Abstract
Bax inhibitor-1 (BI-1) is a novel anti-apoptotic protein. While the effect of BI-1 on apoptosis has been extensively studied, less is known about how BI-1 is related to oncogenesis and the progression, particularly, the invasion and metastasis of cancer. In this study, we investigated the expression and function of BI-1 in human non-small cell lung cancer (NSCLC). RT-PCR and immunohistochemistry were performed on clinical NSCLC samples to detect BI-1 expression. Northern blot and Western blot analysis were performed to detect BI-1 expression of in NSCLC cell lines. Cell proliferation and apoptosis were analyzed by flow cytometry. Our results showed that the overexpression of BI-1 was significantly related to oncogenesis of NSCLC (P < 0.05). Meanwhile, we identified a novel 5’end from normal lung-derived BI-1 transcript, different from any transcript deposit in the Genbank, but we detected no mutations in the coding sequence and the promoter region of BI-1 and no abnormal splicing of the alternative first exon. Ectopic expression of BI-1 changed the proliferation and apoptosis of AGZY83-a and Anip973 cells. In conclusion, BI-1 is overexpressed in NSCLC and promotes the progression and metastasis of NSCLC.
Keywords: Bax inhibitor-1, non-small cell lung cancer, apoptosis, metastasis
Introduction
Bax inhibitor-1 (BI-1) is a highly conserved small transmembrane protein located mostly in the endoplasmic reticulum (ER) [1]. BI-1 was originally identified as an inhibitor of Bax-induced apoptosis protein and plays important role in tissue homeostasis and the regulation of cellular stress [2,3]. It was demonstrated that BI-1 interacted with Bcl-2 and Bcl-XL but not Bax and Bad, and BI-1 suppressed apoptosis induced by Bax, etoposide, staurosporine, and growth factor deprivation, but not by Fas (CD95) [4].
While the effect of BI-1 on apoptosis has been extensively studied, less is known about how BI-1 is related to oncogenesis and the progression, particularly, the invasion and metastasis of cancer. In this study, we investigated the expression and function of BI-1 in human non-small cell lung cancer (NSCLC).
Materials and methods
Cell lines and cell culture
Ten human NSCLC cell lines, L18, 95C, 95D, A549, AGZY83-a, Anip973, GLC-82, PAa, PG and SPC-a1, were provided by Department of Genetics and Cellular Biology of Harbin Medical University. 95C, 95D and PG cell lines were large-cell lung cancer cell lines, and the rest were adenocarcinoma cell lines. Moreover, 95C and 95D were of the same origin but possessed low and high metastatic potential, respectively. Anip973 was isolated from AGZY83-a, but manifested much higher metastatic potential than the parent line. All the NSCLC cell lines were grown in RPMI 1640 medium (GIBCO) containing 10% fetal bovine serum (Hyclone) and 1% penicillin/streptomycin solution. The cells were cultured at 37°C in a humidified incubator with 5% CO2 and grown to 70%-80% confluence before harvested.
Lung tissue
Lung tissue was obtained from the traumatic lung resected from a 32 year-old young man and informed consent was obtained.
Clinical samples
38 tissue specimens for RT-PCR from patients suffering from NSCLC were freshly obtained from the Second and the Third Affiliated Hospital of Harbin Medical University (Table 1). All the surgically excised specimens were stored in liquid nitrogen. All the 3 μm-thick, formaldehyde-fixed, paraffin-embedded sections from 101 primary NSCLC patients for immunohistochemistry were provided by the Pathology Department of the Third Affiliated Hospital of Harbin Medical University. All the cancer tissues and normal controls were histologically confirmed by three senior pathologists. Informed consent was obtained from all subjects.
Table 1.
Immunohistochemistry analysis of BI-1 expression in clinical samples
| Category | Total number | Positive number | Positive percentage (%) | P value |
|---|---|---|---|---|
| Normal lung tissue | 14 | 0 | 0 | P < 0.05 |
| Primary lesion | 101 | 84 | 83.17 | |
| Metastasis lymph node | 38 | 30 | 78.95 |
RT-PCR
Total RNA from the matched tissues were isolated and quantified. For semi-quantitative analysis, the exponential range of amplification of PCR was determined. It was determined that 25 cycles for BI-1 and 23 cycles for the normalization control β-actin yielded products in the exponential range of amplification using the following parameters: 94°C for 3 min; 25 or 23 cycles of 94°C for 40 s, 56°C for 40 s, 72°C for 1 min; and a final extension at 72°C for 10 min. Primers were as follows: BI-1 forward primer, 5’-GAA GAG TGG AGA CTG CTG CAC G-3’ reverse primer, 5’-GGA AAG GCT GGA TGG TCA CTT C-3’, PCR product length 768 bp; β-actin forward primer, 5’-AGC GCA AGT ACT CCG TGT G-3’; β-actin reverse primer, 5’-AAG CAA TGC TAT CAC CTC CC-3’, PCR product length 501 bp. PCR products were separated through a 1.5% agarose gel, visualized and quantitated by ethidium bromide staining.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded human. NSCLC tissue sections. The sections were deparaffinized, hydrated, placed in 10 mM citrate buffer (pH 6.0) and microwaved for 10 min (antigen retrieval). Then the sections were incubated with goat serum, BI-1 polyclonal goat IgG (G19) antibody (1:100 dilution), biotinylated secondary antibody, and HRP-conjugated streptavidin. Finally the sections were stained with DAB and counterstained with haematoxylin. Negative controls were stained as above except the replacement of the first antibody with PBS.
Non-neoplastic lung tissue 5 cm apart from the primary lesion was used as normal control. The staining was evaluated by a semi-quantitative scoring system based on staining intensity and the percentage of cells stained. The following categories were used for scoring: intensity (None, Mild, Moderate, and Strong) and percentage (< 10%, 10%-25%, 25%-50%, and > 50%). Cases were classified as positive if the intensity was strong and at least 10% of cells stained, and if the intensity score was mild or moderate and at least 25% of cells stained. Those cases with no, mild or moderate staining intensity with less than 25% of cell stained or those with strong staining but less than 10% of cells stained were referred to as negative cases.
Northern blot analysis
Total RNA was isolated from AGZY83-a and Anip973 cells, separated on a denaturing agarose gel and transferred to a Hybond-N plus nylon membrane (Amersham Pharmacia Biotech). BI-1 PCR products were purified using QIAquick Gel Extraction Kit (QIAGEN) and subjected to sequencing. BI-1 PCR products were labeled with α-32P dCTP using the random primer labeling system (Invitrogen), purified by ProbeQuant™ G-50 Micro Columns (Amersham Pharmacia Biotech) and hybridized to the multiple tissue Northern membrane (MTN, Clontech) and the membrane prepared previously in ULTRA-hyb buffer (Ambion) at 65°C for 16 h. The membranes were washed at room temperature for 10 min in 2 × SSC and 0.1% SDS (w/v) followed by washing for 10 min in 1 × SSC and 0.1 × SDS (w/v) at 65°C, and exposed to X-ray film at -70°C. To monitor the integrity and equality of RNA samples, the Hybond-N plus nylon membranes were rehybridized with a human β-actin cDNA probe.
Western blot analysis
The whole-cell lysates of NSCLC cell lines were prepared with lysis buffer containing 1 mM ethylenediaminetetraacetic acid, 0.1 M Tris-HCl (pH 7.4), 1% Triton X-100, and protease inhibitors (2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride). Lysates were resolved by SDS-PAGE in Tris-Glycine buffer and electrotransferred to nitrocellulose membrane Hybond-C (Amersham). After blocking with 5% non-fat milk in TBST (10 mM Tris pH 7.4, 142 mM NaCl; 0.1% Tween 20) at room temperature for 2 h, blots were incubated with goat polyclonal antibody against human BI-1 (G19) or goat monoclonal antibody against β-actin (both from Santa Cruz Biotechnology). After incubation with HRP-labeled donkey anti-goat IgG secondary antibody, the membranes were developed using an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia).
5’ RACE
5’ RACE was performed using the BD SMART RACE cDNA Amplification Kit (BD Biosciences, CA) according to the manufacturer’s instructions. Poly (A)+ RNA was isolated from normal lung tissue, reverse-transcribed with 5’-RACE CDS Primer, and amplified in a PCR reaction with 10 × Universal Primer A Mix and BI-1 reverse primer (5’-TCTTCAGGTGCTGCTGCGTTGACGG-3’), then the product was cloned, sequenced and analyzed using the BLASTN program at NCBI.
Mutation analysis of the distal promoter
Mutation analysis of the distal promoter P2 [5] was performed by terminal-sequencing of 452 bp PCR product targeting the according region. The primers used were as follows: BI-1-PF: 5’-ACA CTG CAT GAC AAG ACT AAA GCC-3’; BI-1-PR: 5’-TTC GCC CAG TAC TAG GTT CAG AC-3’. The primers for amplifying P2-derived exon 1 were 5’-TTG GAG AGG CGG GTT AGG AAG AGT G-3’ (located in exon 1) and 5’-CTG CAG CCG CCA CAA ACA TAC AAA-3’ (located in exon 4).
Electrophoretic mobility shift assay (EMSA)
PCR product covering the distal promoter P2 was used as target DNA fragment and the nuclear extract of AGZY83-a, Anip973, 95C and 95D were prepared by treating the cells with hypotonic buffer (0.5% NP-40, 10 mmol/L Hepes, 10 mmol/L KCl, 1 mmol/L DTT, 2 mmol/L PMSF, 30 mg/L leupeptin, 10 mg/L aprotinin, 10 mg/L pepstain, pH 7.9) and splitting the nuclei in hypertonic buffer (5 mmol/L Hepes, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L NaCl, 25% glycerol, 1 mmol/L DTT, 2 mmol/L PMSF, 30 mg/L leupeptin, 10 mg/L aprotinin, 10 mg/L pepstain, pH 7.9) successively. After protein quantification, 15 ng nuclear extract was mixed with 50 ng PCR product, 10 µg salmon sperm DNA in binding buffer (100 mmol/L Tris-HCl, 0.5 mol/L NaCl, 10 mmol/L DTT, 10 mmol/L EDTA, 50% glycerol, pH 7.4), then the results were checked by running on a 1.5% agarose gel.
Plasmid construction and transfection
BI-1 cDNA fragment was amplified from normal lung tissue using the same primers as those used for RT-PCR, purified and ligated into pcDNA 3.1 V5/His TOPO TA vector. The sense and anti-sense constructs were confirmed by sequencing. Stable transformants were generated by transfection of 1 × 105 AGZY83-a and Anip973 cells with 1 μg of the sense and anti-sense constructs using Fugene 6 Transfection Reagent (Roche) according to the manufacturer’s instruction. Cells were plated on 6-well plates in RPMI 1640 medium supplemented with 5% FBS, cultured at 37°C with 5% CO2. Two days after transfection, the cells were incubated in G418-containing selection media. The media was changed every 3 days, and after 2 weeks, single colony was isolated and grown in RPMI 1640 medium supplemented with 5% FBS. When the transfected cells grew to 70-80% confluency, living cells attached to the bottom were collected and subjected to cell growth curve, MTT colorimetry and flow cytometry analysis using routine methods.
Statistics analysis
Data were analyzed using the SPSS version 12 package (SPSS Inc., Chicago, IL, USA). The χ2 test and Fisher’s test were used to analyze the data. The results of Northern blot and Western blot analysis were analyzed by COPI (Closed optical path Image, Image Station 440CF, KODAK Co.). The data were expressed as the mean ± SD, and P < 0.05 was accepted as statistically significant.
Results
BI-1 expression is different among primary cancer, metastatic lymph nodes and normal tissues
By immunohistochemical analysis, we detected very weak and diffuse staining of BI-1 in normal tissues (Figure 1A). In contrast, in tissue sections from NSCLC samples, we detected strong BI-1 staining in epithelial cells which showed brown cytoplasmic staining around the clearly demarcated nuclei (Figure 1B). The results of immunohistochemistry analysis were summarized in Table 1.
Figure 1.
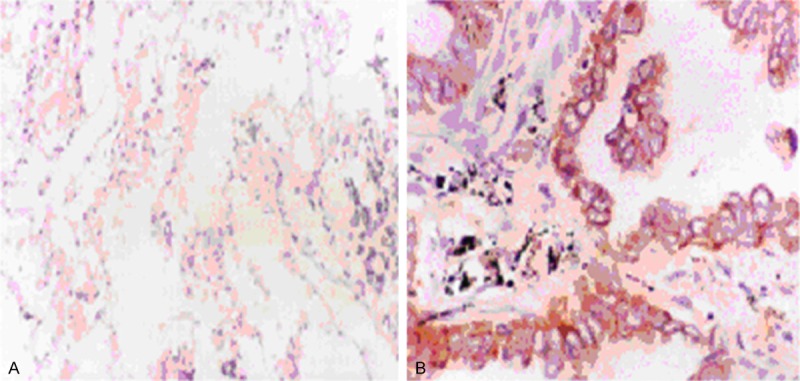
Immunohistochemistry analysis of BI-1 expression in clinical lung samples. All positively stained cells were of epithelial cells origin and showed a brown cytoplasmic staining around the clearly demarcated nuclei. Very weak, diffuse staining of BI-1 was detected in normal tissue (A), while strong staining of BI-1 was detected in tumor tissue (B).
Northern blot analysis of BI-1 expression in AGZY83-a, Anip973 and normal multi-tissues
Next we detected BI-1 mRNA expression in AGZY83-a and Anip973 cells which have the same cell origin but different metastasis potential. By Northern blot analysis we detected two transcripts: one of 2.8 kb and the other of 1.0 kb. Both of the transcripts were more intense in AGZY83-a compared to Anip973 (P < 0.05, Figure 2A). Northern blot analysis of multi-tissue membrane showed that BI-1 was widely expressed in all tissues tested, especially in the kidney, liver, skeletal muscle and placenta, while BI-1 expression in the lung tissue is not so strong (Figure 2B).
Figure 2.
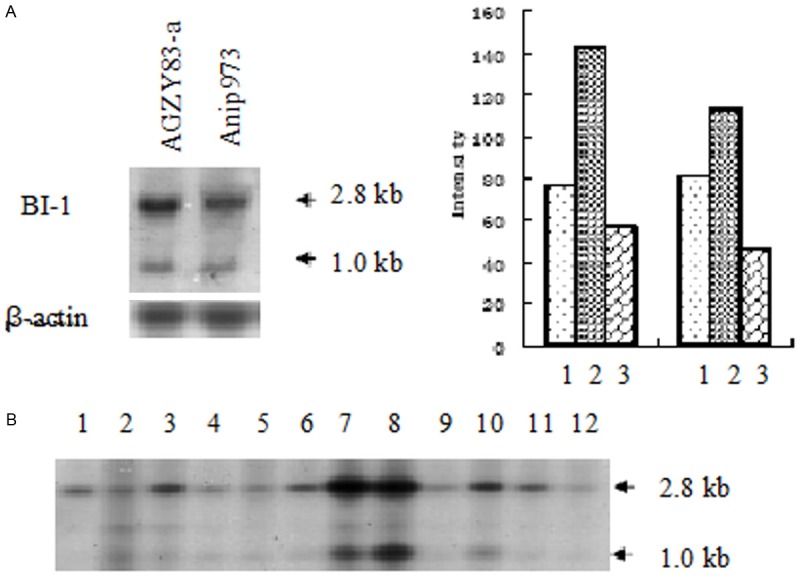
Northern blot analysis of BI-1 expression in lung cancer cells. A: AGZY83-a and Anip973 cells. 1. β-actin; 2. BI-1 2.8 kb; 3. BI-1 1.0 kb. B: Normal multitissue membrane. 1. Brain; 2. Heart; 3. Skeletal muscle; 4. Colon; 5. Thymus; 6. Spleen; 7. Kidney; 8. Liver; 9. Small intestine; 10. Placenta; 11. Lung; 12. peripheral leukocytes. The arrows indicated the 2.8 kb and 1.0 kb transcripts. Both of the transcripts were more intense in AGZY83-a compared to those in Anip973 (P < 0.05).
Western blot analysis of BI-1 expression in NSCLC cell lines
Next we detected BI-1 expression in 10 NSCLC cell lines by Western blot analysis. The results showed that a 26.5 KD band indicating BI-1 was detected in all 10 NSCLC cell lines by BI-1 antobody, although BI-1 expression was weak in Anip973 cells (Figure 3).
Figure 3.
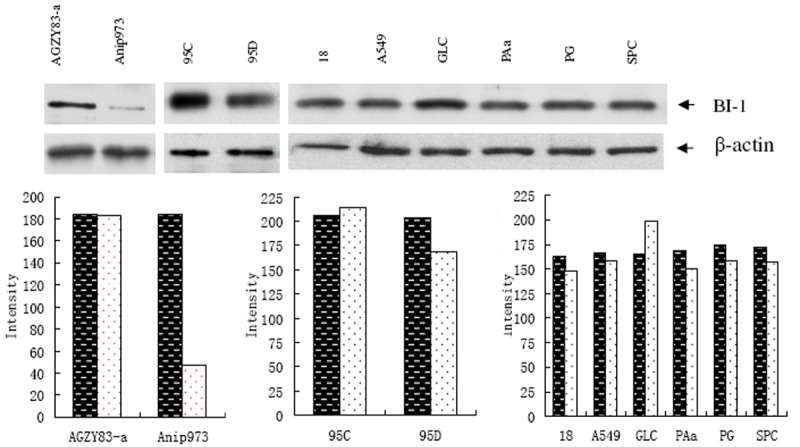
Western blot analysis of BI-1 expression in 10 NSCLC cell lines. Each lane of the blots showed a 26.5 KD band of BI-1, and the immunoreactive bands were significantly stronger in AGZY83-a and 95C than in Anip973 (P < 0.01) and 95D (P < 0.05). β-actin was loading control. The black columns indicated the levels of β-actin while white columns indicated those of BI-1.
A novel transcription start point of BI-1 transcript from human lung tissue
By 5’ RACE, we analyzed the full length mRNA isolated from normal lung tissue. The results showed that it is mostly identical to BC000916 (gi: 13111818), except a specific sequence (5’-AGA GCA CAT CCG GTG TTA GA-3’), 20 bp longer at the 5’ end. To our knowledge, it was the first discovery of this unique transcript in human lung tissue.
No abnormity is detected in the distal promoter region and the 5’ end splicing variant of BI-1 in human lung
BI-1 was reported to have two promoters that generate alternative first exon correspondingly, and the lung-derived transcript originated from the more distal promoter P2 [5]. Thus we further examined whether the mutations in BI-1 promoter region contribute to the regulation of gene expression. The sequencing results revealed no mutation in 452 bp fragment containing P2 from AGZY83-a and Anip973 cells, compared to normal lung. Furthermore, we examine the alternative splicing of the alternative first exon. We used a pair of primers with the sense primer for P2-specific exon 1 and the antisense primer for exon 4. Sequence analysis showed that we got the correct band of 181 bp, which indicated that exon 1 was accurately spliced.
DNA-binding proteins regulate the expression of BI-1
Although there was no TATA element at the usual position, putative binding sites for Sp1 and CAAT-NF1 were identified within the 452 bp fragment of P2. Thus we performed EMSA and the results indicated that each lane showed a decreased original band and a retarded band located at a position larger than 2 kb (Figure 4). When equal amount of PCR product and nuclear extracts from each cell line were used, the shifted bands were much stronger in 95D and Anip973 cells than in 95C and AGZY83-a cells, which suggested that more DNA-binding proteins participated in the regulation of BI-1 expression in 95D and Anip973 cells with higher metastatic potential.
Figure 4.
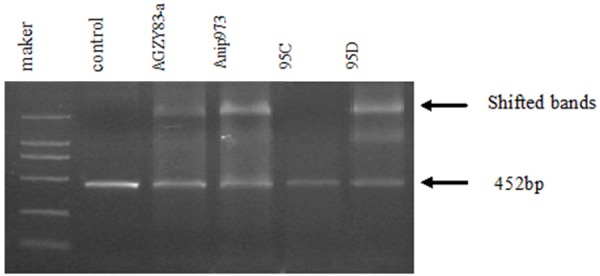
EMSA analysis. Equal amount of untreated PCR product was used as control. A decreased original band and a retarded band larger than 2 kb were detected in almost all the lanes (indicated by arrows), and the shifted bands were much stronger in 95D and Anip973 than in 95C and AGZY83-a.
BI-1 overexpression changes biological behaviors of AGZY83-a and Anip973 cells
AGZY83-a and Anip973 cells were transfected with BI-1 sense and antisense constructs. After selection with G418, AGZY83-a cells transfected with antisense construct (Anti-AGZY) died while other transfected cells were alive and kept in G418 selection medium until colonies were screened out. Cell growth curve and MTT assay revealed that AGZY83-a and Anip973 cells transfected with BI-1 sense construct grew faster and the cells transfected with antisense construct grew slower than the parent cell lines (data not shown). Flow cytometric analysis showed that the ratio of G0-G1 phase was increased in AGZY83-a and Anip973 cells transfected with sense construct (P < 0.05, Figure 5). In addition, the ratio of apoptosis in AGZY-BI-1 cells dropped from 0.59% to 0.04%, while the ratio of apoptosis in Anip-BI-1 cells dropped by 0.05%.
Figure 5.
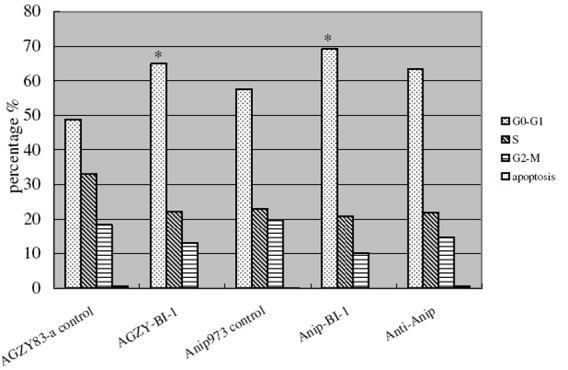
Flow cytometric analysis of cell proliferation and apoptosis. The percentages of apoptotic cells were decreased in AGZY-BI-1 and Anip-BI-1, and the percentages of cell in the different phase of cell cycle varied after transfection. The ratios of cells in G0-G1 were increased after transfection of sense construct both in AGZY83-a and in Anip973. *P < 0.05.
Discussion
As a novel anti-apoptotic protein, Bax inhibitor-1 could promote cell survival, enhance cell vitality, and consequently promote oncogenesis [6]. BI-1 expression has been shown to be upregulated in breast cancer, glioma and prostate carcinoma compared with normal tissues [7-10]. In this report, we examined BI-1 expression in clinical NSCLC samples by RT-PCR and immunohistochemistry analysis. The results validated a significant difference in BI-1 expression between the primary cancer lesion and the adjacent normal lung tissue.
Next, to clarify the role of BI-1 in the metastasis of NSCLC, we employed cell lines with different metastatic potential. Anip973 cell line was derived from AGZY83-a, but gained much higher metastatic potential after being continuously implanted and passaged into the celiac cavity of nude mice. By Northern blot and Western blot, we confirmed the overexpression of BI-1 in AGZY83-a versus Anip973 cells. It seemed to suggest the positive correlation of BI-1 overexpression and low metastatic potential. As the main regulators in the apoptosis pathway, members of the Bcl-2 protein family function to either inhibit (Bcl-2 and Bcl-XL) or promote (Bax and Bak) apoptosis [11,12]. The ratio of Bax homodimers to Bax/Bcl-2 heterodimers is an apoptotic checkpoint [13,14]. BI-1 has been shown to represent a new type of regulator of cell death pathways controlled by Bcl-2 and Bax. Although it interacts directly with Bcl-2 and not Bax, BI-1 can suppress Bax-induced apoptosis. Numerous studies have demonstrated that the inhibition of apoptosis encourages metastasis [15]. Thus we supposed that there may be other apoptosis-related factors which can overwhelm the role of BI-1 in the metastasis.
To understand the molecular mechanisms underlying the differential expression of BI-1 in lung cancer and normal lung, we examined AGZY-83a and Anip973 cell lines for the existence of mutation in the coding sequence, the lung-specific promoter of BI-1, and the abnormal splicing of the corresponding first exon to the downstream exon. The results showed that there were no mutations in the coding sequence and promoter and no abnormal splicing. Several binding sites for ubiquitous factors such as Sp1, AP2, NF-kB, CAAT-CP2 and CAAT-NF1 reside upstream of BI-1 P2 [5]. Therefore, we performed EMSA to detect the sequence-specific DNA-binding proteins on BI-1 promoter and the results showed that there were more DNA-binding proteins retarded in 95D and Anip973 cells than in 95C and AGZY83-a cells. These data suggest a potential mechanism to regulate the differential expression of BI-1 in cells with different metastatic potential. Further studies are needed to identify the specific DNA-binding factors on BI-1 promoter.
To explore the biological function of BI-1 in lung cancer cells, we transfected the sense and antisense BI-1 constructs into AGZY83-a and Anip973 cells. MTT and flow cytometric analysis showed that BI-1 overexpression affected the growth, cell cycle and apoptosis of AGZY83-a and Anip973 cells. These data indicate that BI-1 may promote lung cancer progression and metastasis through the regulation of lung cancer cell proliferation and apoptosis.
In summary, in the present study we demonstrated that BI-1 is overexpressed in the clinical samples and cell lines of NSCLC and BI-1 may promote the progression and metastasis of NSCLC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (NO. 39970396, NO. 30170516), Scientific Research Fund of Heilongjiang Provincial Education Department (NO. 10553044) and Scientific Research Fund of Heilongjiang Provincial Health Department (NO. 2005-150).
Disclosure of conflict of interest
None.
References
- 1.Reimers K, Choi CY, Bucan V, Vogt PM. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr Mol Med. 2008;8:148–56. doi: 10.2174/156652408783769562. [DOI] [PubMed] [Google Scholar]
- 2.Walter L, Marynen P, Szpirer J, Levan G, Günther E. Identification of a novel conserved human gene, TEGT. Genomics. 1995;28:301–304. doi: 10.1006/geno.1995.1145. [DOI] [PubMed] [Google Scholar]
- 3.Robinson KS, Clements A, Williams AC, Berger CN, Frankel G. Bax inhibitor 1 in apoptosis and disease. Oncogene. 2011;30:2391–400. doi: 10.1038/onc.2010.636. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q, Reed JC. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1:337–346. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- 5.Jean JC, Oakes SM, Joyce-Brady M. The Bax Inhibitor-1 Gene Is Differentially Regulated in Adult Testis and Developing Lung by Two Alternative TATA-less Promoters. Genomics. 1999;57:201–208. doi: 10.1006/geno.1999.5761. [DOI] [PubMed] [Google Scholar]
- 6.Glinsky GV, Glinsky VV, Ivanova AB, Hueser CJ. Apoptosis and metastasis: increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett. 1997;115:185–193. doi: 10.1016/s0304-3835(97)04738-1. [DOI] [PubMed] [Google Scholar]
- 7.Van’t Veer LJ, Dai H, Van de vijver MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 8.Schmits R, Cochlovius B, Teitz G, Regitz E, Ketter R, Preuss KD, Romeike BF, Pfreundschuh M. Analysis of the antibody repertoire of astrocytoma patients against antigens expressed by gliomas. Int J Cancer. 2002;98:73–77. doi: 10.1002/ijc.10170. [DOI] [PubMed] [Google Scholar]
- 9.Wdlsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF Jr, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets I prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 10.Grzmil M, Thelen P, Hemmerlein B, Schweyer S, Voigt S, Mury D, Burfeind P. Bax Inhibitor-1 Is Overexpressed in Prostate Cancer and Its Specific Down-Regulation by RNA Inference Leads to Cell Death in Human Prostate Carcinoma Cells. AM J Pathol. 2003;163:543–552. doi: 10.1016/S0002-9440(10)63682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai M, Pan L, Reed JC, Uchimiya H. Evolutionally conserved plant homologue of the Bax inhibitor-1 (BI-1) gene capable of suppressing Bax-induced cell death in yeast(1) FEBS Lett. 1999;464:143–147. doi: 10.1016/s0014-5793(99)01695-6. [DOI] [PubMed] [Google Scholar]
- 12.Driak D, Dvorska M, Bolehovska P, Svandova I, Novotny J, Halaska M. Bad and Bid-potential background players in preneoplastic to neoplastic shift in human endometrium. Neoplasma. 2014;61:411–415. doi: 10.4149/neo_2014_050. [DOI] [PubMed] [Google Scholar]
- 13.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 14.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XM, Gu H, Yan L, Zhang GY. RKIP inhibits the malignant phenotypes of gastric cancer cells. Neoplasma. 2013;60:196–202. doi: 10.4149/neo_2013_026. [DOI] [PubMed] [Google Scholar]


