Abstract
There were limited studies assessing the role of HMGB1 in TB infection. In this prospective study, we aimed to assess the levels of HMGB1 in plasma or sputum from active pulmonary tuberculosis (APTB) patients positive for Mtb culture test, and to evaluate its relationship with inflammatory cytokines and innate immune cells. A total of 36 sputum Mtb culture positive APTB patients and 32 healthy volunteers (HV) were included. Differentiated THP-1 cells were treated for 6, 12 and 24 hrs with BCG at a multiplicity of infection of 10. The absolute values and percentages of white blood cells (WBC), neutrophils, lymphocytes, and monocytes were detected by an automatic blood analyzer. Levels of HMGB1, IL-6, IL-10 and TNF-α in plasma, sputum, or cell culture supernatant were measured by ELISA. The blood levels of HMGB1, IL-6, IL-10 and TNF-α, the absolute values of WBC, monocytes and neutrophils, and the percentage of monocytes were significant higher in APTB patients than those in HV groups (P<0.05). The sputum levels of HMGB1, IL-10, and TNF-α were also significantly higher in APTB patients than those in HV groups (P<0.05). Meanwhile, plasma level of HMGB1, IL-6, and IL-10 in APTB patients were positively correlated with those in sputum (P<0.05), respectively. IL-6 was positively correlated with HMGB1 both in plasma and sputum of APTB patients (P<0.05). HMGB1 and IL-6 is positively correlated with the absolute number of monocytes in APTB patients (P<0.05). BCG induced HMGB1, IL-6, IL-10 and TNF-α production effectively in PMA-treated THP-1 cells. HMGB1 may be used as an attractive biomarker for APTB diagnosis and prognosis and may reflect the inflammatory status of monocytes in patients with APTB.
Keywords: Active pulmonary tuberculosis, infection, cytokines, HMGB1, immunity
Introduction
Tuberculosis (TB) remains a global health problem. It has been estimated that 8.6 million individuals worldwide developed TB and more than 1.3 million died from TB-related disease, in 2012 [1,2]. In China, TB infection incidence has been estimated as 50~124 cases per 100,000 inhabitants per year [1]. The rapid and accurate detection of TB still relies on sputum smear microscopy and culture in a well-managed and equipped laboratory network, although it takes weeks to the result. The prognosis of the TB disease depends on the ability of the host to eliminate the mycobacterium tuberculosis (MTB). In the removal process, innate immune cells, adaptive immune cells and inflammatory cytokines, etc. play important roles.
HMGB1 (previously HMG1; HMG-1; HMG 1; amphoterin; p30), an inflammatory cytokines, functions in nucleus (e.g., nucleosome stability, genome chromatinization, nuclear catastrophe, DNA binding, V (D) J recombination, DNA replication, telomere stability and gene transcription), cytoplasm (e.g., autophagy, unconventional secretory pathway), membranes (e.g., neurite outgrowth, platelet activation, cell differentiation, erythroid maturation, adhesion and innate immunity) and extracellular environment (e.g., inflammation, immunity, migration, invasion, proliferation, differentiation, antimicrobial defense and tissue regeneration) [3-7]. Especially, extracellular HMGB1, released by immune cells (e.g., macrophages, monocytes, neutrophils, DCs, NKs), fibroblasts, or epithelial cells, functions as a DAMP to alert the innate immune system by recruiting inflammatory cells in lung disease (e.g., asthma, COPD, acute respiratory distress syndrome, cystic fibrosis, pneumonia, acute lung injury and lung cancer) [3,8-13]. Notably, extracellular HMGB1 also functions as an immune adjuvant to trigger a robust response of T cells, dendritic cells, and endothelial cells [3,14-18]. It is well established that HMGB1 as a pro-inflammatory cytokine in many inflammatory diseases [3]. Extracellular or blood HMGB1 levels were found increased in patients with juvenile idiopathic arthritis, atrial fibrillation, gastric cancer, and septic shock, and closely associated with the clinical and pathologic features [19-22]. In addition, suppression of HMGB1 release and/or activity significantly decreased the inflammatory response, tissue injury, and death in animals [3,23-25]. These properties of HMGB1 make it an attractive biomarker and therapeutic target. Therefore, there are many translational studies ongoing to assess the feasibility of HMGB1 suppression as therapeutic strategies for inflammatory diseases [3,13,26-28].
Recently, Grover et al. reported that HMGB1, independent of its interaction with receptor for advanced glycation end products (RAGE), enhanced the protective efficacy and cellular immune response of TB subunit vaccines as an adjuvant [29]. Currently, only limited information is available about the role of HMGB1 in TB infection. Hence, in this prospective study, we aimed to assess whether HMGB1 is detectable or mutative both in plasma and sputum of active pulmonary tuberculosis (APTB) patients positive for Mtb culture test, and to evaluate its relationship with inflammatory cytokines (e.g., IL-6, IL-10 and TNF-α) and innate immune cells (e.g., monocytes and neutrophils).
Subjects and methods
Subjects
A total of 36 sputum Mtb culture positive APTB patients and 32 healthy volunteers (HV) were recruited from April 2013 to April 2014 in Dongguan 6th People’s Hospital (Dongguan, China) with the following inclusion/exclusion criteria [30]. All subjects were MTB positive in sputum culture test. Age, gender, tuberculin skin test, fever and smoking status were obtained at the screening evaluation. Subjects with HIV infection, diabetes, cancer, autoimmune diseases, immunosuppressive treatment, or pulmonary tuberculosis history were excluded from the study. The study was approved by the Internal Review and the Ethics Boards of Guangdong Medical College and Dongguang 6th People’s Hospital, and informed consent was obtained from all study subjects.
Plasma and sputum preparation
After registering eligible, patients were asked to rinse their mouth with water and bloods and sputum concomitantly samples were collected. 6 mL blood was drawn into anticoagulated tubes (Improve medical, Guangzhou, china). One third of the blood samples were used to analyze counts and percentages of WBC, neutrophils, lymphocytes, and monocytes by an automatic blood analyzer (UniCel DxC 800 Synchron, Beckman coulter, California, USA). Remaining blood samples were centrifuged at 1500 rpm for 15 min, and plasma samples were collected and stored at -80°C until analysis. Induced sputum collection was done 2 hrs later. After brushing teeth and rinsing mouth, patients were instructed to inhale sterile 3% saline for up to 30 min via a disposable sputum suction device (Hangzhou Medtec Medical Devices, Hangzhou, China) completed by nurses. Sputum samples were collected directly into 50 ml sterile disposable polypropylene centrifuge tubes (Corning incorporated, NY, USA) and processed within 2 hrs of collection as described previously [31] with minor modifications. Briefly, sputum was diluted with an equal amount of Hank’s solution containing 0.1% dithiothreitol and incubated on a rocking platform for 15 min at room temperature to digest mucus. Following digestion, half of the sputum digests were removed for AFB smear and quantitative culture. Remaining sputum digests were centrifuged at 1500 rpm and filtered with 0.22 μm disposable filters (Millipore, MA, USA) to sediment cellular or bacterial constituents and stored at -80°C until analysis. Sputum specimens from healthy control subjects (induced sputum only) were collected and processed following the same procedure as that of APTB patients.
Infection of THP-1 cell line with BCG
M. bovis BCG was obtained from Zheng W. Chen laboratories, University of Illinois College of Medicine, Chicago, Illinois, USA. Mycobacterial strains were grown to mid-log phase in MB7H9 medium supplemented with albumin-dextrose-catalase (1×), glycerol (0.5%) and Tween 80 (0.05%) according to the method described by Huang et al [32]. PBS stocks were prepared and stored at -80°C until further use. The CFU of stocks was enumerated by plating appropriate dilutions in duplicates on MB7H11 agar supplemented with oleic acid-albumin-dextrose-catalase (1×) and glycerol (0.5%) [32]. THP-1 cells were obtained from cell bank of Chinese Academy of Sciences, Shanghai, China, and were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified incubator with 5% CO2. The cells were seeded in 6-well microplates at a concentration of 1×106 cells/well followed by adding 100 nM PMA for 24 hrs to induce the cells differentiation into macrophages. Differentiated THP-1 cells were treated for 6, 12 and 24 hrs with heat-inactivated BCG at a multiplicity of infection of 10. 1 μg/ml lipopolysaccharide (LPS) was used as positive control and PBS was used as negative control. After culture for an indicated period, cell culture supernatants were collected and filtered through a 0.22 μm filter, and stored at -80°C until analysis.
ELISA detection
Cytokines were measured in the plasma, sputum and cell culture supernatant samples using human HMGB1 ELISA Kit (Bluegene Biotech, Shanghai, China) and human IL-6, IL-10 and TNF-α ELISA Kit (RayBiotech, Atlanta, USA) according to the manufacturer’s instructions. Cell culture supernatant was diluted 1:10. Plasma and processed sputum were used as an undiluted specimen.
Statistical analysis
Normality test was first performed to decide whether the data were in normal distribution. Data were shown in Mean ± SEM (standard error of the mean, SEM). Student’s t-test or ANOVA (analysis of variance, ANOVA) were employed to compare the differences of measured data, and Pearson correlation was used to measure the degree of dependency between variables by the GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA, USA). A P value of less than 0.05 was considered as statistical significant.
Results
Characteristics of the subjects included in the study
We prospectively enrolled 68 subjects. Among them, 36 cases were active pulmonary tuberculosis (APTB) and 32 cases were healthy volunteers (HV). The demographic and clinical characteristics for all study subjects are shown in Table 1. No significant difference in terms of age and gender was noted between APTB and HV groups. Among APTB patients, 100% (36/36) of them were MTB positive by sputum smear analysis, 77.8% (28/36) of them were newly APTB patients, and 100% (36/36) of them were positive for tuberculin skin test (TST) (induration diameter ≥10 mm).
Table 1.
Demographics of subjects included in the study
| Groups | APTB | HV | t/x2 | P |
|---|---|---|---|---|
| n | 36 | 32 | ||
| Age, years | 38.550±1.610 | 37.210±1.520 | 0.6010 | >0.05 |
| Male/Female | 20/16 | 17/15 | 0.040 | >0.05 |
| Smoking (ever), n (%) | 5 (13.9) | 3 (9.4) | ||
| New/Relapsed | 28/8 | - | ||
| TST, ≥10 mm, n (%) | 36 (100) | 0 (0) | ||
| AFB Smear-positive, n (%) | 36 (100) | 0 (0) | ||
| Fever, n (%) | 0 (0) | 0 (0) |
TST = Tuberculin skin test; AFB = Acid-fast bacilli.
HMGB1, IL-6, IL-10 and TNF-α are increased both in plasma and sputum of APTB patients
It has been reported that during lung disease like asthma, COPD, acute respiratory distress syndrome, cystic fibrosis, pneumonia and lung cancer, blood HMGB1 levels are increased and correlates with disease severity [3,8-13]. Additionally, HMGB1 production is increased in sputum and contributes to inflammatory response and lung matrix degradation in cystic fibrosis airway disease [11,33]. Therefore, we evaluated the level of HMGB1 in both plasma and sputum, in 36 APTB patients and 32 HV subjects. At the same time, levels of IL-6, IL-10 and TNF-α in plasma and sputum were also analyzed to evaluate inflammatory response in blood and airways or lung parenchyma.
Results showed that plasma level of HMGB1 (1.68-fold) of APTB patients were significantly higher than those in HV groups (Figure 1A). We also found increased plasma levels of IL-6 (1.86-fold), IL-10 (1.44 folds) and TNF-α (1.63-fold) in APTB patients (Figure 1B-D), which was consistent with previously published studies on TB patients [34,35]. Notably, sputum levels of HMGB1 (1.82-fold), IL-10 (1.59-fold), and TNF-α (2.18-fold) were significantly higher than those in HV groups (Figure 1A-D). Interestingly, levels of HMGB1 (1.27-fold), IL-10 (1.30-fold), and TNF-α (1.50-fold) were also significantly higher in sputum than those in plasma in APTB patients. However, sputum level of IL-16 (-1.58-fold) was significantly lower than that in plasma in APTB patients. No significant differences in these cytokines were found between plasma and sputum HV groups (Figure 1A-D).
Figure 1.

Abundance of HMGB1, IL-6, IL-10 and TNF-α both in plasma and sputum of APTB patients. ELISA was performed in concomitantly collected plasma and sputum samples. Plasma and sputum HMGB1 (A), IL-6 (B), IL-10 (C) and TNF-α (D) were significantly increased in APTB patients (n=36) compared to HV (n=32). Data were shown as Mean ± SEM. *, P<0.05; **, P<0.01; ***, P<0.0001.
These results suggested that HMGB1, IL-10, and TNF-α may be involved in a dynamic inflammatory response to TB infection in blood and airways (or lung parenchyma). Especially in airways, released HMGB1, IL-10, and TNF-α may be a useful marker for assessing the airways or lung parenchyma inflammatory response to TB infection.
Plasma level of HMGB1, IL-6 and IL-10 in APTB patients are positive correlate to themselves in sputum, respectively
We further investigated correlations of plasma levels of HMGB1, IL-6, IL-10, and TNF-α with those in sputum, in either APTB patients or HV groups (Figure 2A and 2B). Interestingly, plasma levels of HMGB1, IL-6, and IL-10, but not TNF-α, were positively correlated with those in sputum after TB infection (Figure 2A and 2B).
Figure 2.
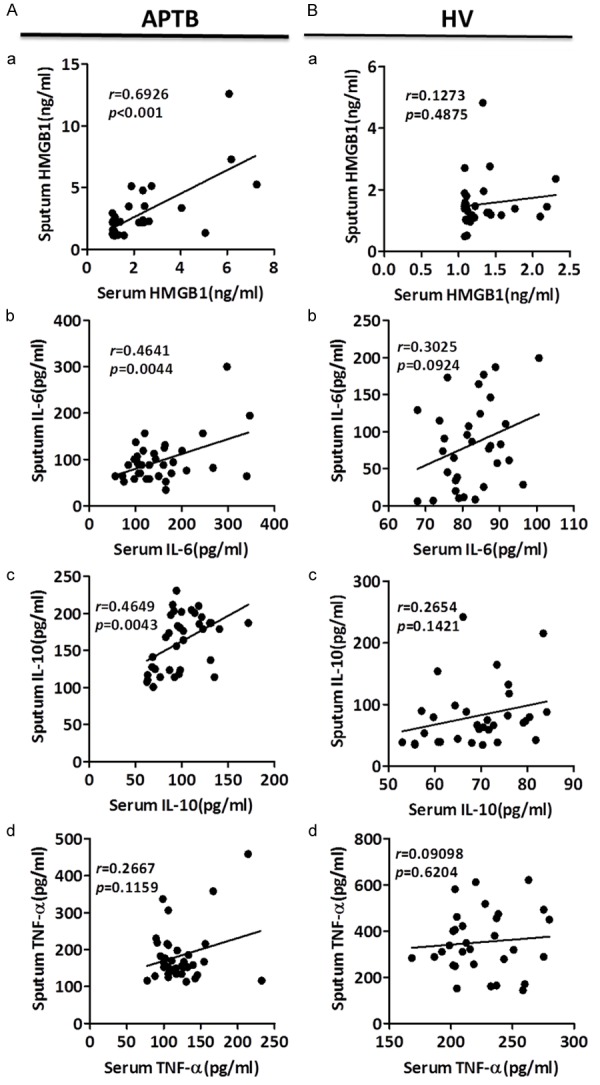
Relationship between the plasma and sputum levels of HMGB1, IL-6, IL-10 and TNF-α in APTB patients and HV subjects. Correlation of plasma and sputum level of HMGB1, IL-6, IL-10 and TNF-α in APTB patients (A) and HV subjects (B) were analysised by Pearson correlation using GraphPad Prism Version 5.0. Plasma levels of HMGB1 (A-a), IL-6 (A-b) and IL-10 (A-c), but not TNF-α (A-d) in APTB patients were positively correlated with those in sputum, respectively. However, plasma levels of HMGB1 (B-a), IL-6 (B-b), IL-10 (B-c) and TNF-α (B-d) in HV subjects did not correlate with those in sputum.
These results suggested that elevated level of HMGB1, IL-6, and IL-10 in plasma may be closely related to the activity of inflammatory response caused by TB infection. They also suggested HMGB1, IL-6, and IL-10 may share some similar response pathway to TB infection, although the abundance of IL-6 levels between plasma and sputum was inconsistent with the abundance of HMGB1 and IL-10 (Figure 1).
IL-6 positively correlates with HMGB1 in both plasma and sputum of APTB patients
In order to explore the relationship among the inflammatory cytokines, correlations of HMGB1, IL-6, IL-10, and TNF-α levels with each other were analyzed in either plasma or sputum (Figure 3A and 3B). Consistent with previously published studies in disease of severe community-acquired infections and bacteraemia [36], plasma level of IL-6 showed a positive correlation with HMGB1 in this study (Figure 3A-a). Furthermore, in line with the findings by Nemeth, et al. [37], there was a positive correlation between plasma level of IL-6 and plasma level of TNF-α in APTB patients (Figure 3A-b). Interestingly, similar to the findings in plasma, sputum level of IL-6 displayed a positive correlation with sputum level of HMGB1 and TNF-α (Figure 3B-a, 3B-b) respectively. While in plasma or sputum of APTB patients, level of HMGB1 had no correlation with level of IL-10 (Figure 3A-c, 3B-c) or level of TNF-α (Figure 3A-d, 3B-d), and level of IL-10 also had no correlation with level of TNF-α (Figure 3A-e, 3B-e) or level of IL-6 (Figure 3A-f, 3B-f).
Figure 3.
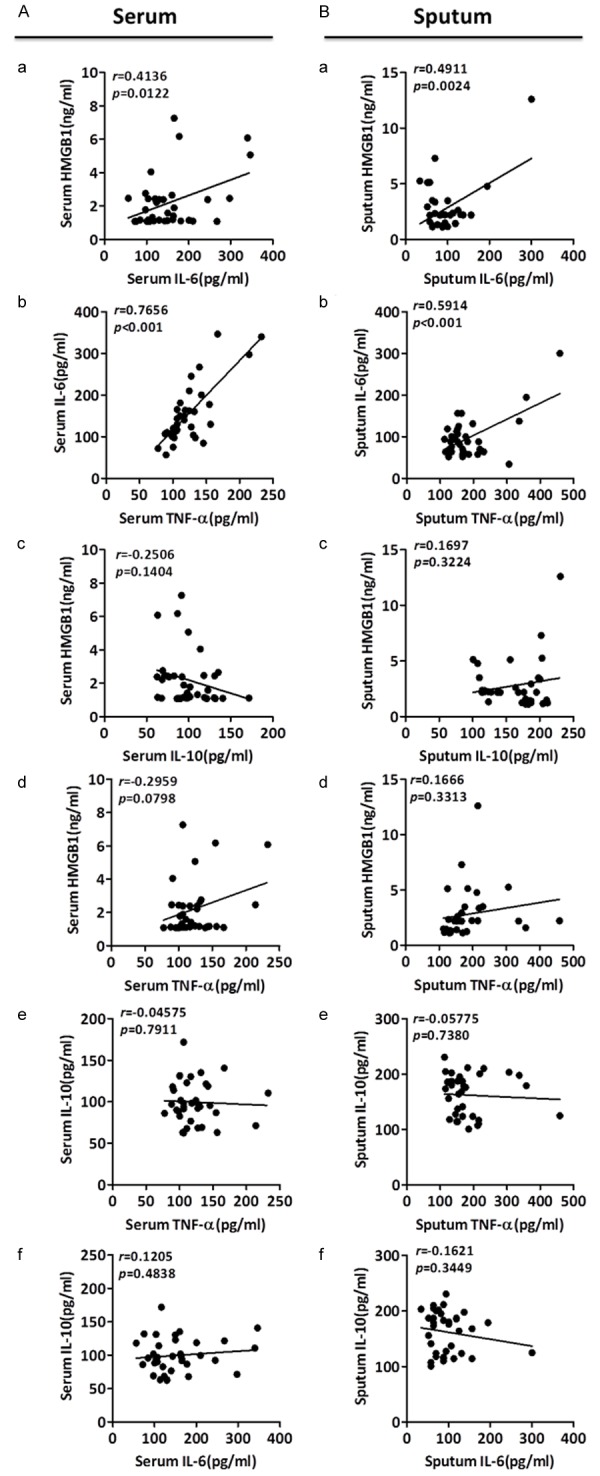
Relationship among the levels of HMGB1, IL-6, IL-10 and TNF-α in plasma and sputum of APTB patients. Correlations among the levels of HMGB1, IL-6, IL-10 and TNF-α in plasma (A) and sputum (B) in APTB patients were analyzed by Pearson correlation using GraphPad Prism Version 5.0. Plasma level of HMGB1 was positively correlated with plasma levels of IL-6 (A-a), and plasma level of TNF-α was positively correlated with plasma level of IL-6 (A-b). There were no significant correlations between plasma HMGB1 level and plasma IL-10 (A-c), between plasma HMGB1 level and plasma TNF-α (A-d), between plasma TNF-α and plasma IL-10 (A-e), and between plasma IL-6 and plasma IL-10 (A-f). Similarly, sputum level of HMGB1 was positively correlated with sputum level of IL-6 (B-a), and sputum level of TNF-α was positively correlated with sputum level of IL-6 (B-b). There were no signification correlations between sputum HMGB1 and sputum IL-10 (B-c), between sputum HMGB1 and sputum TNF-α (B-d), between sputum TNF-α and sputum IL-10 (B-e), and between sputum IL-6 and sputum IL-10 (B-f).
These results further indicated that HMGB1 and IL-6 may share some similar or the same response pathway to TB infection. The above results also indicated the levels of different cytokines are fine-tuned and the delicate balance between the pro-inflammatory and anti-inflammatory cytokine signaling is well established at APTB progression.
HMGB1 handling IL-6 may be associated with monocytes dynamic responses in APTB patients
It is clear that HMGB1 plays a critical role in the regulation of innate and adaptive immune responses [3,37]. Both innate and adaptive immunity play important roles in the host defense against MTB infection [38-43]. In accordance with the findings by Veenstra, et al., we found the absolute WBC, monocytes, and neutrophils counts were significantly higher, but lymphocytes were significantly lower in APTB patients compared to those in HV groups (Table 2). Meanwhile, the percentage of monocytes was significantly higher, but lymphocytes were significantly lower in APTB patients (Table 2). It’s worth noting that the absolute value of blood monocytes was positively correlated to plasma levels of HMGB1 (Figure 4A) and IL-6 (Figure 4B), but not IL-10 (Figure 4C) or TNF-α (Figure 4D).
Table 2.
Blood cell differential of subjects (Mean ± SEM)
| Groups | APTB (n=36) | HV (n=32) | t | P |
|---|---|---|---|---|
| WBC, ×109/L | 7.531±0.313 | 6.014±0.310 | 3.430 | <0.01 |
| Mono, ×109/L | 0.4561±0.0282 | 0.3419±0.02611 | 2.949 | <0.01 |
| Lymp, ×109/L | 1.337±0.221 | 2.031±0.209 | 2.266 | <0.05 |
| Neut, ×109/L | 5.360±0.330 | 4.094±0.368 | 2.569 | <0.05 |
| Mono, % | 7.533±0.683 | 5.259±0.541 | 2.525 | <0.05 |
| Lymp, % | 20.216±2.133 | 30.899±2.744 | 3.109 | <0.01 |
| Neut, % | 60.310±3.852 | 51.630±3.728 | 1.610 | >0.05 |
WBC = white blood cells; Mono = monocytes; Lymp = lymphocytes; Neut = neutrophils. Groups were compared by t test with GraphPad Prism Version 5.0.
Figure 4.
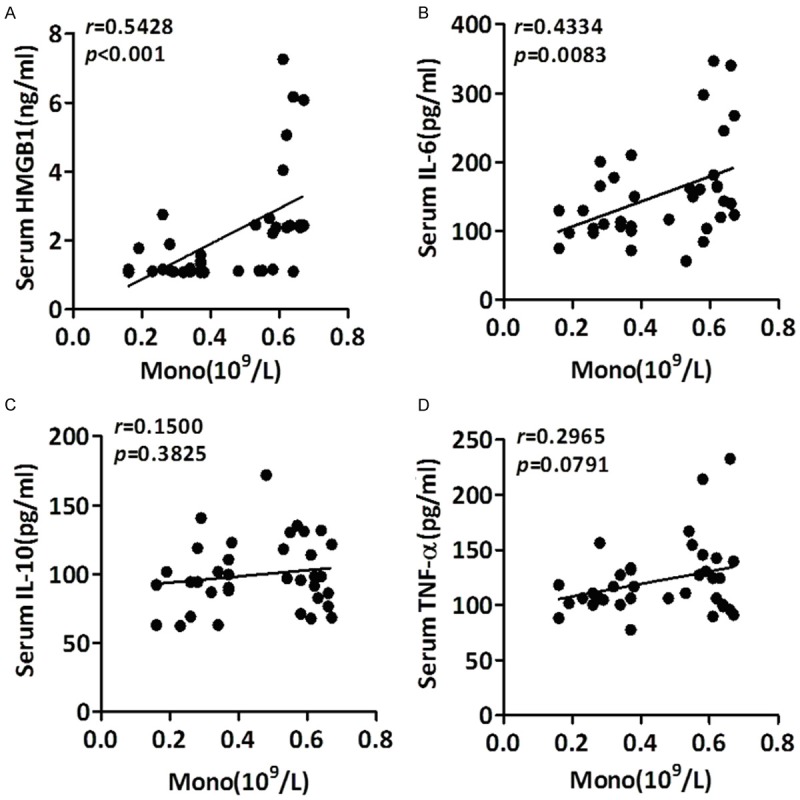
Relationship between the plasma level of HMGB1, IL-6, IL-10 and TNF-α to the absolute values of blood monocytes (Mono) in APTB patients. Correlations of the absolute value of blood monocytes (Mono) in APTB patients with plasma levels of HMGB1 (A), IL-6 (B), IL-10 (C) and TNF-α (D) were analyzed by Pearson correlation using GraphPad Prism Version 5.0. The absolute value of blood monocytes were positively correlated to plasma levels of HMGB1 (A) and IL-6 (B), but not IL-10 (C) and TNF-α (D).
These results suggested that HMGB1 handling IL-6 may be associated with monocytes dynamic responses in APTB patients and also indicated that the elevation of plasma level of HMGB1 and IL-6 may be directly related to the elevation of blood monocytes.
BCG induced HMGB1, IL-6, IL-10, and TNF-α production in PMA-treated THP-1 cells
To better address the relationship of HMGB1, IL-6, IL-10 and TNF-α production with monocytes dynamic responses after TB infection, THP-1 cells that differentiated into a macrophage-like phenotype following the treatment with 100 nM PMA for 24 hrs were used to incubate with BCG at a multiplicity of infection of 10 for 6, 12 and 24 hrs. HMGB1, IL-6, IL-10 and TNF-α were detected in the cell culture supernatant. LPS was used as a positive control and PBS was served as a negative control. As shown in Figure 5, BCG in accordance with LPS induced HMGB1, IL-6 and TNF-α, but not IL-10 production from PMA-treated THP-1 cells in a time-dependent manner. HMGB1 production, at 24 hrs, showed 4-fold increased after BCG stimulated, and near 8-fold increased after LPS stimulation in comparison with un-stimulated group (Figure 5A). In accordance with HMGB1, IL-6 production at 24 hrs showed more than 2-fold increase after BCG stimulation and more than 9-fold increase after LPS stimulation (Figure 5B). However, IL-10 reached the highest level at 12 hrs after BCG (2.4-fold) or LPS (7.5-fold) stimulation and gradually decreased during 12~24 hrs after BCG (1.7-fold) or LPS (4.9-fold) stimulation (Figure 5C). In addition, the baseline TNF-α production in PMA-treated THP-1 cells was at a high level, and both BCG and LPS stimulation showed a near 2-fold increase at 24 hrs (Figure 5D).
Figure 5.
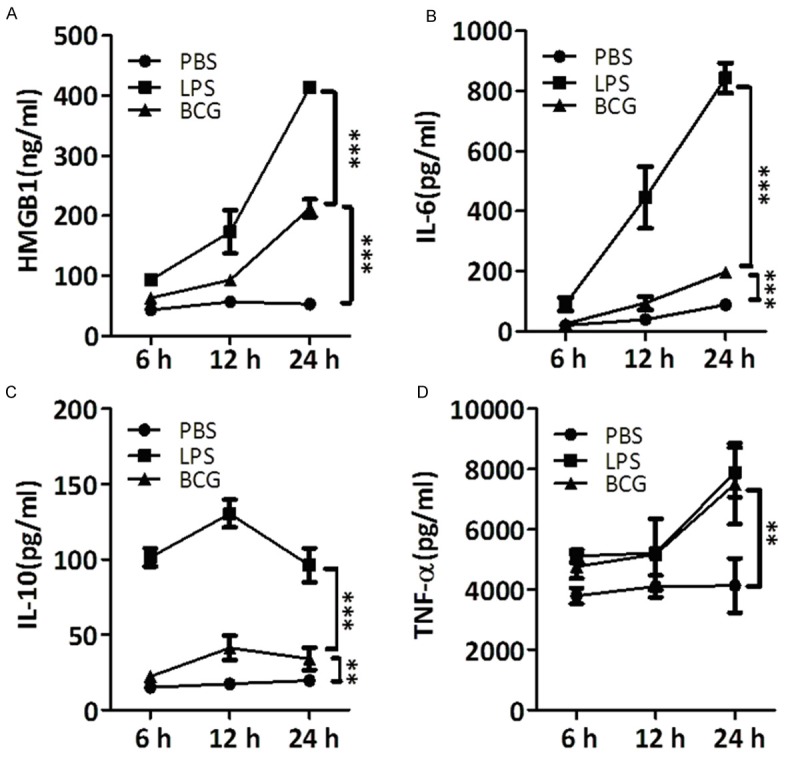
Abundance of HMGB1, IL-6, IL-10 and TNF-α in supernatant of THP-1 cells after BCG infection. PMA differentiated THP-1 cells were infected with BCG. Infection was continued for 6, 12 and 24 hrs and supernatant was collected for the detection of HMGB1, IL-6, IL-10 and TNF-α. LPS was used as a positive control and PBS was used as a negative control. The values and error bars represent average and standard error of the mean (SEM) of three independent set of experiments. *, P<0.05; **, P<0.01; ***, P<0.0001.
These results suggested that monocytes like macrophage generated HMGB1, IL-6, IL-10, and TNF-α after TB infection, but who was the instigator is unknown. These results also further supported HMGB1 handling IL-6 may be associated with monocytes dynamic responses in APTB patients. A recent report showing anti-IL-6 protein inhibits HMGB1 by macrophage also supported our hypothesis [44].
Discussion
The innate cells including macrophages, monocytes, dendritic cells (DCs), mast cells, neutrophils, eosinophils and natural killer cells were the majority producers of HMGB1, IL-10, TNF-α and IL-6 in pulmonary infection [3,45-47]. In this study, for the first time to our knowledge, we found that HMGB1 is detectable both in plasma and sputum of patients positive for sputum Mtb culture test and it was increased in comparison with healthy volunteers. Moreover, elevated levels of IL-6, IL-10 and TNF-α were also detected on the corresponding subjects in plasma and sputum. The blood levels of IL-6, IL-10 and TNF-α had been shown to increase in patients with TB infection [34-37]. Levels of TNF-α and IL-10 were elevated in sputum from TB patients, indicating that their production may be a dynamic response associated with pulmonary infection [48,49]. In contrast to the findings of TNF-α and IL-10 described above, level of IL-6 in sputum did not follow the same pattern as IFN-a, although TNF-α and IL-6 may be produced by macrophages and neutrophils, both of which represented the majority of cells found in sputum from TB patients and healthy volunteers [50].
Interestingly, level of HMGB1, IL-10 and TNF-α, but not IL-6, were significantly higher in sputum than those in plasma in APTB patients, suggesting HMGB1, IL-10, TNF-α but not IL-6 may display a more dynamic inflammatory response to TB infection in airways or lung parenchyma than blood. To better address the role of HMGB1 in the pathogenesis of TB infection, the correction of HMGB1 level between plasma and sputum, and correlations of HMGB1 with other inflammatory cytokines or blood innate immune cells were further evaluated. We showed that HMGB1 level was positively correlated with IL-6 level both in plasma and sputum of APTB patients, at the same time, both HMGB1 and IL-6 were associated with monocytes, but not with neutrophils or lymphocytes (Dates not shown) dynamic responses in APTB patients. These results suggested that HMGB1 may handle IL-6 to involve in a monocytes dynamic inflammatory response to TB infection.
Previous studies have demonstrated HMGB1 mediated the inflammatory response to initiate by TLRs (TLR2, TLR4 and TLR9) in neutrophils, monocytes, and macrophages. And IL-6 depends on a TLR4/MyD88-dependent pathway involving p38 MAPK and NF-κB [46,51,52]. Therefore, HMGB1 handling IL-6 may be associated with monocytes dynamic responses after TB infection.
To better address that HMGB1 and IL-6 could co-produce by monocytes, a PMA-treated THP-1 cells displaying a macrophage-like phenotype were used. Results showed that HMGB1 and IL-6, unlike IL-10 and TNF-α, had a similar generation process in PMA-treated THP-1 cells after BCG infection. The study of the interaction between HMGB1 and IL-6 has been reported in other disease and models. Plasma level of HMGB1 positively correlated with plasma level of IL-6 in patients with suspected community-acquired infections with sepsis, severe community-acquired infections with bacteraemia, neonatal sepsis model, but not in patients with septic shock, patients with severe sepsis and septic shock, patients with bacteraemia only, burned patients, children with febrile seizures, and mouse model of polymicrobial sepsis [36,53-59]. In addition, anti-IL-6R antibody prevented the early loss of transplanted mouse islets in parallel with reduced HMGB1 production by hepatic mononuclear cells [44]. In addition, IL-6 and HMGB1 may form a positive feedback loop to amplify the downstream signals. The concentration of HMGB1 increased as early as the elevation of IL-6 in reducing renal ischemia-reperfusion injury (IRI) [60]. HMGB1 neutralization enhanced hepatic TNF-α, IL-6 mRNA expression during acetaminophen hepatotoxicity [61]. And the L. sanguineus (Ls) IL-6 transcription was significantly induced by recombinant LsHMGB1 in HK leukocytes [62]. These results indicated that HMGB1 may play an important role in immune response by positive feedback to IL-6.
In conclusion, for the first time to our knowledge we showed that the plasma and sputum levels of HMGB1 were significantly increased in MTB infected patients in comparison with healthy donors and significantly correlated with IL-6 level, a pro-inflammatory cytokines, involved in a monocytes dynamic inflammatory response to against TB infection, although the correlation is low.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30971779, 81101553, 81273237), the Key Project of Science and Technology Innovation of Education Department of Guangdong Province (2012KJCX0059), the Science and Technology Project of Dongguan (201450715200503), the Science and Technology Project of Zhanjiang (2013C03012) and the Science and Technology Innovation Fund of GDMC (STIF201110, B2012078, M2013046).
Disclosure of conflict of interest
None.
References
- 1.Zumla A, George A, Sharma V, Herbert N, Baroness MO. WHO’s 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382:1765–1767. doi: 10.1016/S0140-6736(13)62078-4. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
- 3.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in health and disease. Mol Aspects Med. 2014;40C:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessop F, Holian A. Extracellular HMGB1 regulates multi-walled carbon nanotube-induced inflammation in vivo. Nanotoxicology. 2014 doi: 10.3109/17435390.2014.933904. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang HL, Peng LP, Chen WJ, Tang SH, Sun BZ, Wang CL, Huang R, Xu ZJ, Lei WF. HMGB1 enhances smooth muscle cell proliferation and migration in pulmonary artery remodeling. Int J Clin Exp Pathol. 2014;7:3836–3844. [PMC free article] [PubMed] [Google Scholar]
- 6.Yanai H, Taniguchi T. Nucleic acid sensing and beyond: virtues and vices of HMGB1. J Intern Med. 2014;276:444–453. doi: 10.1111/joim.12285. [DOI] [PubMed] [Google Scholar]
- 7.Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20:138–146. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, Min KU, Park HW. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42:958–965. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- 9.Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, Zou F. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17:807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011;44:601–604. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, Mantell LL. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Fei G, Liu Z, Li Q, Xu Z, Ren T. HMGB1 was a pivotal synergistic effecor for CpG oligonucleotide to enhance the progression of human lung cancer cells. Cancer Biol Ther. 2012;13:727–736. doi: 10.4161/cbt.20555. [DOI] [PubMed] [Google Scholar]
- 13.Entezari M, Javdan M, Antoine DJ, Morrow DM, Sitapara RA, Patel V, Wang M, Sharma L, Gorasiya S, Zur M, Wu W, Li J, Yang H, Ashby CR, Thomas D, Wang H, Mantell LL. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. Embo Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz R, Souza CS, Huang CT, Larsson M, Esener S, Messmer D. HMGB1-derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine. 2010;28:7556–7562. doi: 10.1016/j.vaccine.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciucci A, Gabriele I, Percario ZA, Affabris E, Colizzi V, Mancino G. HMGB1 and cord blood: its role as immuno-adjuvant factor in innate immunity. PLoS One. 2011;6:e23766. doi: 10.1371/journal.pone.0023766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagone P, Shedlock DJ, Bao H, Kawalekar OU, Yan J, Gupta D, Morrow MP, Patel A, Kobinger GP, Muthumani K, Weiner DB. Molecular adjuvant HMGB1 enhances anti-influenza immunity during DNA vaccination. Gene Ther. 2011;18:1070–1077. doi: 10.1038/gt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saenz R, Futalan D, Leutenez L, Eekhout F, Fecteau JF, Sundelius S, Sundqvist S, Larsson M, Hayashi T, Minev B, Carson D, Esener S, Messmer B, Messmer D. TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J Transl Med. 2014;12:211. doi: 10.1186/1479-5876-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schierbeck H, Pullerits R, Pruunsild C, Fischer M, Holzinger D, Laestadius Å, Sundberg E, Harris HE. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J Rheumatol. 2013;40:1604–1613. doi: 10.3899/jrheum.120987. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Zhang K, Zhao L, Guo J, Hu X, Chen Z. Increased serum HMGB1 is related to oxidative stress in patients with atrial fibrillation. J Int Med Res. 2013;41:1796–1802. doi: 10.1177/0300060513503917. [DOI] [PubMed] [Google Scholar]
- 21.Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37:957–962. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Ji X, Wu Y, Xuan J, Qi Z, Shen L, Lan L, Li Q, Yin Z, Li Z, Zhao Z. A dual-role of Gu-4 in suppressing HMGB1 secretion and blocking HMGB1 pro-inflammatory activity during inflammation. PLoS One. 2014;9:e89634. doi: 10.1371/journal.pone.0089634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One. 2014;9:e102482. doi: 10.1371/journal.pone.0102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson U, Rauvala H. Introduction: HMGB1 in inflammation and innate immunity. J Intern Med. 2011;270:296–300. doi: 10.1111/j.1365-2796.2011.02430.x. [DOI] [PubMed] [Google Scholar]
- 28.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Grover A, Troudt J, Foster C, Basaraba R, Izzo A. High mobility group box 1 acts as an adjuvant for tuberculosis subunit vaccines. Immunology. 2014;142:111–23. doi: 10.1111/imm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng JC, Lin DZ, Yi LL, Liu GB, Zhang H, Wan WD, Zhang JA, Wu XJ, Xiang WY, Kong B, Chen WZ, Wang CY, Xu JF. An essential role for BTLA in immune memory for αβ T cells against M. tuberculosis in patients with active pulmonary tuberculosis. Am J Transl Res. 2014;6:494–506. [PMC free article] [PubMed] [Google Scholar]
- 31.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang D, Qiu L, Wang R, Lai X, Du G, Seghal P, Shen Y, Shao L, Halliday L, Fortman J, Shen L, Letvin NL, Chen ZW. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J Infect Dis. 2007;195:55–69. doi: 10.1086/509895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O’Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178:822–831. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorenza G, Rateni L, Farroni MA, Bogue C, Dlugovitzky DG. TNF-αlpha, TGF-beta and NO relationship in sera from tuberculosis (TB) patients of different severity. Immunol Lett. 2005;98:45–48. doi: 10.1016/j.imlet.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Gaini S, Koldkjaer OG, Moller HJ, Pedersen C, Pedersen SS. A comparison of high-mobility group-box 1 protein, lipopolysaccharide-binding protein and procalcitonin in severe community-acquired infections and bacteraemia: a prospective study. Crit Care. 2007;11:R76. doi: 10.1186/cc5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeth J, Winkler HM, Boeck L, Adegnika AA, Clement E, Mve TM, Kremsner PG, Winkler S. Specific cytokine patterns of pulmonary tuberculosis in Central Africa. Clin Immunol. 2011;138:50–59. doi: 10.1016/j.clim.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17:374–80. doi: 10.1016/j.coi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Mahan CS, Zalwango S, Thiel BA, Malone LL, Chervenak KA, Baseke J, Dobbs D, Stein CM, Mayanja H, Joloba M, Whalen CC, Boom WH. Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. Am J Trop Med Hyg. 2012;86:690–697. doi: 10.4269/ajtmh.2012.11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Wang Y, Liu X. The role of airway epithelial cells in response to mycobacteria infection. Clin Dev Immunol. 2012;2012:791392. doi: 10.1155/2012/791392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalvani A, Behr MA, Sridhar S. Innate immunity to TB: a druggable balancing act. Cell. 2012;148:389–391. doi: 10.1016/j.cell.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T cells contain early extrapulmonary tuberculosis (TB) dissemination and rapid TB progression and sustain multieffector functions of CD8+ T and CD3- lymphocytes: mechanisms of CD4+ T cell immunity. J Immunol. 2014;192:2120–2132. doi: 10.4049/jimmunol.1301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veenstra H, Baumann R, Carroll NM, Lukey PT, Kidd M, Beyers N, Bolliger CT, van Helden PD, Walzl G. Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders. Clin Exp Immunol. 2006;145:252–260. doi: 10.1111/j.1365-2249.2006.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, Kodama S, Yasunami Y. HMGB1-Mediated Early Loss of Transplanted Islets Is Prevented by Anti-IL-6R Antibody in Mice. Pancreas. 2015;44:166–171. doi: 10.1097/MPA.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 45.Gabrysova L, Howes A, Saraiva M, O’Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014;380:157–90. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 46.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 48.Fraser CK, Lousberg EL, Kumar R, Hughes TP, Diener KR, Hayball JD. Dasatinib inhibits the secretion of TNF-αlpha following TLR stimulation in vitro and in vivo. Exp Hematol. 2009;37:1435–1444. doi: 10.1016/j.exphem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-αlpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro-Rodrigues R, Resende Co T, Johnson JL, Ribeiro F, Palaci M, Sá RT, Maciel EL, Pereira Lima FE, Dettoni V, Toossi Z, Boom WH, Dietze R, Ellner JJ, Hirsch CS. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9:818–823. doi: 10.1128/CDLI.9.4.818-823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama T, Komori A, Nakamura M, Takii Y, Kamihira T, Shimoda S, Mori T, Fujiwara S, Koyabu M, Taniguchi K, Fujioka H, Migita K, Yatsuhashi H, Ishibashi H. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK signaling pathways. Liver Int. 2006;26:467–476. doi: 10.1111/j.1478-3231.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 52.Lin CC, Lee IT, Yang YL, Lee CW, Kou YR, Yang CM. Induction of COX-2/PGE(2)/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: Role of TLR4-dependent NADPH oxidase activation. Free Radic Biol Med. 2010;48:240–254. doi: 10.1016/j.freeradbiomed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 53.Hussein MH, Kato T, Sugiura T, Daoud GA, Suzuki S, Fukuda S, Sobajima H, Kato I, Togari H. Effect of hemoperfusion using polymyxin B-immobilized fiber on IL-6, HMGB-1, and IFN gamma in a neonatal sepsis model. Pediatr Res. 2005;58:309–314. doi: 10.1203/01.PDR.0000169995.25333.F7. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Suzuki T, Ueda Y, Yamada S, Shoji H, Takeuchi M, Ueda S, Matsui T, Adachi H, Okuda S, Yamagishi S. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol Res. 2009;60:515–518. doi: 10.1016/j.phrs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 56.Gaini S, Pedersen SS, Koldkaer OG, Pedersen C, Moestrup SK, Moller HJ. New immunological serum markers in bacteraemia: anti-inflammatory soluble CD163, but not proinflammatory high mobility group-box 1 protein, is related to prognosis. Clin Exp Immunol. 2008;151:423–431. doi: 10.1111/j.1365-2249.2007.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lantos J, Foldi V, Roth E, Weber G, Bogar L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33:562–7. doi: 10.1097/SHK.0b013e3181cd8c88. [DOI] [PubMed] [Google Scholar]
- 58.Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation. 2011;8:135. doi: 10.1186/1742-2094-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014;142:216–226. doi: 10.1111/imm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miura K, Sahara H, Sekijima M, Kawai A, Waki S, Nishimura H, Setoyama K, Clayman ES, Shimizu A, Yamada K. Protective Effect of Neutralization of the Extracellular High-Mobility Group Box 1 on Renal Ischemia-Reperfusion Injury in Miniature Swine. Transplantation. 2014;98:937–43. doi: 10.1097/TP.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 61.Yang R, Zou X, Tenhunen J, Zhu S, Kajander H, Koskinen ML, Tonnessen TI. HMGB1 neutralization is associated with bacterial translocation during acetaminophen hepatotoxicity. BMC Gastroenterol. 2014;14:66. doi: 10.1186/1471-230X-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai J, Xia H, Huang Y, Lu Y, Wu Z, Jian J. Molecular cloning and characterization of high mobility group box1 (Ls-HMGB1) from humphead snapper, Lutjanus sanguineus. Fish Shellfish Immunol. 2014;40:539–544. doi: 10.1016/j.fsi.2014.08.004. [DOI] [PubMed] [Google Scholar]


