. From our current DIGE results, down-regulated FGG plasma level may be related to tumor thrombosis, which suggests that FGG is a useful predictor for ESCC.
Haptoglobin (HP) is an acute phase protein synthesized by liver parenchymal cells. It plays an important role in the regulation of epidermal cell transformation, immune suppression in cancer and angiogenesis. In our study, HP was decreased in the plasma of ESCC, whether it is a potential biomarker for screening ESCC requires further validations.
Hemoglobin subunit alpha (HBA1), one chain of hemoglobin, belongs to the globin family. A previous study revealed that HBA1 was down-regulated in pancreatic cancer [33]. Decreased level of HBA1 in our research indicates that serum HBA1 may represent a clinically interesting diagnostic marker of ESCC.
In summary, this study identified 12 differentially-expressed plasma proteins associated with ESCC by DIGE combined MALDI-TOF/TOF platform, additionally, AHSG and LRG were validated by ELISA. After all, these results might provide a framework for future early diagnosis for ESCC. However, the potential of utilizing these biomarkers for screening and diagnosing ESCC requires further investigations.
Acknowledgements
This work was supported by National Natural Science Founding of China (No. 81202112).
Disclosure of conflict of interest
None.
References
- 1.Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev. 2011;12:2461–2466. [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triboulet JP, Mariette C, Chevalier D, Amrouni H. Surgical management of carcinoma of the hypopharynx and cervical esophagus: analysis of 209 cases. Arch Surg. 2001;136:1164–1170. doi: 10.1001/archsurg.136.10.1164. [DOI] [PubMed] [Google Scholar]
- 6.Cesas A, Bagajevas A. [Combined treatment of esophageal cancer: a review] . Medicina (Kaunas) 2004;40(Suppl 1):161–165. [PubMed] [Google Scholar]
- 7.Pawar H, Kashyap MK, Sahasrabuddhe NA, Renuse S, Harsha HC, Kumar P, Sharma J, Kandasamy K, Marimuthu A, Nair B, Rajagopalan S, Maharudraiah J, Premalatha CS, Kumar KV, Vijayakumar M, Chaerkady R, Prasad TS, Kumar RV, Pandey A. Quantitative tissue proteomics of esophageal squamous cell carcinoma for novel biomarker discovery. Cancer Biol Ther. 2011;12:510–522. doi: 10.4161/cbt.12.6.16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith L, Lind MJ, Welham KJ, Cawkwell L. Cancer proteomics and its application to discovery of therapy response markers in human cancer. Cancer. 2006;107:232–241. doi: 10.1002/cncr.22000. [DOI] [PubMed] [Google Scholar]
- 9.Colantonio DA, Chan DW. The clinical application of proteomics. Clin Chim Acta. 2005;357:151–158. doi: 10.1016/j.cccn.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, Lewis S, Currie I. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. doi: 10.1002/pmic.200390006. [DOI] [PubMed] [Google Scholar]
- 11.Qi Y, Chiu JF, Wang L, Kwong DL, He QY. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics. 2005;5:2960–2971. doi: 10.1002/pmic.200401175. [DOI] [PubMed] [Google Scholar]
- 12.Zhai XH, Yu JK, Lin C, Wang LD, Zheng S. Combining proteomics, serum biomarkers and bioinformatics to discriminate between esophageal squamous cell carcinoma and pre-cancerous lesion. J Zhejiang Univ Sci B. 2012;13:964–971. doi: 10.1631/jzus.B1200066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka K, Yano M, Motoori M, Kishi K, Miyashiro I, Shingai T, Gotoh K, Noura S, Takahashi H, Ohue M, Yamada T, Ohigashi H, Yamamoto T, Yamasaki T, Doki Y, Ishikawa O. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol. 2010;17:2779–2786. doi: 10.1245/s10434-010-1075-3. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/ fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami T, Hoshida Y, Kanai F, Tanaka Y, Tateishi K, Ikenoue T, Obi S, Sato S, Teratani T, Shiina S, Kawabe T, Suzuki T, Hatano N, Taniguchi H, Omata M. Proteomic analysis of sera from hepatocellular carcinoma patients after radiofrequency ablation treatment. Proteomics. 2005;5:4287–4295. doi: 10.1002/pmic.200401287. [DOI] [PubMed] [Google Scholar]
- 17.Swallow CJ, Partridge EA, Macmillan JC, Tajirian T, DiGuglielmo GM, Hay K, Szweras M, Jahnen-Dechent W, Wrana JL, Redston M, Gallinger S, Dennis JW. alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 18.Weivoda S, Andersen JD, Skogen A, Schlievert PM, Fontana D, Schacker T, Tuite P, Dubinsky JM, Jemmerson R. ELISA for human serum leucine-rich alpha-2-glycoprotein-1 employing cytochrome c as the capturing ligand. J Immunol Methods. 2008;336:22–29. doi: 10.1016/j.jim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H, Shinzaki S, Nishikawa T, Ohkawara T, Iwahori K, Ohguro N, Kishimoto T, Naka T. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69:770–774. doi: 10.1136/ard.2009.118919. [DOI] [PubMed] [Google Scholar]
- 20.Heo SH, Lee SJ, Ryoo HM, Park JY, Cho JY. Identification of putative serum glycoprotein biomarkers for human lung adenocarcinoma by multilectin affinity chromatography and LC-MS/MS. Proteomics. 2007;7:4292–4302. doi: 10.1002/pmic.200700433. [DOI] [PubMed] [Google Scholar]
- 21.Hale LP, Price DT, Sanchez LM, Demark-Wahnefried W, Madden JF. Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res. 2001;7:846–853. [PubMed] [Google Scholar]
- 22.Santamaria M, Pardo-Saganta A, Alvarez-Asiain L, Di Scala M, Qian C, Prieto J, Avila MA. Nuclear alpha1-antichymotrypsin promotes chromatin condensation and inhibits proliferation of human hepatocellular carcinoma cells. Gastroenterology. 2013;144:818–828. doi: 10.1053/j.gastro.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Dimberg J, Strom K, Lofgren S, Zar N, Hugander A, Matussek A. Expression of the serine protease inhibitor serpinA3 in human colorectal adenocarcinomas. Oncol Lett. 2011;2:413–418. doi: 10.3892/ol.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano H, Wada S, Eguchi H, Osaki A, Saeki T, Nishiyama M. Quantitative prediction of tumor response to neoadjuvant chemotherapy in breast cancer: novel marker genes and prediction model using the expression levels. Breast Cancer. 2012;19:37–45. doi: 10.1007/s12282-011-0263-8. [DOI] [PubMed] [Google Scholar]
- 25.Kloth JN, Gorter A, Fleuren GJ, Oosting J, Uljee S, ter Haar N, Dreef EJ, Kenter GG, Jordanova ES. Elevated expression of SerpinA1 and SerpinA3 in HLA-positive cervical carcinoma. J Pathol. 2008;215:222–230. doi: 10.1002/path.2347. [DOI] [PubMed] [Google Scholar]
- 26.Schifferli JA, Ng YC, Peters DK. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986;315:488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Liu B, Xin L, Zhang Y, Chen X, Zhu Z, Lin Y. Down-regulated expression of complement factor I: a potential suppressive protein for gastric cancer identified by serum proteome analysis. Clin Chim Acta. 2007;377:119–126. doi: 10.1016/j.cca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JW, Liu H, Shin DH, Yu GI, Hwang JS, Kim ES, Yun JW. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients. Proteomics. 2013;13:2361–2374. doi: 10.1002/pmic.201200550. [DOI] [PubMed] [Google Scholar]
- 30.Xie LQ, Zhao C, Cai SJ, Xu Y, Huang LY, Bian JS, Shen CP, Lu HJ, Yang PY. Novel proteomic strategy reveal combined alpha1 antitrypsin and cathepsin D as biomarkers for colorectal cancer early screening. J Proteome Res. 2010;9:4701–4709. doi: 10.1021/pr100406z. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Kim YT, Park PJ, Shin YS, Kang KN, Kim Y, Kim CW. A novel detection method of non-small cell lung cancer using multiplexed bead-based serum biomarker profiling. J Thorac Cardiovasc Surg. 2012;143:421–427. doi: 10.1016/j.jtcvs.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 32.Tung CL, Lin ST, Chou HC, Chen YW, Lin HC, Huang KJ, Chen YJ, Lee YR, Chan HL. Proteomics-based identification of plasma biomarkers in oral squamous cell carcinoma. J Pharm Biomed Anal. 2013;75:7–17. doi: 10.1016/j.jpba.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Song D, Chaerkady R, Tan AC, Garcia-Garcia E, Nalli A, Suarez-Gauthier A, Lopez-Rios F, Zhang XF, Solomon A, Tong J, Read M, Fritz C, Jimeno A, Pandey A, Hidalgo M. Antitumor activity and molecular effects of the novel heat shock protein 90 inhibitor, IPI-504, in pancreatic cancer. Mol Cancer Ther. 2008;7:3275–3284. doi: 10.1158/1535-7163.MCT-08-0508. [DOI] [PubMed] [Google Scholar]


