Abstract
Gastric cancer is the second leading cause of cancer-related death worldwide. Survivin overexpressed in many human cancers as a member of the inhibitor of apoptosis protein family. We found that all samples of normal gastric tissues did not express the protein of survivin, and however, 65% human gastric cancer samples expressed survivin. Positive expression of survivin correlated with differentiation. The proliferation and migration of gastric cancer decreased after downregulation of surviving by RNA interference. Furthermore, downregulation of survivin caused the cell cycle arrest. These suggest that survivin play an important role in gastric cancer and the use of survivin siRNA might become an effective approach to cancer therapy.
Keywords: Survivin, gastric cancer, proliferation, migration, cell cycle
Introduction
Gastric cancer is the second leading cause of cancer-related death worldwide [1]. In China, the morbidity and mortality of gastric cancer has higher place among malignant tumors [2]. In these years the incidence of gastric cancer still is increasing. Further studies are needed to explore mechanisms for tumorigenesis and progression to help improve patients’ therapy.
Survivin is a 16.5 kD protein with 142 amino acid residues that participate in apoptosis regulation originally as a member of the inhibitor of apoptosis protein (IAP) family [3,4]. Survivin is overexpressed in many human cancers, including gastric cancer [5-9]. In this study, we will further investigate the role of survivin in gastric cancer.
Materials and methods
Samples
Forty cases of gastric cancers and ten cases of normal gastric tissues were obtained on protocols approved by the Affiliated Hospital of Xi’an Medical College. The tissues were paraffin embedded, and cut at a thickness of 4 μm. The slices were mounted on microscope slides. The slices were then fixed in 4% formalin, and stained using H&E to determine the pathological type and grade. The histopathological grade and clinical stage of gastric cancer were defined according to the criteria of the World Health Organization (WHO, 2004). The study protocol was approved by the Ethics Committee of Xi’an Medical College, and informed consent was obtained from the included patients.
Immunohistochemistry
The sections were mounted on microscope slides, air-dried and then fixed in a mixture of 50% acetone and 50% methanol. The sections were then de-waxed with xylene, gradually hydrated with a gradient alcohol series, and washed with PBS. Sections were incubated for overnight with the primary antibody (1:100 dilution survivin, Santa Cruz, CA). Following washings with PBS, sections were incubated for 30 min in the secondary antibody. Tissue sections were then counterstained with hematoxylin, dehydrated, and mounted.
MTT assay
The cell growth was analyzed with the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. In brief, 2 × 103 cells were seeded and cell growth was measured daily until day 5. A volume of 20 μl of MTT (Sigma) solution (5 mg/ml) was then added and cells were further incubated at 37°C for 4 h. Then, the culture medium was removed and replaced with 200 μl of dimethyl sulfoxide (DMSO) to dissolve the formazan product. The optical density (OD) at 570 nm was determined with a microplate reader.
Cell colony formation assay
The cells were seeded in 6-well plates and were incubated at 37°C for 2 weeks. The medium was replaced every 3 days. After washing twice with PBS, the colonies were fixed with ice methanol for 30 min and stained with Giemsa for 10 min. Then, the visible colonies were counted.
Migration assay
Wound healing assay was utilized to analyze cell migration. AGS cells were grown to confluence in 3.5 cm culture dishes in DMEM with 10% FBS for 24 h. The monolayers were then scratched with a 200 μl-pipette tip. Migration of the cells was detected under a microscope. The wound margin distances between the two edges of the migrating cell sheets were measured at 0, 48 h after scratching. The relative migrating distance of cells is measured by the distance of cell migration/the distance measured at 0 h.
Flow cytometry
To analyze the cell cycle 1 × 106 cells were harvested, washed with cold PBS, and fixed in 70% ice-cold ethanol overnight at 4°C. The cells were resuspended in PBS containing 50 μg/ml of PI and analyzed by FCM.
Western blot
Firstly, the proteins were loading and separated using SDS-PAGE. And then the proteins were transferred onto a polyvinylidene fluoride membrane. The membranes were blocked in 5% non-fat milk and incubated overnight at 4°C with primary antibodies. The membranes were then incubated for 1 h at room temperature with secondary antibody. Finally, immunoreactive bands were visualized with enhanced chemiluminescence.
Statistical analysis
The results were expressed as the means ± SE. Statistical analysis was carried out by the One-way ANOVA. The possible associations between FASN expression and clinico-pathological parameters were assessed using χ2 test. Probability P values < 0.05 are considered as significant. All these tests were performed using the Statistical Package for Social Sciences (SPSS) 6.0 for Windows (SPSS Inc.).
Results
Survivin expression in gastric cancer and normal gastric tissues
Immunochemical staining tests showed that survivin was mainly localized in the cytoplasm of esophageal cancer cells (Figure 1). However, all samples of normal gastric tissues did not express the protein of survivin. Survivin was detected in 26/40 (65%) human gastric cancer samples. Positive expression of survivin did not correlate with age, gender, stage and lymph node metastasis (P > 0.05). Surviving expression only correlated with differentiation (Table 1). The positive expression of survivin in low differentiation samples was higher than high differentiation ones (P < 0.05). Thus, immunochemical staining showed that survivin might play an important role in gastric cancer progression.
Figure 1.
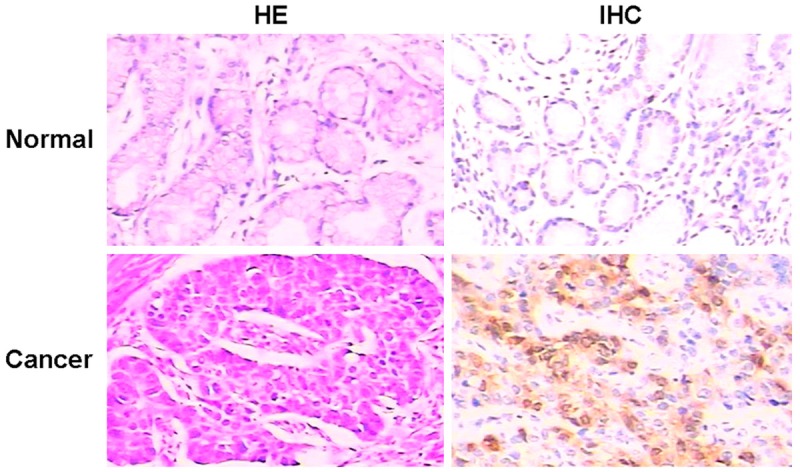
Histological appearance and survivin expression in normal gastric tissues. Survivin was detected in human gastric cancer samples. All cases of normal gastric tissues did not express the protein of surviving (HE or IHC, 200 ×).
Table 1.
Relationship between expression of Survivin and clinicopathological parameters in gastric cancer
| Variable | Cases | Positive (%) | Negative (%) | χ2 | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 28 | 18 (64%) | 10 (36%) | 0.021 | 0.885 |
| Female | 12 | 8 (67%) | 4 (33%) | ||
| Age | |||||
| ≤ 60 | 18 | 12 (67%) | 6 (33%) | 0.040 | 0.842 |
| > 60 | 22 | 14 (64%) | 8 (36%) | ||
| Differentiation | |||||
| High | 12 | 6 (50%) | 6 (50%) | ||
| Median | 14 | 8 (57%) | 6 (43%) | ||
| Low | 14 | 12 (86%) | 2 (14%) | 3.869 | 0.049 |
| Stage | |||||
| I/II | 12 | 6 (50%) | 6 (50%) | 1.695 | 0.193 |
| III/IV | 28 | 20 (71%) | 8 (29%) | ||
| LN metastasis | |||||
| Positive | 26 | 18 (69%) | 8 (31%) | 0.584 | 0.445 |
| Negative | 14 | 8 (57%) | 6 (43%) |
Effect of downregulation of survivin on the growth of AGS cells
To investigate the effect of survivin expression on growth potential, gastric cell AGS was transfected with survivin siRNA and performed the MTT and colony formation assay. Cellls transfected with survivin siRNA significantly decreased the protein levels of survivin compared with those transfected with negative control siRNA (Figure 2A).
Figure 2.
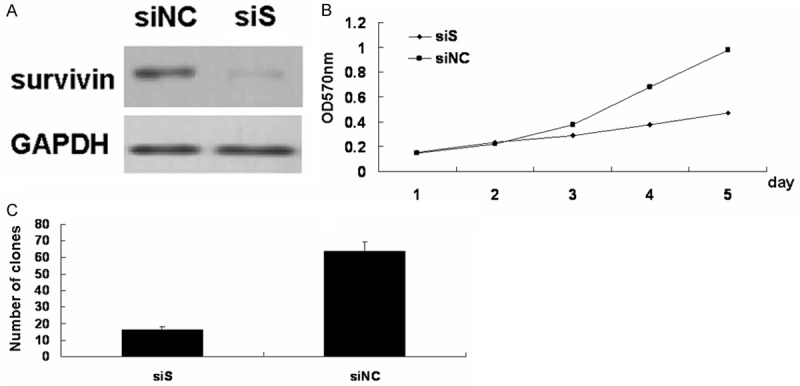
Effect of survivin on AGS cells growth. A. Western blot analysis of survivin protein in siRNA (siS) AGS cells, the negative control (siNC) cells. B. Proliferation of AGS cells was analyzed by MTT. C. Colony formation analysis of AGS cells. (*P < 0.05; siS versus siNC AGS cells).
The proliferation of AGS cells was detected by MTT assay. As shown in Figure 2B, survivin siRNA (siS) AGS cells grew more slowly than the negative control (siNC) cells in a successive 5-day observation. Next, the colony formation was further analyzed. We found that the colony numbers decreased after downregulation of surviving in AGS cells (Figure 2C).
Effect of downregulation of survivin on the migration of AGS cells
The migration ability of AGS cells was measured by wound healing at 24 h after scratching. Downregulation of survivin expression significantly reduced the migration ability of AGS cells, compared with the control group (P < 0.05) (Figure 3).
Figure 3.
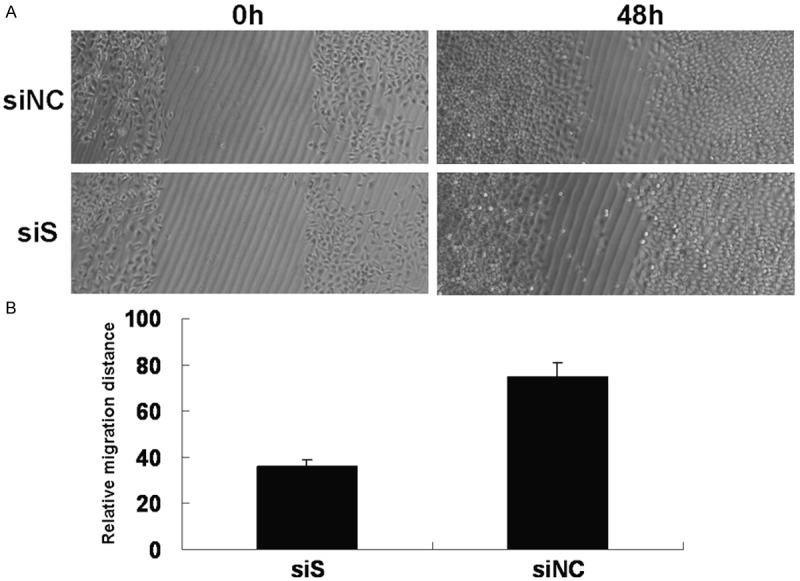
Effect of survivin on AGS cells migration. A. The ability of AGS cells in wound healing was determined by measuring the migration distance at 48 h after scratching. B. Quantitative analysis of the migration distance. (*P < 0.05; siS versus siNC AGS cells).
Effect of downregulation of survivin on the cell cycle of AGS cells
The cell cycle was analyzed after AGS was transfected with survivin siRNA in 48h. An increased fraction of G0/G1 phase was found after downregultion of surviving (Table 2). This result showed that downregulation of survivin arrested cell cycle.
Table 2.
Cell cycle analysis
| G0/G1% | S% | G2/M% | |
|---|---|---|---|
| siNC | 22.34 ± 2.09 | 21.07 ± 2.06 | 56.69 ± 4.38 |
| siS | 65.13 ± 4.24 | 20.24 ± 1.36 | 14.63 ± 1.38 |
Effect of downregulation of survivin on caspase-3, caspase-8 and caspase-9
Furthermore, the caspase-3, caspase-8 and caspase-9 were investigated after knockdown of survivin in AGS cells. The protein level of caspase-3 was significantly decreased, however, the protein levels of caspase-8 and caspase-9 were still stable (Figure 4).
Figure 4.
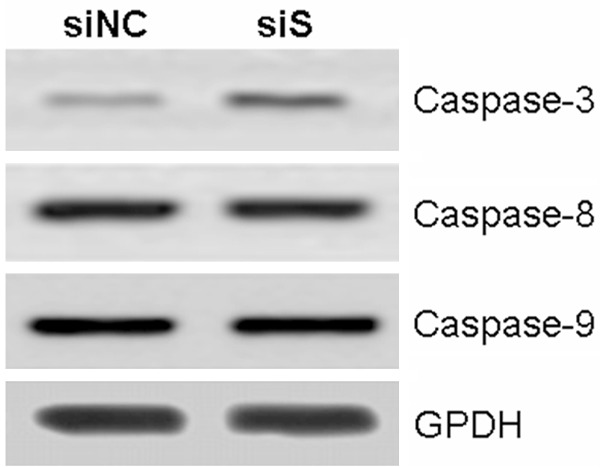
Effect of downregulation of survivin on caspase-3, caspase-8 and caspase-9.
Discussion
Survivin is overexpressed in cancers. Survivin facilitates cancer cell survival and growth by inhibiting apoptosis and promoting mitosis [10-12]. There are some reports that survivin might be a tumor marker in the diagnosis of esophageal cancer, colorectal cancer, non-small cell lung cancer (NSCLC), gastric cancer, and breast cancer [13-17]. Overexpression of survivin is associated with the occurrence and development of gastric cancer [18]. Survivin knockdown increased anti-cancer effects of (-)-epigallocatechin-3-gallate in human malignant neuroblastoma [19]. Knockdown of survivin expression enhanced sensitivity of gastric cancer cells SGC7901 to radiation, cisplatin and 5-FU treatment in vitro and in vivo [20]. Down-regulation of survivin by viral vector (Cys84Ala) also inhibited cell proliferation and induced apoptosis and mitotic catastrophe in colorectal cancer in vitro and in vivo [21].
In this study all samples of normal gastric tissues did not express the protein of surviving, however, survivin was detected in 65% human gastric cancer samples. Moreover, surviving expression correlated with differentiation. These demonstrated that survivin is important in gastric cancer progression. RNA interference in mammalian cells and induce strong inhibition of specific gene expression. In this work RNA interference was used to downregulated the expression of survivin. The proliferation and migration of gastric cancer decreased after downregulation of survivin. Furthermore, downregulation of surviving caused the cell cycle arrest. This suggested that cell cycle arrest in G0/G1 phrase induced the decrease of proliferation and migration of gastric cancer. The decreased protein level of caspase-3 showed the interactive molecule with survivin in gastric cancer. In conclusion, our results suggest that survivin play an important role in gastric cancer and the use of survivin siRNA might become effective approach to cancer therapy.
Acknowledgements
This study was was supported by Scientific Research Program Funded by Shaanxi Provincial Education Department (Program No. 12JK0765), Scientific Program Funded by Xi’an Medical University (Program No. 11FZ07) and Scientific Research Program Funded by The First Affiliated Hospital of Xi’an Medical University (Program No. XYFY10-04).
Disclosure of conflict of interest
None.
References
- 1.Ueda SM, Mao TL, Kuhajda FP, Vasoontara C, Giuntoli RL, Bristow RE, Kurman RJ, Shih IeM. Trophoblastic neoplasms express fatty acid synthase, which may be a therapeutic target via its inhibitor C93. Am J Pathol. 2009;175:2618–2624. doi: 10.2353/ajpath.2009.081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 4.Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92:212–216. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 6.Tsuburaya A, Noguchi Y, Yoshikawa T, Saito A, Doi C, Okamoto T. An anti-apoptosis gene, survivin and telomerase expression in gastric cancer. Hepatogastroenterology. 2002;46:1150–1152. [PubMed] [Google Scholar]
- 7.Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984–1988. doi: 10.3748/wjg.v10.i13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiaoyun L, Nanzhi L. The correlation of Survivin expression in gastric cancer tissue and the expression of caspase-3 and p53. Journal of Huazhong University of Health Sciences and Technology. 2004;33:724–725. [Google Scholar]
- 9.Cao W, Yang W, Li H, Lou G, Jiang J, Geng M, Xi W. Using Detection of Survivin-Expressing Circulating Tumor Cells in Peripheral Blood to Predict Tumor Recurrence Following Curative Resection of Gastric Cancer. J Surg Oncol. 2011;103:110–115. doi: 10.1002/jso.21777. [DOI] [PubMed] [Google Scholar]
- 10.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 11.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 12.Altieri DC. Molecular circuits of apoptosis regulation and cell division control: the survivin paradigm. J Cell Biochem. 2004;92:656–663. doi: 10.1002/jcb.20140. [DOI] [PubMed] [Google Scholar]
- 13.Cao M, Yie SM, Wu SM, Chen S, Lou B, He X, Ye SR. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis. 2009;26:751–758. doi: 10.1007/s10585-009-9274-7. [DOI] [PubMed] [Google Scholar]
- 14.Stanilov N, Miteva L, Mintchev N, Stanilova S. High expression of Foxp3, IL-23p19 and survivin mRNA in colorectal carcinoma. Int J Colorectal Dis. 2009;24:151–157. doi: 10.1007/s00384-008-0588-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen YQ, Zhao CL, Li W. Effect of hypoxia-inducible factor-1alpha on transcription of survivin in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28:29. doi: 10.1186/1756-9966-28-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen C, Hu L, Xia L, Li Y. The detection of circulating tumor cells of breast cancer patients by using multimarker (Survivin, hTERT and hMAM) quantitative real-time PCR. Clin Biochem. 2009;42:194–200. doi: 10.1016/j.clinbiochem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Yie SM, Lou B, Ye SR, Cao M, He X, Li P, Hu K, Rao L, Wu SM, Xiao HB, Gao E. Detection of survivin-expressing circulating cancer cells (CCCs) in peripheral blood of patients with gastric and colorectal cancer reveals high risks of relapse. Ann Surg Oncol. 2008;15:3073–3082. doi: 10.1245/s10434-008-0069-x. [DOI] [PubMed] [Google Scholar]
- 18.Miyachi K, Sasaki K, Onodera S, Taguchi T, Nagamachi M, Kaneko H, Sunagawa M. Correlation between surviving mRNA expression and lymph node metastasis in gastric cancer. Gastric Cancer. 2003;6:217–224. doi: 10.1007/s10120-003-0255-2. [DOI] [PubMed] [Google Scholar]
- 19.Hossain MM, Banik NL, Ray SK. Survivin knockdown increased anti-cancer effects of (-)-epigallocatechin-3-gallate in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Exp Cell Res. 2012;318:1597–610. doi: 10.1016/j.yexcr.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen X, Zheng JY, Shi H, Zhang Z, Wang WZ. Survivin Knockdown Enhances Gastric Cancer Cell Sensitivity to Radiation and Chemotherapy In Vitro and in Nude Mice. Am J Med Sci. 2012;344:52–8. doi: 10.1097/MAJ.0b013e318239c4ee. [DOI] [PubMed] [Google Scholar]
- 21.Tu SP, Cui JT, Liston P, Huajiang X, Xu R, Lin MC, Zhu YB. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of surviving Cys84Ala mutant. Gastroenterology. 2005;128:361–375. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]


