Abstract
Neogrifolin, a natural biologically active substance isolated from the edible bodies of the mushroom Albatrellus confluens, has been shown to possess several pharmacological properties. No studies were investigated against osteosarcoma cancer. Hence, in this study, we investigated the apoptosis-inducing effects and the mechanisms of neogrifolin on human osteosarcoma cells. Our results demonstrated that neogrifolin induced concentration- and time-dependent suppression of proliferation. Further, induction of apoptosis in U2OS and MG63 osteosarcoma cell lines were also observed. Neogrifolin induced the release of cytochrome c accompanied by activation of caspase-9, caspase-3 and cleavage of poly (ADP-ribose) polymerase (PARP). In addition, z-VAD-fmk, a universal inhibitor of caspases, prevented caspase-3 activation and PARP cleavage and inhibited neogrifolin-induced cell growth inhibition. Furthermore, neogrifolin treatment resulted in a reduction of phosphorylated AKT level, FOXO transcription factor, and glycogen synthase kinase 3 (GSK3). Knockdown of GSK3 with siRNA inhibited the apoptotic effects of neogrifolin. On the other hand, neogrifolin treatment also down-regulated the expression of the inhibitor of apoptosis protein (IAP) in both osteosarcoma cells. Collectively, our results suggested that neogrifolin is a potential candidate for osteosarcoma.
Keywords: Neogrifolin, osteosarcoma, AKT signaling pathway
Introduction
Osteosarcoma is an aggressive cancerous neoplasm arising from primitive transformed cells of mesenchymal origin that exhibit osteoblastic differentiation and produce malignant osteoid [1,2]. The highest incidence of osteosarcoma is in the second decade of life, which suggests a relationship between bone growth and tumor development [3]. One of the critical steps for normal skeletal development and bone formation is the proliferative expansion of mesenchymal cells, osteoprogenitors, and immature osteoblasts. Gene and viral therapy, a highly promising strategy for the treatment, has shown some therapeutic effects, but the therapeutic effect in vivo is less obviously than in vitro, still having been a lack of real breakthrough [4]. Osteosarcomatous is due to involve the high heterogeneity caused by the interaction of multifactor [5].
Osteosarcoma is found to be the most common histological form of primary bone cancer comprising 2.4% of all malignancies in pediatric patients, and approximately 20% of all primary bone cancers [6,7]. About 8% of all cases occur in the skull and jaw, and another 8% in the pelvis [8]. Neoplastic cells undergo osteoblastic differentiation and form tumoral bone, and have the high incidence, the high malignant degree, the high rate of disability and the high-speed migration. There is a preference for origination in the metaphyseal region of tubular long bones with 42% occurring in the femur, 19% in the tibia, and 10% in the humerus [9,10]. The osteosarcomatous patient must face to resects the trouble bone, even amputation for saving his life. And sometimes amputation is the inevitable way of the large focal lesion, but the five-year survival rate of these osteosarcomatous amputation is only 17.4% to 21.8% to the early and mid-term patients [11].
The use of effective adjuvant chemotherapies plays an important role in controlling osteosarcoma [12,13]. Despite the latest advances, the prognosis for patients with osteosarcoma is still poor. In order to ensure a cure, it is necessary to develop more effective adjuvant treatments. The treatment for osteosarcoma consists of neo-adjuvant chemotherapy, delayed wide resection and adjuvant chemotherapy adapted to tumor tissue removed by surgery. The survival rate for patients with bone osteosarcoma has improved mostly thanks to the addition of systemic chemotherapy following surgical removal of the tumor [14]. The primary treatment is a combination of surgery and chemotherapy. The major problems associated with chemotherapy are the cytotoxic effects [15]. Thus, safe and more effective anticancer treatments are needed for patients with osteosarcoma.
Mushrooms (macrofungi) accumulate a variety of cytotoxic secondary metabolites including polyphenols, terpenoids, and alkaloids [16,17]. Medicinal mushrooms such as Ganoderma lucidum, Phellinus linteus, and Coriolus versicolor have an established history of use in traditional Asian therapies [18,19]. Several polysaccharides and polysaccharide conjugates have been commercialized for the clinical treatment of patients undergoing anticancer therapy. Neogrifolin, a natural biologically active substance isolated from the edible bodies of the mushroom Albatrellus confluens [20]. Two closely related naturally occurring biologically active phenolic compounds, grifolin and neogrifolin were isolated from Albatrellus ssp. (Basiciomycetes) [21-23]. Such compounds were derived from tetraketides and belong to the group of polyketide-terpenoids. Grifolin, neogrifolin and their derivatives possess not only antimicrobial properties [24,25] but also antioxidative [26], tyrosinase inhibiting [27] and cytotoxic activities [28] as well as activity on the vanilloid receptors [29]. No research reports are available so far in the literature based on osteosarcoma studies. Based on the above mentioned facts, we decided to investigate the mechanism of the chemopreventive effects of neogrifolin in human osteosarcoma cell lines. We examined the involvement of AKT and its substrates FOXO and GSK3 and inhibitor of apoptosis protein (IAP) families in the apoptotic effects of neogrifolin in these cells.
Materials and methods
Materials
Neogrifolin was obtained from reputed chemical suppliers (Netsun USA, California, USA). Anti-phospho-AKT (Ser 473), Anti-phospho-AKT (Thr 308), anti-PARP, anti-caspase-3 antibodies, z-VAD-fmk were purchased from Santa Cruz Biotechnology (Santa Cruz, CA USA). Hoechst H33258, Propidium iodide (PI), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), monoclonal mouse anti-β-actin antibody and heat-inactivated fetal bovine serum were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Negative control RNA, GSK-3α/βsiRNA, anti-phospho-GSK3, anti-phospho-FKHRL1, anti-cytochrome c antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA). Rhodamine dye was purchased from Alexis Corp (San Diego, CA, USA).
Cell culture and methods
Human osteosarcoma cell lines MG63 and U2OS were obtained from American Type Culture Collection, (Rockville, MD, USA). Cells were grown in DMEM with 10% (v/v) fetal calf serum, streptomycin100 μg/ml, and penicillin 100 U/ml. Cultures were maintained at 37°C in a humidified incubator in an O2 atmosphere of 95% and 5% CO2.
Determination of cell viability by MTT assay
The cell viability was assessed by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. 104 cells was plated on a 96-well plate in the presence or absence of indicated concentrations of neogrifolin for 6-24 h, then added 100 μl of 0.5 mg/ml MTT to each well. After 4 h incubation at 37°C, 100 μl of dimethyl sulfoxide (DMSO) was added to each well to allow the formed formazan crystals to dissolve. The optical density (OD) value at 565 nm was measured.
Hoechst nuclear staining assessing the percentage of apoptotic cells
Apoptotic morphology was studied by staining the cells with Hoechst 33258 stain. Cells were seeded on cover slips on a 6-well plate in the presence or absence of 50 μM neogrifolin. After 24 h incubation, the cover glasses were carefully washed with phosphate-buffered saline (PBS) and stained with 20 μg/ml of Hoechst 33258 for 10 min. Later, the cells were washed in PBS and observed under a fluorescence microscope (Leica Microsystems AG, Wetzlar, Germany).
Flow cytometry
After neogrifolin-treatment for 4 h, 8 h and 24 h, cells suspension was prepared by trypsinization, and was centrifuged at 1,000 rpm for 5 min at 4°C. Pellets were then rinsed with ice-cold PBS and fixed with 70% ethanol for 24 h. Then the samples were stained with staining buffer (PBS containing 50 μg/ml of propidium iodide, 10 μg/ml RNase A, 0.1% sodium citrate and 0.1 Triton X-100) for 30 min at room temperature in the dark. DNA content was analyzed by flow cytometry (EPICS XL, BECKMAN COULTER, USA). The populations containing less DNA than G1 populations (sub-G1 peak) were considered as apoptotic cells [30].
Cytochrome c release assay
Cytochrome c release from mitochondria was assayed by following the method as reported previously [31]. In this experiment, cells were treated with and without neogrifolin centrifuged at 1000 g. Cell pellets were suspended in 5 volumes of a hypotonic buffer containing 20 mM HEPES-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 20 μg/ml leupeptin, 10 μg/ml aprotinin, and 250 mM sucrose for 15 min on ice. Cells were homogenized by passing them 15-20 times through a 22-gauge, 1.5-inch-long needle. The lysates were centrifuged at 1000 g for 5 min at 4°C and the supernatants were collected and centrifuged at 12,000 g for 15 min. The resulting mitochondrial pellets were resuspended in lysis buffer. Supernatants were transferred to new tubes and centrifuged again at 12,000 g for 15 min, and the resulting supernatants, which represented cytosolic fractions, were separated. The protein from the cytosolic fraction of each sample was used to perform western blotting with anti-cytochrome c antibody.
Mitochondrial membrane potential measurement
Determination of mitochondrial membrane potential was performed as described previously [32]. At first, 1 × 106 MG63 or U2OS cells/ml were incubated with 10 μM rhodamine 123 for 10 min at 37°C. After incorporation of fluorescent probe, the cells were incubated up to 4 h with or without neogrifolin. After incubation, the cells were washed twice with phosphate-buffered saline, harvested by centrifugation, and then resuspended in 1.5 ml phosphate-buffered saline. The fluorescent intensity of each cell suspensions was measured at an excitation wavelength 480 nm and an emission wavelength 530 nm in a Perkin Elmer L15B fluorescence spectrophotometer. The fluorescence intensity was recorded in arbitrary units representing the mitochondrial transmembrane potential.
GSK-3 siRNA transfection
GSK-3 siRNA transfection was used to knock down the expression of GSK-3α/β as described in the literature method [33]. In this study, MG63 and U2OS cells were transfected with GSK-3 siRNA or negative control RNA at concentration of 50 nM using Lipofectamine 2000 according to the manufacturer (Invitrogen, Life Technologies, Inc.). After transfection of about 24 h, the cells were exposed to 50 μM neogrifolin for 24 h and then were harvested to perform western blotting and flow cytometry analysis.
Western blot analysis
Western blot experiments were performed by following this reported method [34]. In brief, cells were washed with PBS (mM: NaCl 130, KCl 2.5, Na2HPO4 10, KH2PO4 1.5, pH 7.4), analysed with solubilization buffer (mM: Tris-Cl 50, NaCl 150, 0.02% NaN3, 1% Nonidet P-40, 0.1% SDS, 0.5% sodiumdeoxycholate, NaVO3, 5 μg/ml leupeptin and 1 μg/ml aprotinin). After centrifugation, the supernatants were collected and equivalent protein concentrations were separated by SDS-PAGE. The separated proteins were electrotransferred to PVDF membranes (Millipore, Bedford, MA, USA). After blocking with PBST (PBS containing 0.05% Tween 20) containing 5% nonfat milk for 1 h, each membrane was incubated with primary antibodies 1 h at room temperature or overnight at 4°C, and then the membranes were probed with the appropriate secondary peroxidase-conjugated antibodies (HRP-linked anti-rabbit secondary antibody and HRP linked anti-biotin antibody, 1 h at room temperature). The immunoblots were visualized by enhanced chemiluminescence.
Statistical analysis
The experimental data was analyzed by SPSS11.5 statistical software. All the data were expressed in the form of mean ± standard deviation. Statistical analysis was performed by applying a one-way analysis of variance followed by the Student t test. P values of less than 0.05 indicated statistical significance. Each experiment is repeated for three times.
Results
Neogrifolin suppressed the proliferation in human osteosarcoma cells
Two experiments were carried out to find the inhibition effect of neogrifolin. In the first experiment, U2OS and MG63 cell lines were incubated with 0, 5, 10, 25, 50, or 100 μM of grifolin for 24 h. In the second experiment, U2OS and MG63 cell lines were incubated with 50 μM neogrifolin for 4 h, 8 h and 24 h. At the end of incubation the cell survival rates were determined by MTT methods. Cell viability is expressed as the percentage of cell survival compared with the control. Data were obtained from five independent experiments. *P < 0.05, **P < 0.01 compared to the control group. The results suggested that neogrifolin treatment evoked cells growth inhibition in concentration- and time-dependent manners in both human osteosarcoma cell lines. The result is depicted in Figure 1.
Figure 1.
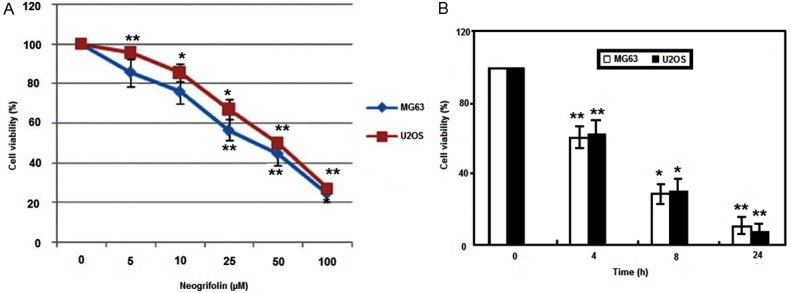
A. U2OS and MG63 cell lines incubated with 0, 5, 10, 25, 50, or 100 μM of neogrifolin for 24 h. B. U2OS and MG63 cell lines incubated with 50 μM neogrifolin for 6 h, 12 h and 24 h.
Neogrifolin induced apoptosis in osteosarcoma cells
To determine whether the growth inhibition induced by neogrifolin in human osteosarcoma cells was caused by apoptosis, MG63 and U2OS cell lines were treated with 50 μM neogrifolin for 24 h, and the morphological changes were examined with Hochest 33258 staining. As shown in Figure 2, the nuclei of cells were round and homogeneously stained in the control group, however, 50 μM neogrifolin treatment induced nuclei condensation or apoptotic bodies formation in two cell lines. Furthermore, we analyzed the sub-G1 population of cells treated with 50 μM neogrifolin for 4, 8 and 24 h. Figure 3 demonstrated that neogrifolin treatment induced apoptosis in a time-dependent manner (Figure 3). The apoptotic population was measured as the percentage of total cell populations with sub-G1 DNA content. These results shown are representative of six independent experiments. All these results suggested that neogrifolin treatment suppresses the growth of osteosarcoma cells by inducing apoptosis.
Figure 2.

Hoechst 33258 fluorescent staining to detect apoptotic morphology in U2OS and MG63 osteosarcoma cell lines treated with 50 μM neogrifolin for 24 h compared with untreated cells (× 200).
Figure 3.
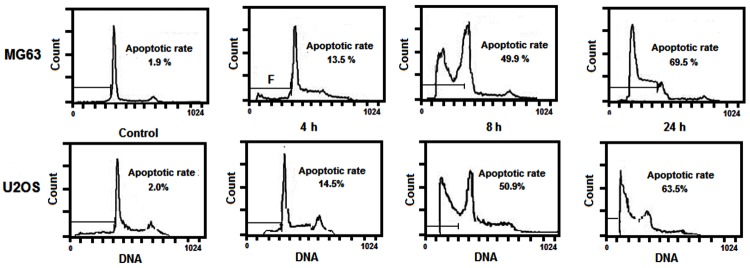
Flow cytometric analysis of cell apoptosis induced by 50 μM neogrifolin treatment for 6 h, 12 h and 24 h in U2OS and MG63 osteosarcoma cell lines.
Effects of neogrifolin treatment on mitochondrial membrane potential and cytochrome c release in osteosarcoma cells
In this experimental study, we examined the effects of neogrifolin treatment on mitochondrial membrane potential and cytochrome c release in osteosarcoma cells. The effect of neogrifolin on the mitochondria membrane potential is shown in Figure 4A. From the Figure 4A, it can be said that neogrifolin treatment induced the loss of the mitochondria membrane potential in two tested cells. In addition we tested the cytochrome c release. Release of cytochrome c from mitochondria to cytosol has been suggested to be involved in apoptosis process induced by various apoptotic inducers. Figure 4B showed whether the release of cytochrome c is involved in the apoptotic effect of neogrifolin in MG63 and U2OS osteosarcoma cells. It can be inferred from Figure 4B, that treatment with 50 μM neogrifolin for 24 h induced the translocation of cytochrome c from the mitochondria to cytosol in two osteosarcoma cell lines. The results shown are representative of five independent experiments and the data are expressed as mean ± SEM of 5 determinations, *P < 0.01 and **P < 0.001 compared with control group.
Figure 4.
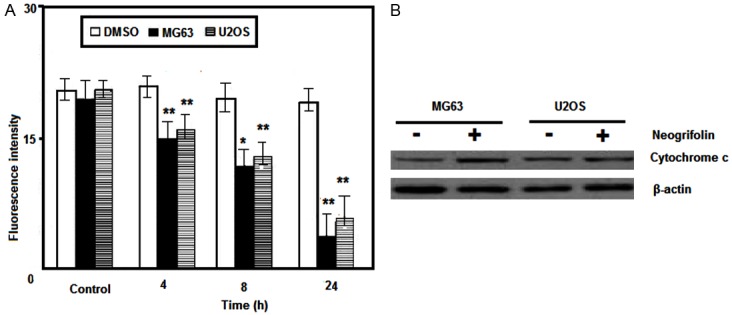
A. Neogrifolin induced the loss of mitochondrial membrane potential in time-dependent manner. B. Neogrifolin increased the release of cytochrome c to cytosol.
Neogrifolin induced caspases-dependent apoptosis in osteosarcoma cells
Further downstream in the apoptotic pathway, we investigated the effects of neogrifolin treatment on the activation of caspases-9 and caspase-3 and PARP. As shown in Figure 5A and 5B, neogrifolin treatment activated caspases-9 and caspase-3 and PARP as determined by the cleavage of caspases-9 and caspase-3 and PARP. The cleavage of caspase-3 and PARP was abolished by the pre-treatment with universal caspases inhibitor z-VAD-fmk. Moreover, z-VAD-fmk pre-incubation blocked the neogrifolin induced cell death in MG63 and U2OS osteosarcoma cells (Figure 5C). All these results suggested that neogrifolin activated caspase 9/3 and induced PARP cleavage.
Figure 5.
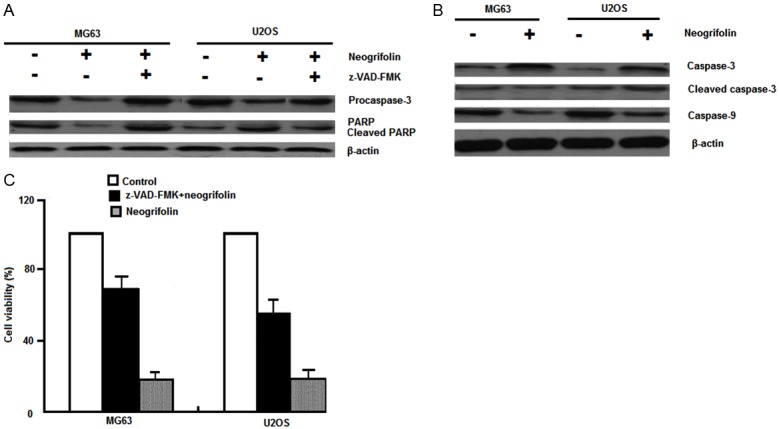
A and B. 50 μM Neogrifolin treatment for 24 h induced activation of caspase-9, caspase-3, and cleavage of PARP in osteosarcoma cells. z-VAD-fmk, a universal inhibitor of caspases, prevented caspase-3 activation and PARP cleavage. C. Cell growth inhibition induced by neogrifolin was abolished by z-VAD-fmk preincubation in osteosarcoma cells.
Dephosphorylation of AKT and its substrates induced by neogrifolin
In order to determine whether the Akt activity is associated with apoptotic effects of neogrifolin, we carried out the protein expression and phosphorylation level of Akt after neogrifolin treatment. MG63 and U2OS cells were treated with various doses of neogrifolin as indicated for 24 h. Neogrifolin treatment decreased the phosphorylation level of Akt at both Thr308 and Ser473 sites (Figure 6A). In both the cells, Akt is found to be constitutively activated. We then examined the effect of neogrifolin on the phosphorylation level of two Akt downstream targets: Fork head transcription factors (FKHR) and GSK3 in MG63 and U2OS osteosarcoma cell lines. As shown in Figure 6B and 6C, constitutive phosphorylation of FKHR and GSK3 was seen in two osteosarcoma cell lines and the phosphorylation level was decreased by the treatment with neogrifolin. All these results suggested that neogrifolin inhibited constitutively active AKT signaling pathway in osteosarcoma cell lines. The results shown are representative of five independent experiments.
Figure 6.
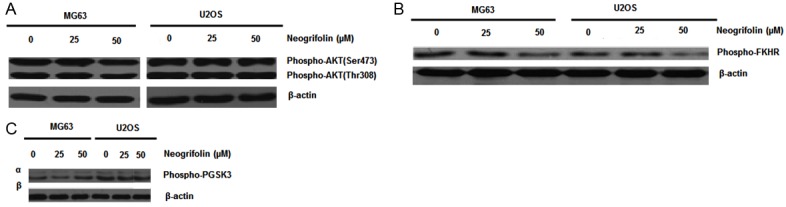
Equal amounts of protein from each sample were separated on SDS-PAGE and immunoblotted with phospho-AKT (ser473) and phospho-AKT (Thr 308) (A), phosphor FKHR (B), or phospho-GSK3-α/β (C) β-actin (control).
Knockdown of GSK3 with siRNA inhibited neogrifolin-mediated apoptosis
To further prove the role of GSK3 in the neogrifolin-induced apoptosis, MG63 and U2OS cells were transiently transfected with GSK3 siRNA or negative control RNA for 24 h. Cell were then harvested to perform western blot and after transfection, MG63 and U2OS cells were treated with neogrifolin for another 24 h. The cell viability and apoptotic rates were measured by MTT and flow cytometry methods, respectively. As shown in Figure 7A, GSK3 siRNA transfection significantly knocked down the expression of GSK3. Knockdown of GSK3 inhibited the apoptotic effects of neogrifolin in the two tested osteosarcoma cells (Figure 7B and 7C). The results shown are representative of five (A) or six (B and C) independent experiments.
Figure 7.
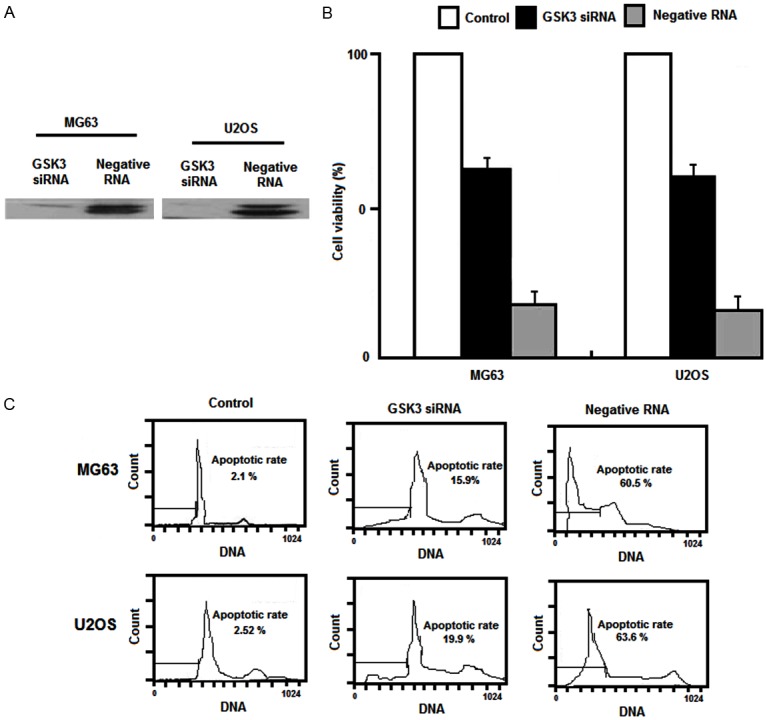
A. Western blot. B. Cell viability and C. Flow cytometry method results.
Neogrifolin reduced the expression of IAP protein family
Inhibitors of apoptosis proteins (IAP) have been shown to be able to inhibit cell apoptosis and have direct effects on caspase-9 and caspase-3 [35]. Therefore, we also determined the effects of grifolin treatment on the expression of IAP family members. As shown in Figure 8, 50 μM grifolin treatment for 24 h decreased the expression of cIAP1, XIAP and survivin in MG63 and U2OS osteosarcoma cells.
Figure 8.
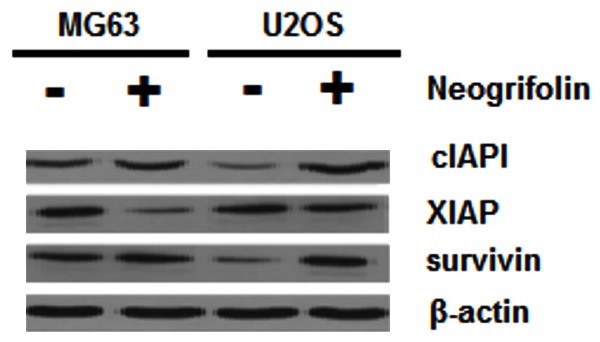
U2OS and MG63 cells treated with and without 50 μM neogrifolin against cIAP1, XIAP, surviving and β-actin antibodies.
Discussion
In this study, our results demonstrated that neogrifolin decreased cell viability in both MG63 and U2OS osteosarcoma cell lines in dose and time-dependent fashions. The predominant mode of cell death in these cells was found to be apoptosis. This was determined by characteristic changes in cell morphology with Hochest 33258 staining and by the presence of sub-G1 peak with flow cytometry method. During apoptosis, the permeability of mitochondrial membrane increased, leading to the loss of membrane potential and release of cytochrome c to cytosol and the released cytochrome c binds to Apaf-1, and then this compound activates caspase-9, triggering caspase-3 activation resulting in the cleavage of PARP [36,37].
In this study, the release of cytochrome c, activation of caspase-9 and caspase-3, and the cleavage of PARP were observed after neogrifolin treatment. In addition, z-VAD-fmk, a universal inhibitor of caspases, preventedcaspase-3 activation and PARP cleavage and inhibited neogrifolin-induced cell growth inhibition. These data indicated that release of cytochrome c mediated caspases activation and PARP cleavage is involved in the apoptotic effects of neogrifolin in osteosarcoma cells. The Akt/mTOR signal transduction is critical to control processes integral in cancer development, such as protein translation, growth, metabolism, therapeutic resistance, and survival. This pathway is frequently over-expressed or over-activated in osteosarcoma, providing a strong rationale to target it in cancer therapy [38,39]. Our present results showed that Akt is constitutively phosphorylated at both Ser473 and Thr 308 in the two tested osteosarcoma cell lines.
Neogrifolin inhibited AKT phosphorylation at these two sites. As the downstream targets of Akt, GSK3 and FKHR family of transcription factors have been reported to be involved the regulation of the cells survival. Akt promotes cells survival by phosphorylating GSK3 and FKHR family of transcription factors, which inactivates them and prevents the apoptosis [40,41]. Therefore, we next examined the phosphorylation levels of GSK3 and FKHR in neogrifolin treated and untreated osteosarcoma cells. The results showed that the constitutive phosphorylation of GSK3 and FKHR were observed in the two osteosarcoma cell lines and the phosphorylation levels were inhibited by neogrifolin treatment. To further confirm the role of GSK3 in neogrifolin-mediated apoptosis, we knock down the expression of GSK3 by siRNA transfection. We found the GSK3 knockdown significantly inhibited the apoptotic effects of neogrifolin in two osteosarcoma cell lines. Our data suggested that Akt signaling pathway play critical role in regulating the growth and survival of osteosarcoma cells. Neogrifolin treatment inactivates Akt signaling pathway thereby inhibiting these cells growth. One mechanism of Akt promoting cells survival is by up-regulating many survival genes including inhibitors of apoptosis proteins (IAPs) [42]. IAPs have been demonstrated to be able to inhibit the activity of caspases and prevent the apoptosis induced by various stimuli.
Survivin is important for osteosarcoma cell survival, growth, and apoptosis resistance, and down-regulating survivin induces osteosarcoma cell apoptosis and growth inhibition [43,44]. Survivin is a structurally unique member of the inhibitor of apoptosis protein family, which is important for mitotic progression, cancer survival, and apoptosis inhibition. Its marked expression in cancers versus normal tissues and its association with unfavorable disease outcome have made survivin a promising new target for anti-cancer interventions [45,46]. Our present study showed that osteosarcoma cell lines expressed IAPs including cIAP1, XIAP and survivin. Neogrifolin treatment decreased the expression of these proteins. It suggested that the down-regulation of IAPs is also involved in the apoptosis process induced by neogrifolin treatment.
In conclusion, research report showed that AKT and its downstream targets FKHR and GSK3 are constitutively active in MG63 and U2OS osteosarcoma cell lines. Neogrifolin-induced inhibition of Akt signaling pathway leads to apoptosis in osteosarcoma cells through cytochrome c release from the mitochondria and activated caspases and down-regulation of IAPs (cIAP1, XIAP and survivin). Collective results suggested that neogrifolin may act as a promising antitumor agent against human osteosarcoma.
Disclosure of conflict of interest
None.
References
- 1.Bramwell VH. The role of chemotherapy in the management of non-metastatic operable extremity osteosarcoma. Semin Oncol. 1997;24:561–571. [PubMed] [Google Scholar]
- 2.Haydon RC, Luu HH, He TC. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res. 2007;454:237–246. doi: 10.1097/BLO.0b013e31802b683c. [DOI] [PubMed] [Google Scholar]
- 3.Cotterill SJ, Wright CM, Pearce MS, Craft AW. Stature of young people with malignant bone tumors. Pediatr Blood Cancer. 2004;42:59–63. doi: 10.1002/pbc.10437. [DOI] [PubMed] [Google Scholar]
- 4.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4:218–227. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirrmacher V, Ahlert T, Probstle T, Steiner HH, Herold-Mende C, Gerhards R, Hagmüller E, Steiner HH. Immunization with virus-modified tumor cells. Semin Oncol. 1998;25:677–696. [PubMed] [Google Scholar]
- 6.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–989. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 7.Bao PP, Zheng Y, Wang CF, Gu K, Jin F, Lu W. Time trends and characteristics of childhood cancer among children age 0-14 in Shanghai. Pediatr Blood Cancer. 2009;53:13–16. doi: 10.1002/pbc.21939. [DOI] [PubMed] [Google Scholar]
- 8.Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchiya H, Kanazawa Y, Abdel-wanis ME, Asada N, Abe S, Isu K, Sugita T, Tomita K. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group study. J. Clin. Oncol. 2002;20:3470–3477. doi: 10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Corradi D, Wenger De, Bertoni F, Bacchini P, Bosio S, Goldoni M, Unni KK, Sim FH, Inwards CY. Multicentric osteosarcoma: clinicopathologic and radiographic study of 56 cases. Am J Clin Pathol. 2011;136:799–807. doi: 10.1309/AJCP0V0OATKCNAZP. [DOI] [PubMed] [Google Scholar]
- 11.Afonso CL, Tulman ER, Lu Z, Zsak L, Kutish GF, Rock DL. The genome of fowlpox virus. J Virol. 2000;74:3815–3831. doi: 10.1128/jvi.74.8.3815-3831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical Chemotherapy Compared With Immediate Surgery and Adjuvant Chemotherapy for Nonmetastatic Osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 13.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21:vii320–vii325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 14.Gobin B, Moriceau G, Ory B, Charrier C, Brion R, Blanchard F, Redini F, Heymann D. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immune competent murine models. PLoS One. 2014;9:e90795. doi: 10.1371/journal.pone.0090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAllister TW, Ahles TA, Saykin AJ, Ferguson RJ, McDonald BC, Lewis LD, Flashman LA, Rhodes CH. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep. 2004;6:364–371. doi: 10.1007/s11920-004-0023-y. [DOI] [PubMed] [Google Scholar]
- 16.Ye M, Luo X, Li L, Shi Y, Tan M, Weng X, Li W, Liu J, Cao Y. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, induces cell-cycle arrest in G1 phase via the ERK1/2 pathway. Cancer Lett. 2007;258:199–207. doi: 10.1016/j.canlet.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Russo A, Piovano M, Clericuzio M, Lombardo L, Tabasso S, Chamy MC, Vidari G, Cardile V, Vita-Finzi P, Garbarino JA. Putrescine-1,4-dicinnamide from Pholiota spumosa (Basidiomycetes) inhibits cell growth of human prostate cancer cells. Phytomedicine. 2007;14:185–191. doi: 10.1016/j.phymed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Moradali MF, Mostafavi H, Ghods S, Hedjaroude GA. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi) Int Immunopharmacol. 2007;7:701–724. doi: 10.1016/j.intimp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Cui SW, Cheung PCK, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol. 2007;18:4–19. [Google Scholar]
- 20.Ding ZH, Dong ZJ, Liu JK. Albaconol: a novel prenylated resorcinol (=benzene-1,3-diol) from Basidiomycetes Albatrellus confluens. Helv Chim Acta. 2001;84:259–262. [Google Scholar]
- 21.Hirata Y, Nakanishi K. Grifolin, an antibiotic from a Basidiomycete. J Biol Chem. 1950;184:135–143. [PubMed] [Google Scholar]
- 22.Vrkoč J, Buděšinský M, Dolejš L. Phenolic meroterpenoids from the Basidiomycete Albatrellus ovinus. Phytochemistry. 1977;16:1409–1411. [Google Scholar]
- 23.Besl H, Hoefle G, Jendrny B, Jägers E, Steglich W. Pilzpigmente, XXXI. Farnesylphenole aus Albatrellus-Arten (Basidiomycetes) Chem Ber. 1977;110:3770–3776. [Google Scholar]
- 24.Zechlin L, Wolf M, Steglich W, Anke T. Antibiotika aus Basidiomyceten, XII. Cristatsäure, ein modifiziertes Farnesylphenol aus Fruchtkörpern von Albatrellus cristatus. Liebigs Ann Chem. 1981;12:2099–2105. [Google Scholar]
- 25.Hashimoto T, Quang DN, Nukada M, Asakawa Y. Isolation, synthesis and biological activity of grifolic acid derivatives from inedible mushroom Albatrellus dispansus. Heterocycles. 2005;65:2431–2439. [Google Scholar]
- 26.Nukata M, Hashimoto T, Yamamoto I, Iwasaki N, Tanaka M, Asakawa Y. Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochemistry. 2002;59:731–737. doi: 10.1016/s0031-9422(02)00050-x. [DOI] [PubMed] [Google Scholar]
- 27.Misasa H, Matsui Y, Uehara H, Tanaka H, Ishihara M, Shibata H. Tyrosinase inhibitors from Albatrellus confluens. Biosci Biotechnol Biochem. 1992;56:1660–1661. [Google Scholar]
- 28.Yang XL, Qin C, Wang F, Dong ZJ, Liu JK. A new meroterpenoid pigment from the Basidiomycete Albatrellus confluens. Chem Biodivers. 2008;5:484–489. doi: 10.1002/cbdv.200890047. [DOI] [PubMed] [Google Scholar]
- 29.Hellwig V, Nopper R, Mauler F, Freitag J, Ji-Kai L, Zhi-Hui D, Stadler M. Activities of prenylphenol derivatives from fruitbodies of Albatrellus ssp. on the human and rat vanilloid receptor 1 (VR1) and characterisation of the novel natural product, confluentin. Arch Pharm. 2003;336:119–126. doi: 10.1002/ardp.200390008. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HN, Zhou JG, Qiu QY, Ren JL, Guan YY. ClC-3 chloride channel prevents apoptosis induced by thapsigargin in PC12 cells. Apoptosis. 2006;11:327–336. doi: 10.1007/s10495-006-3980-2. [DOI] [PubMed] [Google Scholar]
- 31.Uddin S, Hussain AR, Al-Hussein KA, Manogaran PS, Wickrema A, Gutierrez MI, Bhatia KG. Inhibition of Phosphatidylinositol 3-kinase/AKT-signaling promotes apoptosis of primary effusion lymphoma cells. Clin Cancer Res. 2005;11:3102–3108. doi: 10.1158/1078-0432.CCR-04-1857. [DOI] [PubMed] [Google Scholar]
- 32.Lin HI, Lee YJ, Chen BF, Tsai MC, Lu JL, Chou CJ, Jow GM. Involvement of Bcl-2 family, cytochrome c and caspase 3 in induction of apoptosis by beauvericin in human non-small cell lung cancer cells. Cancer Lett. 2005;230:248–259. doi: 10.1016/j.canlet.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 33.Pang RP, Zhou JG, Zeng ZR, Li XY, Chen W, Chen MH, Hu PJ. Celecoxi induces apoptosis in COX-2 deficient human gastric cancer cells through Akt/GSK3β/NAG-1 pathway. Cancer Lett. 2006;251:268–277. doi: 10.1016/j.canlet.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Zhou JG, Ren JL, Qiu QY, He H, Guan YY. Regulation of intracellular Cl-concentration through volume-regulated ClC-3 chloride channel in A10 vascular smooth muscle cells. J Biol Chem. 2005;280:7301–7308. doi: 10.1074/jbc.M412813200. [DOI] [PubMed] [Google Scholar]
- 35.Kornacker M, Verneris MR, Kornacker B, Scheffold C, Negrin RS. Survivin expression correlates with apoptosis resistance after lymphocyte activation and is found preferentially in memory T cells. Immunol Lett. 2001;76:169–173. doi: 10.1016/s0165-2478(01)00186-9. [DOI] [PubMed] [Google Scholar]
- 36.Chan DW, Son SC, Block W, Ye R, Khanna KK, Wold MS, Douglas P, Goodarzi AA, Pelley J, Taya Y, Lavin MF, Lees-Miller SP. Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase. J Biol Chem. 2000;275:7803–7810. doi: 10.1074/jbc.275.11.7803. [DOI] [PubMed] [Google Scholar]
- 37.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 39.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 40.Romashkova JA, Makarov SS. NF-kappa B is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 41.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altieri DC, Marchisio PC. Survivin apoptosis: An interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327–1333. [PubMed] [Google Scholar]
- 43.Wu YF, Liang XJ, Liu YY, Gong W, Liu JX, Wang XP, Zhuang ZQ, Guo Y, Shen HY. Antisense oligonucleotide targeting surviving inhibits growth by inducing apoptosis in human osteosarcoma cells MG-63. Neoplasma. 2010;57:501–506. doi: 10.4149/neo_2010_06_501. [DOI] [PubMed] [Google Scholar]
- 44.Zou J, Gan M, Mao N, Zhu X, Shi Q, Yang H. Sensitization of osteosarcoma cell line SaOS-2 to chemotherapy by downregulating survivin. Arch Med Res. 2010;41:162–169. doi: 10.1016/j.arcmed.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 46.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]


